Isolation and Characterization of Starch obtained from Cocoyam cultivated at Akungba Akoko, Ondo State, Nigeria
Jacob Olalekan Arawande*1,2 and Adeleke Omodunbi Ashogbon2
1Chemistry Department, University of Medical Sciences, Nigeria
2Department of Chemical Sciences, Adekunle Ajasin University, Nigeria
Submission: October 25, 2018; Published: January 10, 2019
*Corresponding author: Dr. Jacob Olalekan Arawande, Chemistry Department, University of Medical Sciences, PMB. 536, Ondo-City, Ondo-State, Nigeria
How to cite this article: AJacob O A, Adeleke O A. Isolation and Characterization of Starch obtained from Cocoyam cultivated at Akungba Akoko, Ondo State, Nigeria. Nutri Food Sci Int J. 2019. 8(2): 555732. DOI:10.19080/NFSIJ.2018.08.555732.
Abstract
This research was carried out to determine the yield, chemical composition, functional, pasting and gelatinization properties of starch isolated from cocoyam cultivated at Akungba Akoko. Cocoyam starch was isolated from matured corms of cocoyam and was analyzed for percentage yield, chemical composition, functional and pasting properties. The percentage yield of starch in the cocoyam was 11.47±0.11%. The starch contained moisture content, crude protein, lipid, ash, amy lose, amylopectin and phosphorus of 15.05±0.19%, 0.05±0.00%, 0.07±0.00%, 0.21±0.02%, 23.50±0.02%, 76.50±0.01% and 0.76±0.18mg/100g respectively. The pH, bulk density, dispersibility, water binding capacity and water absorption index of 7.19±0.02, 0.88±0.09 g/ml, 86.50±0.18%, 72.13±0.26% and 162.51±0.42% accordingly. The swelling power of the starch ranged between 0.79±0.02 and 7.96±0.10 while its solubility ranged between 6.12±0.09g/100g and 14.03±0.14 g/100g. The peak, trough, breakdown, setback and final viscosities of the starch were 480.63±0.02, 245.75±0.09, 234.88±0.18, 123.75±0.26 and 368.38±0.42 RUV respectively while its peak time and pasting temperature were 5.01±0.00 minutes and 84.23±0.01℃. The onset, peak and conclusion temperatures of the starch were found to be 81.30±0.11℃, 85.20±0.19℃ and 93.00±0.21℃ accordingly and its enthalpy of gelatinization was 12.10±0.13J/g. Cocoyam starch possessed excellent qualities that can make it useful for food and non- food applications.
Keywords: Chemical composition; Functional properties; Pasting properties; Gelatinization properties; Cocoyam starch
Introduction
Akungba Akoko is a town located in Akoko land, Ondo-State, Nigeria where cocoyam is one of the commonest cultivated plants. The cocoyam cultivated in this part of the country is given much attention because it is a common food usually consumed especially during the period of “food scarcity” when the normal yam is out of stock around January to March before the availability of early year maize in April/May. Due to much availability of cocoyam at this period of the year, it is being underutilized thereby leading to wastage because most people in the town usually cultivate the plant since farming is the major preoccupation in the town. Though a State University is located in the ancient town, most workers of the University don’t reside in the town as a result of lack of regular supply of electricity hence workers prefer to live in nearby towns and city.
Starch is a renewable, biodegradable, edible polymer as a major storage polysaccharide in plants with natural abundance next to cellulose and chitin. Starch plays an important role in food products, either as a major component or as a food additive [1]. It is an important naturally occurring polymer of glucose, with diverse applications in food and polymer science, found in roots, rhizomes, seeds, stems, tubers and corms of plants, as microscopic granules having characteristic shapes and sizes [2]. Annual worldwide starch production is 66.5 million tons and the growing demand for starches has created interest in identifying new sources and modifications or derivatives of this polysaccharide [3]. The different botanical sources of starches are cereal, legume, root and tuber [4]. Starch is the basic source of energy for majority of the world’s population. In human nutrition, starch plays a major part in supplying the metabolic energy that enable the body to perform its different functions [5]. It is found accompany with other reserved such as protein, fats, lipids and some phosphates. Starch consists of major and minor components. The major components of starch are amylose (normally 20-30%) and amylopectin (normally 70-80%) [6] while the minor components are lipids, protein, phosphorus and mineral [7]. Although, these components are at very low level in the starch granules but they play an important role in the physicochemical properties of starch [8]. Utilization of starch in both food and non-food industries depends on its physical, chemical and functional properties [9]. These properties are unique for different crops and varieties. Therefore, understanding the physiochemical and functional properties of starch from different sources can help in utilization of starch for the different applications [10].
Though much research works have been reported on starch and cocoyam. Aboubakar studied physiochemical, thermal properties and microstructure of taro (Colocasia esculenta) flours and starches. Adebayo & Itiola [11] reported the evaluation of breadfruit and cocoyam starches as exodis-integrants in a paracetamol tablet formulation. The proximate composition and selected functional properties of African breadfruit and sweet potato flour blends was studied by Akubo [12]. Oladebeye et al. [13] researched on functional, thermal and molecular behaviours of ozoned-oxidized cocoyam and yam starches. Physicochemical properties of starches of sweet potato (Ipomea batata) and red cocoyam (Colocasia esculenta) cormels was reported Oladebeye, et al. [14]. Ashogbon [15] worked on physicochemical properties of bambarra groundnut starch and cassava starch blends. However, there is no or little information on the yield and properties of starch isolated from cocoyam cultivated in Akungba Akoko, Ondo- State, Nigeria. Therefore, the focus of this work is to isolate and determine the chemical composition, functional, pasting and gelatinization properties of starch obtained from cocoyam species prevalent at Akungba Akoko, Ondo-State, Nigeria with the view of enhancing the utilization of cocoyam that is yearly wasted in this part of the country.
Materials and Methods
Source of materials
Twenty big tubers of cocoyam were bought at Ibaka Market, Akungba Akoko, Ondo- State and all chemicals used were of good analytical grade purchased from a reliable vendor in Akure, Ondo- State, Nigeria.
Starch isolation and percentage yield
The method described by Akanbi, et al. [16] & Agboola, et al. [17] was used for starch isolation and percentage yield. The cocoyam tubers were weighed and peeled. The peeled cocoyam tubers were washed, sliced and crushed with Home Flower blender (Model NO: HFB-3489). The blended pulp was suspended in 5 litres of distilled water at room temperature for two hours and the suspended pulp was sieved using muslin clothe which retained the fibre. The fibre was rewashed to remove adhering starch. The extracted starch was allowed to sediment for two hours and the supernatant was decanted off. The extracted starch was washed with 5 litres of distilled water thrice until white and odourless starch was obtained. This was finally suspended in distilled water and left for another 24 hours and the supernatant was finally decanted off. The sediment was re-suspended in distilled water and centrifuged at 300 revolutions per minute for 15 minutes. The resulting wet starch was dried in an oven at 40℃ for 2 days. The dried starch was weighed, ground to powder, packaged in glass jar and stored for analysis. The percentage yield was calculated.
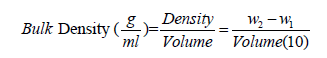
Determination of chemical composition of cocoyam starch
The moisture content, ash content, crude protein, lipid and phosphorous of cocoyam starch was determined by AOAC international standard method [18] while the amylose and amylopectin contents were determined by method described by Takahashi & Seib [19].
Determination of functional properties of cocoyam starch
The functional properties of cocoyam starch determined were pH, bulk density, dispersibility, water binding capacity, water absorption index, swelling power and water solubility index.
Determination of pH: 5g of cocoyam starch was weighed in triplicate into a beaker and 20ml of distilled water was added and mixed together. The resulting suspension was stirred for 5 minutes and left to settle for 10 minutes. The pH of the supernatant was measured using a calibrated pH meter [5].
Determination of bulk density: This was determined by the method of Wang & Kinsella [20] as modified by Ashogbon & Akintayo [4]. 10ml capacity graduated measuring cylinder was weighed (W1) and the cylinder was filled with the starch sample by gently tapping the bottom of the cylinder on the laboratory bench several times until there was no other contraction of the powdery starch level after filling to the 10ml mark. The weight of the measuring cylinder with its content was obtained (W2).
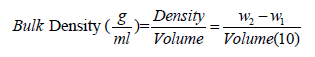
Determination of starch dispersibility: This was determined by the method described by Kulkarrni et al. [21] as modified by Akanbi et al. [16]. 10g of starch sample was accurately weighed into measuring cylinder and 100ml of distilled water was added to it and it was left to stand for 3 or 4 hours during which pure and sediment layers were formed. The lower layer meniscus from upper layer meniscus gave the dispersibility.
Determination of water binding capacity: This was carried out using the Medcaf and Gillies [22] method. 37.5ml of distilled water was added into 2.5 ml of the cocoyam starch and was centrifuged for 10minutes at 3000rpm. Then the weight of the centrifuge tube and content was determined after decanting the water and allowed to drain for another 10 minutes, the bound water was determined by the change in weight. It was calculated by the formula,
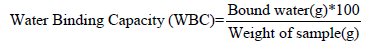
Determination of water absorption index: This was determined by modified method of Ruales et al. [23]. 2.5g of starch was suspended in 30ml of distilled water at 30℃ in a centrifuge tube, stirred for 30 minutes intermittently and then centrifuged at 3000rpm for 10 minutes. The supernatant was decanted, and the weight of the gel formed was recorded. The water absorption index (WAI) was the calculated as gel weight per gram dry sample.
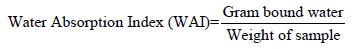
Determination of swelling power and water solubility index: Swelling power (SP) and water solubility index (WSI) determination were carried out in the temperature range of 60- 90℃ at 10℃ interval using the method of Takashi and Seib [19]. 1g of starch sample was accurately weighed and 50ml of distilled water was added and gently mixed together. The slurry was heated in a water bath at 55℃, 65℃, 75℃, 85℃ and 95℃ for 15minutes. During heating, the slurry was gently stirred to prevent clumping of the starch. After 15 minutes, the tubes containing the paste were centrifuged at 3000rpm for 10minutes using SPETRA U.K (Merlin 503) centrifuge. The supernatant was immediately decanted after centrifuging. The weight of the sediment was taken and recorded. The moisture content of the gel was thereafter determined to get the dry matter content of the gel.
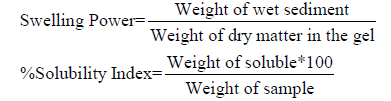
Determination of pasting properties of cocoyam starch: The pasting properties of cocoyam starch was analysed using a Rapid Visco Analyzer (Newport Scientific, RVA Super 3, and Switzerland). Starch suspension containing 9% w/w dry starch basis; 28g total weight) was equilibrated at 30℃ for 1 minute, heated at 95℃ for 5.5 minutes at a rate of 6℃/minutes, held at 95℃ for 5.5 minutes, cooled to 50℃ at a rate of 6℃/minute and finally held at 50℃ for 2 minutes. Parameters recorded were pasting temperature (PT), peak viscosity (PV), trough viscosity (TV), final viscosity (FV) and peak time (Pt). Breakdown viscosity (BV) was calculated as the difference between PV minus TV, while total setback viscosity (SV) was determined as FV minus TV. All determinations were performed in triplicate and expressed in rapid viscosity unit (RVU) [24].
Determination of gelatinization properties of cocoyam starch (Thermal analysis): The gelatinization properties of cocoyam starch were studied using a Differential Scanning Calorimeter (DSC-Q100, TA Instruments, New Castle, DE, USA). Cocoyam starch slurries were prepared at 1:3 dry starch/water ratios, hermetically sealed using a DuPont encapsulation press (DuPont Co., Delaware, USA) and reweighed. The sample was heated at a rate of 5℃/min from 20 to 100℃. Onset Temperature (To), peak temperature (Tp), conclusion temperature (Tc) and enthalpy of gelatinization (ΔH) and all determinations were performed in triplicate [14].
Result and Discussion
Percentage yield and chemical composition of cocoyam starch
The percentage yield and chemical composition of cocoyam starch is presented in Table 1. The percentage yield of cocoyam starch was 11.47±0.11% and this value is lower than 14.26% starch yield obtained from breadfruits [16]. The moderately low starch value is of significant importance in domestic and industrial food utilization. The moisture content of the cocoyam starch was 15.05±0.19%, which is higher than 9.36±0.02% reported by Oladebeye et al. [25]. The high moisture content of the starch suggests its instability against microbial activity. Hence the starch needs to be further dried in order to be within the 10% stipulated standard of the revised regulation of the Standards Organisation of Nigeria [26] so as to prolong its shelf life over a long storage period. Akubo [12] reported that the lower the initial moisture content of a product to be stored, the better the storage stability of the product. The crude protein, lipid and ash of cocoyam starch were 0.05±0.00%, 0.07±0.00% and 0.21±0.02% respectively. The ash and fat contents as reported by Oladebeye et al. [25] were 1.88 ±0.02% and 0.52±0.01%, respectively. The lipid and protein contents are minor component of starch and they are very important because of their anti-swelling effect and influence on the pasting parameters [16]. The low value of lipid content of cocoyam starch may suggest that the starch and other products made from it are not susceptible to rancidity. The amylose and amylopectin contents of cocoyam starch were 23.05±0.02% and 76.50±0.01% accordingly. The amylose content was lower than 81.07±0.04% reported for red cocoyam tubers by Oladebeye et al. [14]. However, the amylose content was within 18-24% reported by Jane and 16.65±0.15% -30.85±0.63% reported by Aboubukar et al. [27]. The difference in value might be as a result of difference in species and agricultural environment where the plants were cultivated. The amylose and amylopectin contents of starches are significant as they affect pasting, gelatinization, retrogradation, swelling power and enzymatic vulnerability of starches [28]. Phosphorus was 0.76±0.18mg/100g and this value falls within 0.76mg/100g and 1.36mg/100g reported by Aboubukar et al., [27]. Phosphorus has been reported to be covalently linked to starch and affects its properties [29].

Functional properties of cocoyam starch
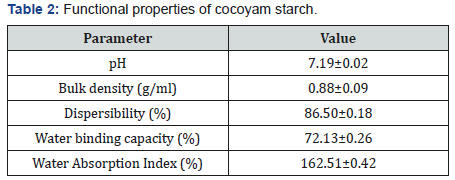
Table 2 depicts the functional properties of cocoyam starch. The pH value of the cocoyam starch was 7.19±0.02 which is slightly higher than 6.513±0.0058 reported by Akanbi et al. [16] for breadfruit starch. Though the pH value was within the range 7.38±0.04 and 7.03±0.02 reported for bambarra groundnut starch and cassava starch blends by Ashogbon [15]. The pH is an important property in starch industrial applications, being generally used to measure the degree of acidity or alkalinity of liquid media. The cocoyam starch has bulk density of 0.88±0.09g/ml. The bulk density is a measure of the degree of coarseness of starch sample. The bulk density of cocoyam starch was the same with the 70% bambarra groundnut starch and 30% cassava starch blend (that is 0.88±0.01g/ml) reported by Ashogbon [15]. However, the bulk density value was higher than 0.72±0.01 and 0.75±0.01g/ ml reported for white cocoyam corms and cormels respectively by Oladebeye et al. [25]. The high value of cocoyam starch bulk density connotes that less packaging space is required for it. Bulk density, as a function of suitability of starch as disintegrants and binder in tablet formulation, suggests that cocoyam starch as alternative binder and disintergrants in pharmaceuticals.
Values represent means of triplicate determination ± standard deviation.
The value of dispersibility of cocoyam starch obtained is 86.50±0.18% and this value is much higher than 40.667±0.5774% obtained for breadfruit starch as reported by Akanbi et al. [16]. But the value is within 83.00±0.03 and 87.00±0.06% reported for bambarra groundnut starch and cassava starch blends by Ashogbon [15]. Dispersibility is a measure of reconstitution of flour or flour blends in water, the higher the dispersibility the better the flour reconstitutes in water [21]. Cocoyam starch has water binding capacity and water absorption index of 72.13±0.26% and 162.51±0.42% respectively. The water binding capacity and water absorption index of cocoyam starch is higher than that of breadfruit starch (water binding capacity of 8.267±0.2309% and water absorption index 104.933±0.2309%) as reported by Akanbi et al. [16]. Oladebeye et al. [14] reported that water binding capacity for sweet potato and red cocoyam starch as 84.91±0.02% and 82.74±0.03% accordingly and these values are higher than that obtained for cocoyam starch.
Swelling power and water solubility index of cocoyam starch
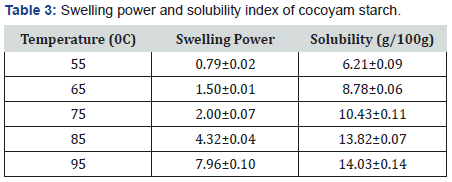
Table 3 presents the swelling power and solubility index at different temperatures of cocoyam starch. The swelling power and solubility index of cocoyam starch increases with increase in temperature of the starch, but there was a sudden increase from 75℃ to 95℃. Swelling power of cocoyam starch ranged between 0.79±0.02 and 7.96±0.10 over a temperature range of 55℃ to 95℃. Cocoyam starch solubility ranged between 6.21±0.09 and 14.03±0.14g/100g over a temperature range of 55℃ to 95℃. The swelling power has been related to the associative binding within starch granules and apparently, the strength and character of the micellar network is related to the amylose content of the starch, low amylose content, produces high welling power [17]. Swelling power and solubility are measures of the magnitude of the interaction between starch chains within the amorphous and crystalline domain [13]. The swelling power of starches is of great significance in tablet and capsule formulations as it is believed that disintegrants works through a swelling and wicking action [11]. As a result, starches with higher swelling power would be expected to release the active pharmaceutical ingredient from its compacts at a faster rate, where starch acts as a disintegrant. Solubility represents the amount of solubilized starch molecules present at a certain temperature.
Values represent means of triplicate determination±standard deviation.
Pasting properties of cocoyam starch
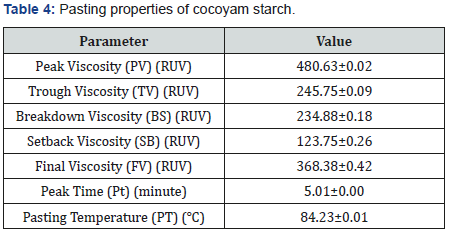
The pasting properties of cocoyam starch are presented in Table 4. The peak viscosity and trough viscosity of cocoyam starch are 480.63±0.02 RUV and 245.75±0.09 RUV respectively while its breakdown viscosity and setback viscosity are 234.88±0.18 RUV and 123.75±0.26 RUV accordingly. The peak viscosity of cocoyam starch is higher than the values obtained for bambarra groundnut starch and cassava starch blends but lower than 553.75±0.10 RUV for pure cassava starch as reported by Ashogbon [15]. Both values of breakdown viscosity and setback viscosity for cocoyam starch were higher that values (7.92 and 40.08 accordingly) obtained for breadfruit starch by Akanbi et al. [16]. The trough viscosity of cocoyam starch is lower than 247.04±0.20 RUV for bambarra groundnut starch but higher than 162.58±0.10 RUV for cassava starch [15]. Breakdown viscosity is a measure of granule disruption or lesser affinity of starch to resist shear force during heating while the setback viscosity is a measure of the degree of retrogradation of starch, mainly amylose [30], implying that a high setback viscosity value means a high tendency of starch to retrogradation. The final viscosity, peak time and pasting temperature of cocoyam starch are 368.38±0.42 RUV, 5.01±0.00 minutes and 84.23±0.01℃ respectively and the values for peak time and pasting temperature are higher than values obtained for bambarra groundnut starch and cassava starch as reported by Ashogbon [15]. Oladebeye et al. [14] reported lower values for pasting temperature and peak time for sweet potato and red cocoyam starches. The final viscosity value of cocoyam starch was lower than that of bambarra groundnut starch but higher than that of cassava starch [15]. The final viscosity indicates the ability of starch samples to form past [31]. The peak time is a measure of the rate at which equilibrium is attained between swelling and polymer leaching, and rupture and polymer alignment [13].
Values represent means of triplicate determination ± standard deviation. RUV = Rapid Visco Unit (Unit of Viscosity); BV = PV – TV; SV = FV – TV; PV = Peak Viscosity; TV = Trough Viscosity; BS = Breakdown Viscosity; SB = Setback Viscosity; FV = Final Viscosity; Pt = Peak Time; PT = Pasting Temperature.
Gelatinization properties of cocoyam starch
Table 5 depicts the gelatinization properties of cocoyam starch. The onset temperature, peak temperature and conclusion temperature of cocoyam starch are 81.80℃ 0.110C, 85.20℃ 0.190C and 93.00℃ 0.210C respectively while its enthalpy of gelatinization is 12.10±0.13 J/g. Oladebeye et al. [14] gave the onset temperature, peak temperature and conclusion temperature of cocoyam starch are 77.24±0.11℃, 79.52±0.01℃ and 86.14±0.58℃ respectively and all these values are lower than the obtained values. However, the enthalpy of gelatinization for cocoyam starch reported by Oladebeye et al. [13] is higher (15.19±3.12 J/g) than obtained value. The differences in these values may be as a result of differences in the agricultural environments in which the cocoyam were planted. The gelatinization temperature and enthalpy of starches depend on the microstructure and degree of crystallinity within the granules, and also on the granule size and amylose-to-amylopectin ratio [32].
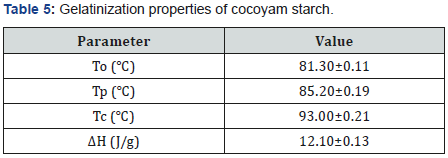
Values represent means of triplicate determination ± standard deviation. To = Onset Temperature; Tp = Peak Temperature; Tc = Conclusion Temperature; ΔH = Enthalpy of Gelatinization.
Conclusion
The results of the research work on cocoyam starch obtained at Akungba - Akoko, Nigeria revealed that it has an array of chemical, functional, pasting and gelatinization properties that makes it suitable in many areas for food and non-food applications. With the values of functional properties obtained for cocoyam starch, it is very useful as binder in pharmaceutical, cosmetic and paper industries. The cocoyam starch needed to be further dried so as to stay unspoiled due to its high moisture content when stored over a long period. Further research work can be conducted if the cocoyam starch is blended with other starches from other sources in different proportion and observed the effect of blending on chemical, functional, pasting and gelatinization properties of blended starch.
References
- Chung-Jen C, Yu-Chin S, An-IY (2010) Physico-chemical characteristics of media-milled corn starch. J Agric Food Chem 58(16): 9083-9091.
- James MG, Denyer K, Myers AM (2003) Starch synthesis in cereal endosperm. Curr Opin Plant Biol 6(3): 215-22.
- Peroni FHG, Rocha TS, Franco CML (2006) Some structural and physicochemical characteristics of tuber and root starches. International Journal of Food Science and Technology 12(6): 505-513.
- Ashogbon AO, Akintayo ET (2013) Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch/Starke 66(1-2): 41-57.
- Benesi IR (2005) Characteristics of Malawian cassava germplasm for diversity, starch extraction and its native and modified properties. PhD Thesis, Department of Plant Science, University of Free State, South Africa.
- Tetchi FA, Rolland-Sabate A, Amani NG, Colona P (2007) Molecular and physiochemical characteristics of starches from yam, cocoyam, cassava, sweet potato and ginger produced in Ivory Coast. Journal of Science, Food and Agriculture 87: 1906-1916.
- Vandeputte GE, Delcour JA (2004) From sucrose to starch granule to starch physical behaviour: A focus on rice starch. Carbohydrate Polymer 58: 245-266.
- Sherry XX, Qiang L, Steve WC (2005) Food Chemistry, Physical Properties and Applications, CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW.
- Betancur-ancona DA, Chel-Guerrero LA, Bello-Perez LA, Davilla-Ortiz G (2002) Isolation and functional properties. Starch/Starke 54: 303-309.
- Morton S (2002) Functional properties of starch: A review. Starch/ Starke 55: 7-12.
- Adebayo AS, Itiola SO (1988) Evaluation of breadfruit and cocoyam starches as exodis-integrants in a paracetamol tablet formulation. Pharmacy and Pharmacology Communications 4(8): 385-389.
- Akubo PI (1997) Proximate composition and selected functional properties of African breadfruit and sweet potato flour blends. Journal of Plant Foods for Human Nutrition 1997, 51(1): 53-60.
- Oladebeye AO, Oshodi AA, Amoo IA, Karim AA (2013) Cocoyam functional, thermal and molecular behaviours of ozoned-oxidized and yam starches. Food Chemistry 141(2): 1416- 1423.
- Oladebeye AO, Oshodi AA, Oladebeye AA (2009) Physicochemical properties of starches of sweet potato (Ipomea batata) and red cocoyam (Colocasia esculenta) cormels. Pakistan Journal of Nutrition 2009, 8(4): 313-315.
- Ashogbon AO (2014) Physicochemical properties of bambarra groundnut starch and cassava starch blends. African Journal of Food Science 8(6): 322-329.
- Akanbi TO, Nazamid S, Adebowale AA (2009) Functional and pasting properties of a tropical breadfruit (Artocarpus altilis) starch from Ile- Ife, Osun State, Nigeria. International Food Research Journal 16: 151- 157.
- Agboola SO, Akingbala JO, Oguntimi GB (1990) Processing of cassava starch for adhesives production. Starch/Starke 42(1): 12-15.
- AOAC (2005) Official methods of analysis of the Association of Analytical Chemists18th edition, Washington, DC, USA.
- Takahashi S, Seib PA (1988) Paste and gel properties of prime corn and wheat starches with and without native lipids. Cereal Chemistry 65(6): 474-483.
- Wang JC, Kinsella JF (1976) Functional properties of novel proteins: alfaafa leaf protein. Journal of Food Science 41(2): 286-289.
- Kulkarni KD, Kulkarni DN, Ingle UM (1991) Sorghum malt-based weaning formulations: preparation, functional properties and nutritive value. Food Nutrition Bulletin 13(4): 322-327.
- Medcalf DG, Gilles KA (1965) Wheat starches I. comparison of physicochemical properties. Cereal Chemistry 42: 558-564.
- Ruales J, Valencia S, Nair B (1993) Effect of processing on the physicochemical characteristics of quinoa flour. Starch/Starke 45(1): 13-19.
- Phimolsiripol Y, Siripatrawan U, Henry CJK (2011) Pasting behaviour, textural properties and freeze-thaw stability of wheat flour-crude maiva nut (Scaphium scaphigerum) gum system. Journal of Food Engineering 105: 557-562.
- Oladebeye AO, Oshodi AA, Imanah JE, Oladebeye AA (2010) Physicochemical properties and pasting behaviours of selected tropical tubers. Nigerian Journal of Chemical Research 15: 35-42.
- Son (1988) Standards Organisation of Nigeria. Nigeria Industrial Standard for starch.
- Aboubakar NY, Njintang YN, Scher J, Mbofung CMF (2008) Physiochemical, thermal properties and microstructure of taro (Colocasia esculenta) flours and starches. Journal of Food Engineering 86(2): 294-305.
- You S, Izidorczyk MS (2002) Molecular characteristics of barley starches with variable amylose content. Carbohydrate Polymer 49: 33- 42.
- Tekede Y, Tokunaga N, Tekeda C, Hizukuri S (1986) Physicochemical properties of sweet potato starches. Starch/Starke 38(10): 345-350.
- Karim AA, Norziah MH, Seow CC (2000) Methods for the study of starch retrogradation: A review. Food Chemistry 71: 9-36.
- Kaur M, Singh N (2005) Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chemistry 91: 403-411.
- Sandhu KS, Kaur M, Singh N, Lim SA (2008) comparison of native and oxidized normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. LWT 41(6): 1000-1010.






























