Characterization and Nutritional Evaluation of Detarium Senegalense Seed Oil -Based Diet in Male Wistar Rats
Nwozo SO1*, Bajehson JR1, Waryo T2 and Iwuoha EI2
1 Department of Biochemistry, Nutritional and Industrial Biochemistry Unit, University of Ibadan, Nigeria
2Department of Chemistry, University of Western Cape, South Africa
Submission: June 18, 2018; Published: October 09, 2018
*Corresponding author: Nwozo SO, Department of Biochemistry, Nutritional and Industrial Biochemistry Unit, University of Ibadan, Ibadan, Nigeria.
How to cite this article: Nwozo SO Bajehson JR, Waryo T, Iwuoha EI. Characterization and Nutritional Evaluation of Detarium Senegalense Seed Oil -Based Diet in Male Wistar Rats. Nutri Food Sci Int J. 2018; 7(4): 555720. DOI: 10.19080/NFSIJ.2018.07.555720.
Abstract
Ofo seed oil (OSO) was examined for chemical composition, physicochemical characterization, oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) in-vitro and varying percentage OSO (0.5%, 1.0%, 1.5% and 10%) compounded-feed in male rats were evaluated on growth, toxicity, lipid profile and antioxidant level following continual ingestion for eight weeks. vegetable oil VO and OSO feed had higher fat content than commercial rat chow. The major fatty acids in OSO are approximately 78.5% unsaturated fats, namely linoleic and oleic acids from GC/MS. OSO had saponification, iodine and peroxide values of 131.5±2.53 (mg KOH/g, 75.0±1.05 mgI2/100g and 3.5±0.03m Eq/Kg respectively; solidification, smoke point and flash point temperatures were 19-21 ⁰C, 186 ⁰C and 205 ⁰C respectively; ORAC value of 64.0±3.76 (μmol/TE/g) and FRAP value of 3.10±0.78 (μmol/AA/g). Thirty six rats were divided into 6 groups of 6 animals, Group 1(control); Group 2 (10%VO); Groups 3-6 (0.5%, 1.0%, 1.5% and 10%) OSO. Groups 2 - 5 had 53-27% increase in body-weight compared to control with 69%. OSO feeding decreased total-cholesterol, LDLc, artherogenic index; increased tissue GSH, GPx and GST and decreased MDA compared to groups 1and 2. Tissue protein concentration and sperm morphology data were not statistically different from control while groups 2-5 had significant differences in bent/curved mid piece and bent/curved tail compared to groups 1 and 6. There was portal congestion, diffuse vascular degeneration and mild cellular infiltration in the liver of OSO feed rats, while kidney and testis had normal tissue histology.
Keywords: OSO; Fatty acids; Feeding; Artherogenic index; Antioxidants; Growth; Histology
Abbrevations: ORAC: Oxygen Radical Absorbance Capacity; FRAP: Ferric Reducing Antioxidant Power Activity; CDNB: 1-Chloro-2,4-dinitrobenzene; GPx: Glutathione Peroxidase; Tg: Triglycerides; HDLc: High Density Lipoprotein Cholesterol; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase
Practical Application
Animal husbandry practices is fast changing from the conventional household type and traditional nomadic herds men style of moving cattle from one part to another in the dry season in search of arable pasture to farms were hundreds of cattle are raised. This change with the ever increasing need to feed the growing population necessitates that feeds be compounded for animals and cheap source is from foods that are under-utilized by man. Detarium senegalense probably because of labour involved in the processing is fast moving out of conventional staple food. This work intended to encourage the utilization of OSO as an alternate source for compounding animal feed and as vegetable oil for man.
Introduction
Adequate nutrient content of major and minor food components plays a very crucial role as health could become delicate if it is insufficient either in the quantity or quality of intakes especially in the vulnerable groups such as growing children, the elderly, pregnant and lactating women [1]. Over time intake of diets lacking the essential food nutrients and or insufficient in quantity to meet dietary requirements could lead to malnutrition and accompanying biochemical changes. Prior to growth retardation in malnourished animals, metabolic changes would take place in a bid for the organism to adapt to prevailing conditions such as increased liver concentration of lipids or serum proteins [2,3]. Fats and oils are an integral part of a healthy diet and oils rich in polyunsaturated fatty acids are more desirable compared to those high in saturated fats. Fats and oils are important components of cell membranes, precursors of needed biological lipids as well as energy source for metabolic activity. Humans and animals can synthesize saturated and some monounsaturated fatty acids but cannot create double bonds at positions 3 and 6 as they lack the desaturases [4]. Thus, essential fatty acids (EFAs) cannot be synthesized in-vivo and most be obtained from the diet. Deficiency of EFAs is rare but could occur especially in infants on diets lacking EFAs. Concentrations of fatty acids in the blood depends on both dietary intake and metabolism [5,6]. Nutritionally, healthy oils are rich in polyunsaturated fatty acids, they do not only provide energy, but are nutritional essential play a major role in lowering serum cholesterol levels by the redistribution of cholesterol between the plasma and the tissues [7]. Modulating serum levels of lipids are important in coronary heart disease, type 2 diabetes and other disease conditions. Fats and oils are also important as they provide the antioxidative vitamin E which can also aid in preventing lipid peroxidation, an event involved in several ailments development. Commonly available oils in the market are olive, canola, sunflower, corn, rapeseed, coconut, peanut and palm nut. Increasing world population and accompanying rise in animal husbandry in the event of providing enough food has given rise to the need for alternate sources of oil for both nutritional and industrial purposes.
Detarium senegalense JF Gmelin (Caesalpinaceae) is a tree crop that gets to about 36m in height with buttressed bole and it is native to Tropical region of Africa. Common English name is tallow tree and in Eastern Nigeria, it is called Ofo. The leaves are eaten as vegetable for soups, enema for dysentery and as eye wash for conjunctivitis. The Seeds are used as a thickener in soups and stews in Eastern Nigeria. The fruit pulp is rich in vitamins [8], soluble dietary fiber present in it is in the form of mucus and lignans [9] and a water soluble non-starch polysaccharide has been reported in seed flour of the plant [10]. Most seed oils contain antinutritional factors which might limit its nutritional potential, thus the oil might need purification to enhance its suitability for consumption. Sequel to this, we characterized OSO and evaluated the effect of its continual ingestion for eight weeks on growth, markers of organ toxicity, serum lipid profile, tissue lipid peroxidation, tissue antioxidant levels, sperm characteristics / morphology and histopathology in male rats as this would provide information on biological utilization of the feed component which cannot be gotten from proximate and chemical constituents alone.
Materials and Methods
Seed source and preparation
Fresh Ofo seeds were purchased from Ojoo market, Ibadan on the market day; seeds were identified and authenticated By Mr Donatus Esiemakhai in the Herbarium of the Department of Botany, University of Ibadan, Ibadan, Nigeria. The seeds were air dried in the laboratory at normal room conditions and properly dried seeds were ground using Corona hand mill in Nutrition/industrial unit postgraduate laboratory in the Department of Biochemistry, University of Ibadan and subjected to Soxhlet extraction using nHexane for 6 hours in the Laboratory. The solvent was removed using rotary evaporator at 50 °C and we checked oil for presence of the solvent by flashing test between 80-100 ⁰C using an aliquot. Percentage yield of oil was 12.24% and the very pale brownish yellow oil obtained was stored at 4 °C until it was used.
Feed formulation
Normal control diet feed was prepared using soybean (27%), whole maize flour (Zea mays) (59%), vegetable oil (10%) and salt/vitamin mix (4%) was from Sigma-Aldrich Co. Ltd., Poole Dorset, UK. Same ingredients were used for test diet except Detarium senegalense seed oil was used wholly for 10% OSO feed and varying percentages (1.5%, 1.0%, and 0.5%) were supplemented with commercially consumed vegetable oil (VO) to make up the difference. Total oil composition was 10% for all formulated feeds used in this study. The different feed ingredients were thoroughly mixed, made into pellets for easy handling by animals and oven dried to prevent the growth of mold. Fully dried feeds were kept in previously labeled air tight bags and were stored at 4 °C to prevent microbial spoilage.
Proximate analysis of the feeds
Feeds were analyzed for proximate content as follows. Ash and mineral composition was by arching in a muffle furnace at 550 °C for 4 h. Total dietary fiber was determined by an enzymatic gravimetric method and crude oil content was assayed by extraction of the lipids with n-hexane in a Soxhlet extractor and was weighed to calculate the percentage. Standard micro Kjeldahl digestion apparatus was used to obtain percentage nitrogen content and this was used to obtain percentage protein by multiplying nitrogen content by a factor of 5.71 (this takes into account the non-protein nitrogen) and has been approved for calculating the crude protein content. Moisture content was determined by drying to a constant weight at 100 °C for 24h.
Characterization and physicochemical properti OSO
OSO was evaluated by physical examination like color, whether the oil was in liquid state of semi-solid form at room temperature and the density, acid value, peroxide value, saponification number and iodine numbers were determined by methods described by AOAC [11]. The GC/MS of the oil was done on Water GCT equipped with CTC CombiPAL autoanalyzer using HP5 MS column. Flow rate of the carrier gas (helium) was 1-2mls/min, 2μL of sample was injected, injection temperature was 280 ⁰C and scanning range from 15 - 700m/z. Samples were dissolved in dichloromethane and Peaks were identified using authentic standards from computer literature search based on fragmentation patterns, while peak area provided information on the percentage of each fatty acid component.
Ferric reducing antioxidant power activity (FRAP)
The ferric reducing antioxidant power assay of Ofo seed oil was done on a Multiscan Spectrum plate reader by using the method developed by Benzie & Strain [12]. Assay principle uses antioxidants as reductants in a redox-linked colorimetric method which measures the reduced oxidant at low pH, ferric tripyridyltriazine (FeIII-TPTZ) complex is reduced to the ferrous form, forming an intense blue colour with an absorption maximum at 593nm. The change in absorbance is directly related to the reducing power of the electron donating antioxidants present in the reaction mixture.
Oxygen radical absorbance capacity (ORAC)
The oxygen radical absorbance capacity of Ofo seed oil was determined using fluorescein (C20H10Na2O5) as the fluorescent probe and 2,2'-Azobis(2-methylpropionamidine) dihydrochloride (AAPH) as peroxyl radical generator based on the method of Prior et al., 2003 [13]. This was performed on a Fluoroskan plate reader with fluorescence filters for an excitation wavelength of 490nm and emission wavelength of 530nm at 37 ⁰C. Trolox calibration solutions were used as standard and ORAC values are calculated using a regression equation and data were expressed a micromoles of Trolox equivalents (TE) per milligram of sample
Animals used and protocol approval
Thirty-six male albino rats (Wistar strain) weighing between 60 - 80g was obtained from the animal house in the Department of Veterinary Physiology, University of Ibadan. Rats were transferred and allowed to acclimatize for two weeks, been maintained on the standard normal diet with water ad-libitum in the Biochemistry Department Animal House under normal room temperature before the commencement of the experiment. Animals were distributed randomly into six different groups of six rats each. Group 1: control on commercial rat chow from Ladokun Feed; Group 2 on 10% VO diet; Groups 3-5 were fed compounded diet using (0.5%, 1.0% and 1.5%) OSO made up to 10% using VO and Group 6 had 10% OSO compounded feed. All compounded feeds had 10% oil and animal feeding was done daily for sixty days. Animal care and handling were according to protocol of National Institute of Health (NIH publication, number 82- 23, revised 1985) and experimental design for our study was approved by Institutional Animal Care and Use Committee of University of Ibadan, Ibadan.
Sample collection
Animals were sacrificed by cervical dislocation and blood was obtained using 2ml syringe by cardiac puncture into clean bottles and allowed to clot. These were spun at 3000rpm for 10 minutes; the supernatant (serum) was removed and stored at 4 ⁰C. The liver and kidney were quickly removed, weighed, washed with 1.15% KCl, homogenized in 56mM Tris-HCl buffer (pH 7.4) containing 1.15% potassium chloride and the homogenate was centrifuged at 10,000rpm for 15 minutes to obtain post mitochondrial fraction (PMF) at 4 ⁰C.
mitochondrial fraction (PMF) at 4 ⁰C.
Serum and tissue homogenate protein concentration was determined using the method Lowry et al., [14] with bovine serum albumin standard. Extent of lipidperoxidation was evaluated by measuring thiobarbituric acid reactive substances (TBARS) using the method of Varshney & Kale [15]. Superoxide dismutase (SOD) activity was assayed using the method of Misra & Fridovich [16]. Microsomal catalase (CAT) activity was determined by using hydrogen peroxide as described by Claiborne [17]. Briefly, the reaction mixture contained phosphate buffer (0.01 M, pH 7.0), tissue homogenate and 2M H2O2. The reaction was stopped by the addition of dichromateacetic acid reagents (5% potassium dichromate and glacial acetic acid were mixed in a ratio of 1:3). Reduced glutathione (GSH) level was determined by measuring the rate of formation of the chromophoric product in a reaction between DTNB (5,5´-dithiobis- (2-nitrbenzoic acid) and free sulphydryl groups at 412 nm as described by Beutler et al., [18] and glutathione-stransferase (GST) activity was determined using 1-Chloro-2,4- dinitrobenzene (CDNB) at 340 nm was determined according to the method of Habig et al., [19]. Glutathione peroxidase (GPx) activity was measured according to the method of Hafeman et al., [20] based on the degradation of H2O2 in the presence of GSH. Total Cholesterol (Tc), Triglycerides (Tg) and high-density lipoprotein cholesterol (HDLc) were estimated using Randox kit. Serum urease was estimated at 546nm using the amount of urea in serum that is hydrolyzed to ammonia by urease [21]. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed using Randox kit.
Tissue histopathology
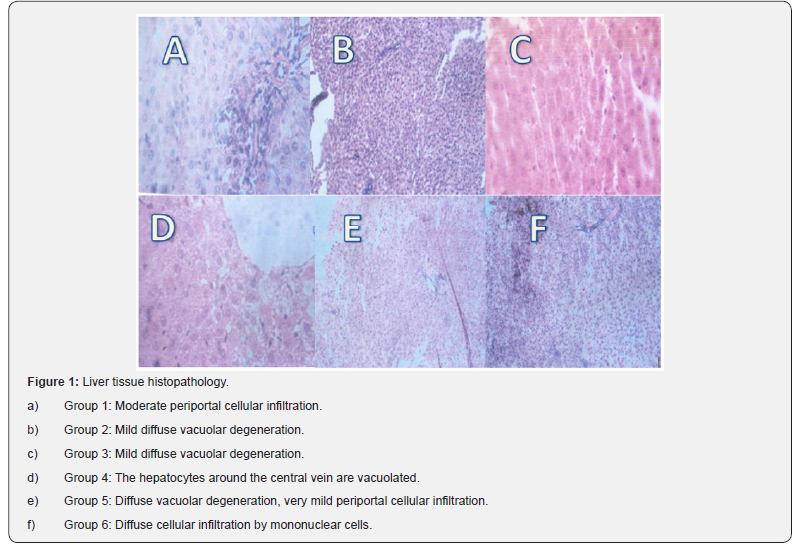
After sacrifice, the liver, kidney and testis tissues were removed and small bits from each was fixed in 10% formaldehyde solution and sent to Veterinary Anatomy Department, University of Ibadan, for histopathology examinations. Briefly, the tissues were washed by dehydration in increasing gradient of ethanol and finally cleared in toluene. The tissues were then embedded in molten paraffin wax. Sections were cut at 5μm thickness and stained with hematoxylin and eosin. The slides were photographed with an Olympus UTU1X-2 camera connected to an Olympus CX41 microscope (Tokyo, Japan)
Sperm analysis
Sperm motility, live/dead count, sperm morphology and concentration were analyzed. Sperm motility was by examining it under the microscope using a drop of semen incubated in Tris/ citric acid/fructose medium. Semen suspension was stained with 1% eosin B and 5% nigosin and it was examined under the microscope, percentages of stained and unstained cells were estimated. Sperm count was done using haemocytometer and the morphology was by microscopy.
Result and Discussion
OSO yield and characterization
Fats and oils are essential as source of energy, precursors of hormones, carriers of fat soluble vitamins and growth promoters. Knowledge of exact chemical composition, purification and chemical modifications further enhances health benefits of oils [22]. Percentage yield of OSO extracted on dry matter basis was approximately 12.24 %, hence OSO is an average oil producing seed compared to oily seeds such as groundnut oil with over 40% yield. OSO is faintly brownish yellow in color, with no characteristic smell and a liquid at room temperature. Data obtained for physiochemical and chemical composition characterization from GC/MS are shown on Table 1. Solidification temperature ranged from 19-21 ⁰C, while smoke point and flash point temperatures were 186 ⁰C and 205 ⁰C respectively. Low smoke point, flash point temperatures of 186 and 205 ⁰C (Table 1) indicate that the oil is ignitable and it could breakdown easily at high temperatures; hence the oil should not be used at very high temperature for deep frying. Acid value is a measure of age, quality, stability and suitability for consumption of any oil [23-24]. Low acid value (2.8mg KOH/g) of OSO indicates reduced susceptibility to enzymatic degradation and the reduced iodine value an indicator of unsaturation and lowered susceptibility to hydrolysis and oxidative spoilage on storage [25]. The saponification value (161.50±2.53 mgKOH/100g) of OSO was slightly low compared to other edible oils (palm oil 196-205mgKOH/100g, corn oil 187-196mgKOH/100g, ground nut oil 188-196mgKOH/100g, coconut oil 253mgKOH/100g and palm kernel oil 247mgKOH/100g) [26]. In-vitro antioxidant assay, mainly ORAC and FRAP of OSO was 64.0±3.76 (μmol/ TE/g) and 3.10±0.78 (μmol/AA/g) respectively. OSO had very low values of ORAC and FRAP and the oil is not be a rich source of antioxidants compared to other edible oils such as Olive oil with 42.18mmolTE/g [27]. The predominant chemical constituents, which are mostly triglycerides in OSO was oleic and linoleic acids and was approximately 80% by composition, this result was similar to that of Sowemimo et al., [28]. OSO has high concentration of monounsaturated fatty acids and this supports our physicochemical data as the oil remained in the liquid state at room temperature throughout the study duration. Unsaturated fatty acids in the diet are not only nutritionally essential but are healthy lipids as they play key role in the redistribution of plasma cholesterol between the plasma and tissues [7].

Proximate content of feed, changes in bodyweight and lipid profile
Proximate content of control and test diets were done, there were no significant differences except in percentage fat. 10% OSO and 10% VO diets had approximately 41 and 27% more fat than control diet. Data obtained for serum lipid levels, body weight and organ weight are shown on (Table 2). All animals gained weight but there was almost 50g difference between rats on test diets VO or OSO (groups 2 & 6) compared to group 1 (control) which had 69% gain in weight. The Group 1 rats had the largest kidney, liver and testis weights compared to other groups; however, the kidney of rats on VO+OSO diet decreased significantly compared to control. The reduced weight gain in test animals compared to control might be attributed to a decrease in feed-acceptability, reduced feedintake and increased early satiety of high fat diet compared to commercial rat chow 10% OSO feed had over 50% fat content compared to commercial rat chow. Thus the decrease in bodyweight gain of rats in groups 2-6 maybe due to decreased feed-intake, reduction in the intake of drinking water, dietinduced anorexia or systemic toxicity. Consequently, rats on compounded-diet all had lower organ weights compared to control, possible as a result of lowered bodyweights of the rats, and hence organs were not swollen or inflamed OSO at the concentration used in this study is not nephrotoxic [29]. VO fed rats had elevated serum triglyceride, total cholesterol, HDLc and LDLc levels compared to groups 1 and 6 on control and 10%OSO diets respectively. The increase in Triglyceride, total cholesterol and LDLc was further elevated in rats on combined OSO and VO diet (groups 3, 4 and 5), while HLDc decreased in the same group. Consequently rats on combined oil diet had higher artherogenic index relative to groups 1 and 6. Also compared to group 2, the animals on OSO and VO+OSO had comparable total cholesterol and triglyceride values. Earlier evidence has shown that serum lipid levels can be altered by dietary intakes [5,6,30]. Serum lipids levels are a pointer to the metabolism of lipids and it could be used to predict predisposition to coronary heart disease (CHD). Dietary lipids have been shown to alter serum lipid levels [31]. An elevation in serum triglyceride levels enhances atherogensis and could trigger heart attack. High levels of LDLc are responsible for the development of atherosclerosis as it induces inflammations by producing leukocytes and cytokines in the endothelia tissue. OSO feeding elicited reduction in LDLc, total cholesterol and triglyceride compared to both control and VO only fed rats and could therefore protect against cardiovascular diseases development [32].
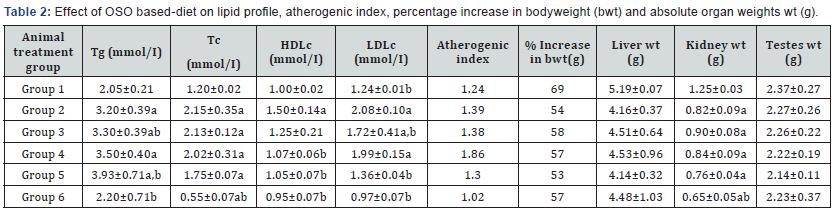
Data are mean ± S.D of six rats; a,b are significant when compared to groups 1 and 2 respectively at ρ<0.05.
OSO feeing and oxidative stress
Antioxidants help to remove free radicals and chelate metal ions, thereby protecting the body by preventing tissue dysfunction by removal of oxidants in the body, thus modulating the levels of reactive oxygen species (ROS) present [33]. Hence, antioxidants scavenge ROS and could hinder lipid peroxidation. OSO feeding in rats caused an increase in the activities of SOD, CAT, GPx but decreased GSH and GST compared to control in the liver as shown on Table 3. Groups 2-5 rats on had higher values of SOD, CAT and GPx compared to group 6 on only OSO. Lipid peroxidation increased significantly in group 2 and 5 rats compared to group 1 (control), also group 6 on only OSO had lower liver MDA values than both control and VO given rats. The measurement of MDA is a well-established mechanism for evaluating cellular damage in both plant and animal tissue and is used as an index of oxidative stress in cells and tissues. Oxidative stress in biological membranes might alter the structure and function, as well as cause a total loss of biological function.
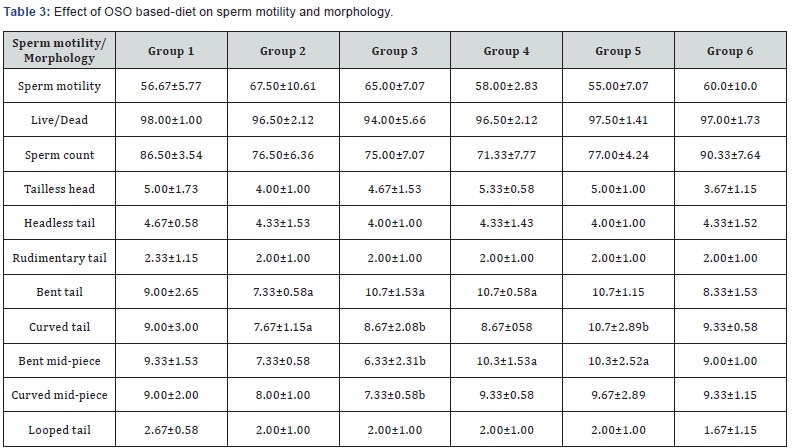
Data are mean ± S.D of six rats; a,b are significant when compared to groups 1 and 2 respectively at ρ<0.05.
OSO and sperm motility/morphology
Sperm motility and morphology data are on Table 4 and we observed that continual ingestion of OSO for eight weeks did not affect both parameters. It has been observed that oxidative stress is been linked to many conditions, such as increased levels of LDLc which could lead to hyperlipidermia [34]; male infertility and alterations in both sperm motility and morphology [35,36] and several other disease conditions. In this study it was only in rats on VO and VO+OSO (groups 3, 4 and 5) that we observed statistically significant differences in sperm morphology for bent tail, curved tail, bent mid-piece and curved mid-piece compared to both groups 1 and 6, hence OSO feeding does not alter both sperm motility and morphology.
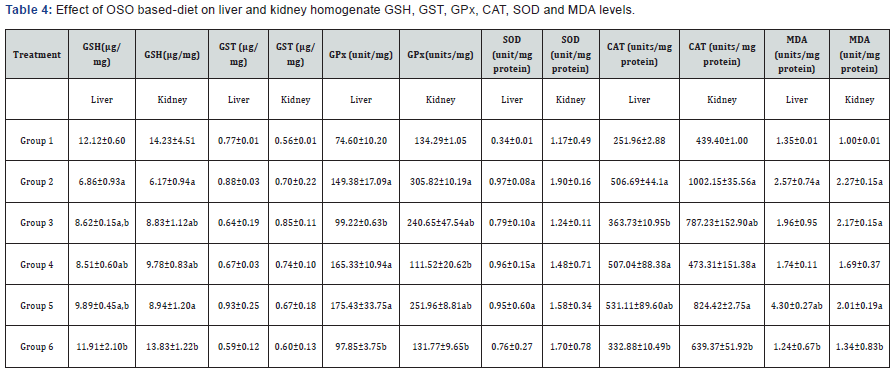
Data are mean ± S.D of six rats; a,b are significant when compared to groups 1 and 2 respectively at ρ<0.05.
OSO and markers of tissue toxicity
Toxicological effects of OSO feeding were evaluated on serum, liver, kidney and testis. Serum urea, serum and tissue protein concentrations are values of AST and ALT levels in OSO fed rats. Serum ALT levels were lowest in rats fed either VO or VO+OSO diets, with control rats having higher values than OSO only. AST increased in groups 1 and 6, while animals in group 2 on VO had reduced values with group 4 rats having the lowest. Aminotransferases could serve to predict tissue damage as ALT and AST are usually present in the organs and are only found in copious amounts in the blood after tissue injury as they are released into cellular flow from affected organs [37]. OSO feeding caused a decrease in AST and ALT compared to control rats, thus implying that OSO-base diet could protect the integrity of the liver. OSO feeding caused an increase in urea level compared to control, group3 had the highest value and group 6 on OSO only had urea level which was slightly lower than group 2 on VO diet. Kidney protein concentration decreased by 50% rats in groups 2 & 5, with group 6 on 10%OSO diet having the highest protein concentration. Liver protein concentration similarly decreased by over 60% in group 5 while rats in group 6 on 10%OSO diet had only 10% decrease relative to control. Serum protein levels was not affected in this study and there were no statistical differences, but kidney and liver protein concentrations decreased in test diet fed groups compared to control, only in group 6 did protein content increase relative to control. Serum urea levels increased slightly in groups 6 and 2 rats compared to group 1 on control feed but it was not statistically significant. Fluctuations in serum and organ protein concentration might serve as pointer to nutrient utilization and disease state [29], especially as there were no significant differences in body weights of rats at onset of experiment and the only difference in test diet was the oil used.
OSO feeding and tissue histopathology
Photomicrograph of liver, kidney and testis of rats fed OSO was done but only that of the liver is shown in Figures 1, as kidney and testis tissue did not have any lesions, just normal tissue histology and were not shown for simplicity of data, however liver tissue was affected by ingestion of OSO either alone or augmented with VO in this study. 10%OSO fed group 6 rats had diffuse cellular infiltration by mononuclear cells, group 2 0n VO only diet had mild diffuse vacuolar degeneration, rats in group 3-5 on combined VO/OSO diet had mild diffuse vacuolar degeneration, hepatocytes around the central vein were vacuolated and showed diffuse vacuolar degeneration, very mild periportal cellular infiltration respectively [Table 4].
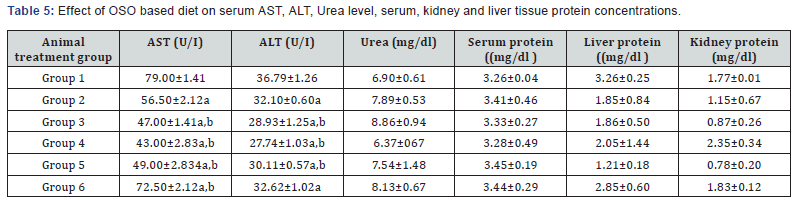
Data are mean ± S.D of six rats; a,b are significant when compared to groups 1 and 2 respectively at ρ<0.05.
Conclusion
We have shown that OSO, though not an oil rich plant, with low ORAC and FRAP values, that continual ingestion of OSO compounded feed for sixty days is not hepatotoxic or nephrotoxic, it could produce healthy lipid profile in rats; however, there is need for caution based on histopathology results of the liver tissue.
Acknowledgment
We acknowledge Ms. Fanie Rautenbach of Antioxidant Laboratory, Cape Peninsula University of Technology, Cape Town for fluorometric ORAC and spectrophotometric FRAP assays, Mr. Lucky Mokwena of Mass Spectrophotometry Unit in Stellenbosch University, Cape town for GC/MS data and OPCW for short term fellowship (L/ICA/168239/11) in SensorLab, Chemistry Department, University of Western Cape, Cape town which made the characterization of the oil possible.
References
- World Health Organization (2003) Organização de Alimentos e Agricultura das Naçõe Unidas. Manual das necessidades nutricionais humanas. São Paulo: Atheneu, Brazil.
- Natali MRM, Miranda Neto MH, Orsi AM (2000) Effect of hypoproteic diet supply on adult rat Wistar rat (Rattus Norvegicus). Acta Sci 22(2): 567-571.
- Sano A, Uchida R, Saito M, Shioya N, Komori Y, et al. (2007) Beneficial effects of grape seed extract on malondialdehyde- modified LDL. J Nutri Sci Vitaminol 53(2): 174-182.
- Food and Nutrition Board (2002) Institute of Medicine. Dietary fats: total fats and fatty acids. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acid. National Academy Press Washington, USA, pp. 422-541.
- Davidson MH (2013) Omega-3-faty acids: new insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid. Curr Opin Lipidol 24(6): 467-474.
- Nakamura MT, Nara TY (2004) Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr 24: 345-376.
- Hu FB, Manson JE, Willet WC (2001) Types of dietary fat and the risk of coronary heart disease: A critical review. JAM Coll Nutr 20(1): 5-19.
- Nkafamiya II, Modibbo UU, Manji A J, Haggai D (2007) Nutrient content of seeds of some wild plants. African JBiotech 6(14): 1665-1669.
- Akpata MI, Miachi OE (2001) Proximate composition and selected functional properties of Detarium macrocarpum. Plant Foods Human Nutr 56(4): 297-302.
- Onyechi UA, Judd PA, Ellis PR (1998) African plant foods rich in nonstarch polysaccharides reduce postprandial blood glucose and insulin concentrations in healthy human subject. Br J Nutr 80(5): 419- 428.
- AOAC (1990) AOAC Official methods of analysis (15th Ed) Association of Official 13. Analytical Chemists. Washington DC, USA.
- Benzie IFF, Strain JJ (1999) Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology 299: 15-27.
- Prior RL, Hoang H, Gu L,Wu X, Bacchiocca M, et al. (2003) Assays for hydrophilic and lipophilic antioxidant capacity (ORACFL¬) of plasma and other biological and food samples. J Agric Food Chem 51(11): 3273-3279.
- Lowry OH, Rosenbrough NJ, Farr AI, Randall RJ (1951) Protein Measurement with Folin-Phenol reagent. J Biol Chem 193(1): 265 -275.
- Varshney R, Kale RK (1990) Effects of Calmodulin antagonists on radiation- induced lipid peroxidation in microsomes. Int J Radiat Biol 58(5): 733-743.
- Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 24(10): 3170-3175.
- Claiborne A (1989) Catalase activity. In: Handbook of Methods for Oxygen Radical Research, Greenwald AR (Ed), CRC Press, Florida, USA, pp. 234-242.
- Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of Blood glutathione. J Lab Clin Med 61: 882-888.
- Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22): 7130-7139.
- Hafeman DG, Sunde RA, Hoekste WG (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104(5): 580-587.
- Fawcett JK, Scott JF (1960) A new semimicro method for determination of urea. J. Clin. Pathol. 13: 156-159.
- Asif M (2010) A review on purification and chemical modification of oils and fats T. Ph. Res 4: 16-27.
- Kardash E, Turyan YI (2005) Acid value determination in vegetable oils by indirect titration in aqueous alcohol media. Croatia Chem Acta 78: 99-103.
- Akubugwo IE, Chinyere GC, Ugbogu AE (2008) Comparative studies on oils from some common plant seeds in Nigeria. Pak J Nutr 7: 570-573.
- Popoola TOS, Yangomodou OD (2006) Extraction, properties and utilization potentials of cassava seed oil. Biotechnology 5: 38-41.
- FAO/WHO (1963) Fats, oil and related products, food standard program in codex alimentarius commission. Food and Agricultural Organization of the United Nations, World Health Organization, Rome, Italy, pp. 33-35.
- Sano A, Uchida R, Saito M, Shioya N, Komori Y, et al. (2007) Beneficial effects of grape seed extract on malondialdehyde- modified LDL. J Nutri Sci Vitaminol 53(2): 174-182.
- Sowemimo AA, Pendota C, Okoh B, Omotosho T, Idika N, et al. (2011) Chemical composition, antimicrobial activity, proximate analysis and mineral content of the seed of Detarium senegalense JF Gmelin African J. Biotechnology 10(48): 9875-9879.
- Oladiji AT, Abodunrin TP, Yakubu MT (2010) Toxicological evaluation of Tetracarpedium conophorum nut-based diet in rats. Food and Chem Tox (48): 898.
- Nwozo SO, Orojobi BF, Adaramoye OA (2011) Hypolipidemic and antioxidant potentials of Xylopia aethiopica seed extract in hypercholesterolaemic rats. Journal of Medicinal Foods 14(1-2): 114- 119.
- Gilani GS, Ratnayaka WMN, Brooks SPJ, Botting HG, Plouffe LJ, et al. (2002) Effects of dietary protein and fat on cholesterol and fat metabolism in rats. Nutr Res 22: 297-311.
- De Guzman G, Ysrael M, Dacanay A, Dimaano J (2013) Comparative hypolipidemic properties between the lyophilized fruit juice of Morinda citrifolia L. (Rubiaceae) and lyophilized commercial noni juice in triton and atherogenic diet-induced dyslipidemic rats. Intl J Pharm Teach Prac 4(2): 631-635.
- Ochsendorf FR (1999) Infections in the male genital tract and reactive oxygen species. Human Reprod Update 5(5): 399-420.
- Martins PJF, Azzalis LA, de Oliviera AC, Fernades L, Tufik S, et al. (2013) Effect of chow and liquid diet on liver integrity and antioxidant defense in sleep deprived male rats, Nutrition and Food Sciences 3: 5.
- Agarwal A, Saleh RA (2002) Role of oxidants in male fertility: rationale, significance and treatment. Urol Clin North Am 29(4): 817-827.
- Vernet P, Aitkenm RJ, Drevet JR (2004) Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 216(1-2): 31-39.
- Shahjahan M, Sabitha KE, Jamu M, Shyamala-Devi CS (2004) Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Indian J Med Res 120(3): 194-198.






























