Abstract
Lifestyle modification and poor nutrition have led to a major shift in eating patterns. Herbal foods have gained extensive consideration because of the presence of abundant phytonutrients in them. Among the beverage products, Tea is mostly liked by individuals from different age group. However, their nutritional quality can further be improved by incorporating healthy ingredients like liquorice root and fennel seed powder. Liquorice root has functional and therapeutic potential to treat asthma due to the presence of bioactive components like Glycyrrhizin. The present project has been designed to find out quality characteristics of herbal teamix incorporated with liquorice root and fennel seed powder. For the purpose, liquorice root and fennel seed powder were prepared and evaluated for mineral, total phenolic content, total flavonoid, antioxidant analysis, and bioactive component. The results of the mineral content of liquorice root and fennel seed powder showed that it holds (1203mg) calcium, (1737mg) potassium, (372mg) magnesium. Liquorice root possessed total phenolic content (873mg GAE/100g) and total flavonoid content (270mg RE/100g). Antioxidant (DPPH) value was (70%), and bioactive compound (31.7g) and Fennel seed possess total phenolic content (13.47mg GAE/100g) and total flavonoid content (0.04mg RE/100g). Antioxidant (DPPH) value was (78%). Liquorice root powder was mixed with fennel seed powder in different combinations. All formulations were converted into herbal teamix followed by their compositional and phytochemical analysis. The demonstration of mineral analysis of all treatments of herbal teamix from T0 to T5 showed ranges of calcium (118.17 to 489.98mg), potassium (40.45 to 680.99mg), magnesium (1.94 to 157.31mg) mg/100g respectively. The TPC results illustrated that the highest content (853.05mg) was found in T0, and lowest (516.73mg) in T5. The highest TFC (268.24mg) was also present in T0. Antioxidant activity ranged from (68.46mg) for T0 to (72.27) mg for T5. The product was also assessed for color. With the decrease in the liquorice powder, color of herbal teamix was also decreased. Afterwards, sensory acceptability of the product was judged for different attributes like color, taste, aroma, mouthfeel, and overall acceptability. Based on composition and sensory analysis, herbal teamix containing 70% liquorice root powder and 30 of fennel seed powder (T4) was preferred as best treatment. From the results it was clear that there was a need to use underrated but versatile herbs like liquorice root and fennel seeds to develop value added products with high nutritional value.
Introductıon
Asthma is a complex lung disease that affects approximately 300 million people worldwide. It is distinguished by variable airflow obstruction and Airway Hyper Responsiveness (AHR) resulting in episodic and reversible bronchoconstriction as a reaction to a variety of environmental stressors such as allergens. Extrinsic asthma and intrinsic asthma are its two types. Extrinsic asthma often known as allergic asthma is caused by allergens. Intrinsic asthma also known as non-allergic asthma can be triggered by a number of causes such as aspirin, lung infection, exercise, cold, stress and obesity [1].
Asthma is a serious disease that can endanger a person’s life and have a major effect on their everyday lives. It is caused by an interaction of genetic and environmental variables that can combine to influence the disease’s progression. One of the main reasons could be changes in dietary patterns caused by an excessive intake of highly processed and refined foods and a reduced consumption of vegetables and fruits. Diets with these features are more likely to result in obesity, which is a serious public health concern and ultimately becomes a risk factor for asthma. Obese persons are more likely to suffer from asthma have more frequent and severe signs and complications and have a worse quality of life and less respond to asthma therapies [2].
Liquorice root (Glycyrrhiza glabra) is a perennial herb or subshrub that grows 3 to 7 feet high with a long, cylindrical and branched form [3]. Liquorice grows naturally in Baluchistan, Chitral and Hindu Kush Himalayan areas. Its Chitral roots are thick and its quality is similar to Chinese liquorice [4]. Countries that produce liquorice roots are Iran, Afghanistan, China, Pakistan, Iraq, Azerbaijan, Uzbekistan, Turkmenistan and Turkey [5]. Between 2009 and 2018, global exports of liquorice commodities totaled more than 246, 234 tones and worth at more than USD 1.735 billion [6].
The bioactive component glycyrrhizic acid was identified primarily in the woody parts of the roots. Other similar substances such as soyasaponins and flavonoids are found in every part of the plant [7]. It has been utilized as a flavoring agent and sweetener in Asian countries as well as a traditional herbal treatment [8]. For asthma, 1.0 to 5.0g of dried root powder liquorice three times per day is recommended. A standardized liquorice extract dosage of 250 to 300mg three times a day (containing 20% glycyrrhizin acid) is recommended in asthma. Glycyrrhizin glabra roots were found to relax the tracheal smooth muscle and its effect on asthma were studied in-vivo and in vitro [9].
Fennel (Foeniculum vulgare) is herbaceous plant of Apiaceae family. It’s a hard perennial herb with feathery leaves and yellow flowers [10]. Fennel seeds are widely grown throughout Europe, the Middle East and the Mediterranean regions for their peculiar sweet and anise flavor [11]. The seeds are native to Southern Europe and are a common ingredient in China, Turkey and India. India is the world’s largest fennel producer with over 153,000 tons of fennel in 2016-17 and Syria, Egypt, Turkey, Germany, Spain and Pakistan are the other major fennel producers [12]. Its major components of have been identified as trans-anethole, fenchone, estragole (methyl chavicol) and phellandrene [13]. Its essential oil also promotes the contraction of the tracheal smooth muscle, which may aid in mucus expectoration [14].
Medicinal herbs and seeds are the blessing of nature which
have been an integral part of human history and culture for
centuries. Tea is one of the most popular drinks around the globe
and equally liked by everyone. It is a major part of snack time
especially in Asia. It is made by dipping powder in hot water to get
an extract. The production and consumption of tea is increasing
dramatically. The extract of the liquorice compound glycyrrhizin
relieves symptoms of asthma. Both of these compounds have
many therapeutic uses because of their phytochemicals profile
and it is preferable for people to drink in asthma. There is no
source that provides as quick relief as my product will be. The goal
is to provide patients with a source that provides immediate relief
from that issue. The main objectives of this research include:
• Development of liquorice root and fennel seed based
herbal teamix
• Nutritional and phytochemicals analysis of teamix
• Checking consumer acceptability through the sensory
evaluation
Materials and Methods
The present study has been carried out in different laboratories
of National Institute of Food Science and Technology, UAF. In this
study, liquorice root and fennel seeds based herbal teamix was
developed and evaluated for its beneficial health benefits. The
protocol has been described as:
Procurement of Raw Materials
The raw ingredients like fennel seeds and liquorice roots were procured from the local vendors in Faisalabad.
Preparation of Raw Materials
Liquorice roots and fennel seeds were first cleaned and
washed to remove any foreign entities and dust. Any damaged
or discolored roots and seeds were separated by manual
sieving. Afterwards, liquorice roots were completely dried using
dehydrator. Then liquorice roots and fennel seeds were grinded
into powdered form. Samples were stored in airtight jar for
further study (Figure 1).
f1
Analysis of Liquorice Root and Fennel Seed Mineral analysis
Mineral content (magnesium, potassium and calcium) of herbal teamix was accessed by using the method of AOAC [15]. Wet digestion of the materials was done for this purpose so, it took a 2g sample of herbal teamix and added 7ml HNO3 and 3ml HCIO3 to a beaker. Then all the chemicals were mixed and heated on a hot plate until it was colorless or golden brown. After 30 minutes, it had cooled. It was still a 2ml solution but now the volume has been increased with distilled water to 100ml. it has now been purified on filter paper and put in a bottle. Calculations were made by using an atomic absorption spectrophotometer for calcium concentration and a UV visible spectrophotometer for magnesium and potassium levels.
Total Phenolic Content (TPC)
The ratio of phenolics in the sample’s extract was measured
using Folin-Ciocalteu technique. Total phenolic content was
determined according to protocols used by Naz et al. [16] To
create a 2mg/ml standard gallic acid solution, weigh 0.1g of gallic
acid and mix with distill water (50ml). To analyze the herbal
teamix samples, 40mL of the sample extract or gallic acid standard
was combined with 1.8ml Folin- Giocalteu reagent, which had
been previously 10-fold diluted using distilled water. Afterward,
1.2ml of sodium bicarbonate (7.5%) was transferred to mixture.
The resulting mixture was cooled down for 5 minutes at room
temperature. After 60 minutes of standing at room temperature, a
spectrophotometer was used to detect the wavelength at 765nm.
The results were expressed as mg gallic acid equivalents per gram
of sample analyzed.
T = C x V /M where,
T = total contents of phenolic compound in mg GAE/g plant
extract.
C = the concentration of gallic acid calculated from calibration
curve in mg/mL.
V = the volume of extract in mL.
M = the weight of plants extracts in grams.
Total Flavonoid Content (TFC)
The total flavonoid contents s of the samples was determined by following the method described by Rehman et al. [17] using Catechin as a standard. The procedure likely involved the following steps. A known amount of Catechin was dissolved in ethanol to create a standard solution of known concentration. The banyan fruit powder samples were prepared for analysis. This likely involved extracting the flavonoids from the samples using an appropriate solvent or extraction method. A series of working standard solutions of Catechin with varying concentrations were prepared by diluting the standard solution with ethanol. These solutions would cover a range of concentrations to create a calibration curve. The absorbance of each standard solution and the sample solutions was measured as a specific wavelength using a spectrophotometer. The wavelength chosen is typically the one at which the flavonoids absorb light most efficiently. Using the calibration curve and the corresponding absorption values of the sample solution, the concentration of flavonoids in the banyan fruit powder samples was determined. The results were given in milligrams of catechin equivalent per unit of sample (e.g., per gram). To ensure accuracy and precision, all calculations and measurements were likely recorded in triplicates, meaning each sample was analyzed three times independently. By using Catechin as a standard, the flavonoid contents s in the banyan fruit powder samples could be quantified based on its equivalent Catechin contents s. This method allows for a reliable comparison of flavonoid levels in different samples and provides valuable information about the antioxidant potential of the banyan fruit powder sample.
Antioxidant analysis
The antioxidant potential of the sample was evaluated using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, as described by Shahid et al. [18]. To perform this technique, 1 ml of the DPPH (0.004%) in methanol solution was added to 3 ml sample. Later, the prepared solution was kept in dark for thirty minutes. The absorbance was recorded at λ 517nm. A low absorbance of reaction combination suggests strong radical scavenging activity. The solution empty of plant extract served as the control group [19]. The test was repeated three times to avoid any human error. To determine DPPH percentage inhibition in radical samples, the following formula was followed

A0 = Absorbance of blank.
A1 = Absorbance of sample.
Ascorbic acid was used as a positive control.
Bioactive component analysis
The glycyrrhizic acid concentration of all the liquorice extracts was determined using a High-Performance Liquid Chromatography (HPLC) equipment (PerkinElmer, Series 200, Shelton, CT) in accordance with the procedures of Tian et al. [20]. Prior to HPLC analysis, all of the sample vials were filtered and vortexed using a gyromixer utilized a 25mL sample loop Reodyne injection valve. The UV wavelength was set to 252nm and the flow rate was 1.0mL/min. For a subsequent HPLC examination, all solvents were filtered using a disposable syringe filter unit (0.2lm). A column (a Shimpack C18 column with a mobile phase of methanol-water (70:30, vol/vol, containing 1% acetic acid) was used to analyze Glycyrrhizic acid.
Preparation of Herbal Teamix
Herbal teamix were prepared using a modified recipe of Xu et al. [21]. Liquorice root and fennel seed were properly weighed and mixed together in polythene teabags and stored for further analysis. Five formulations of herbal teamix were prepared along with control (Table 1) (Figure 2).
T0 = 100% liquorice root powder act as control.
T1= 90% liquorice root powder, 10% fennel seed powder.
T2= 85% liquorice root powder, 15% fennel seed powder.
T3= 80% liquorice root powder, 20% fennel seed powder.
T4= 70% liquorice root powder, 30% fennel seed powder.
T5= 60% liquorice root powder, 40% fennel seed powder.
Analysis of Herbal Teamix Mineral analysis
Mineral analysis of herbal teamix was calculated to patronage their standard protocol [15] as discussed before.
Phytochemical analysis
TPC [22], TFC [23] and anti-oxidant potential [24] of herbal teamix were assessed using their respective methods as demonstrated earlier.
Color analysis
Colorimeter was used to analyze the color of herbal teamix as determined by [25]. Utilizing criteria of lower and upper limits, a calorimeter was calibrated (51cm for dark and 151cm for light). Petri plate was entirely filled with product sample and the surface was smoothed by removing sample from the plate’s upper surface. In order to get the most reflection, the color meters photocells were used to emerge the sample and the display reading was recorded and compared with the standards.
Sensory evaluation
Sensory characterization of herbal teamix was performed for color, aroma, taste, mouthfeel and overall acceptability by using the 9-Point Hedonic scale [26]. Postgraduate student of NIFSAT were selected as a judge to find the best treatment of herbal teamix. The range of the scale was from 1 (extremely disliked) to 9 (extremely liked). Herbal teamix were placed with labeled in a different cup. These cups were placed in front of the judges so that they could give scores whether they liked the product or not. To increase the validity of analysis, the judges were given water in between the samples to rinse their mouth.
Statistical Analysis
To highlight the significant level, the data from the characterization and production of herbal teamix was statistically analyzed using the Completely Randomized Design (CRD) and ANOVA [27].
Results and Discussion
Liquorice root and fennel seeds were the two main research ingredients used in this study. Firstly, these were cleaned and washed to remove the dust and dirt particles. Afterwards, dried both the ingredients followed by grinding them into powder. These powders were analyzed for selected minerals, phytochemicals (TPC, TFC and DPPH) and bioactive components. Afterwards, herbal teamix was developed by using liquorice root and fennel seed in different proportions. It was also subjected to mineral and phytochemical analysis and color. Furthermore, a sensory evaluation was done for color, aroma, taste, mouthfeel and overall acceptability. T4 (Herbal teamix composed of 70% liquorice root and 30% fennel seed powder) was chosen based on its chemical composition and sensory qualities. Discussion of the study’s different results are given below:
Analysis of Liquorice Root and Fennel Seed Mineral analysis
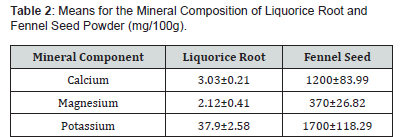
Means and standard deviation for mineral analysis of liquorice root and fennel seed has been presented in Table Analysis showed that in liquorice root (mg/100g), presence of calcium was recorded to be 3.03±0.21, magnesium 2.12±0.14, potassium scored 37.9±2.58 and fennel seeds (mg/100g mg) Ca, K content were reported to be magnesium 370±26.82, calcium 1210±83.99 and potassium 1700±118.29 respectively. From these results it was observed that liquorice root contained potassium in higher quantity while the presence of magnesium is very low, but this indicates that overall liquorice root is quite a good source of minerals while fennel seed contains high higher level of potassium calcium and a significant amount of magnesium as well. Recommended value of potassium is less than 2000mg/day so, both these ingredients could not cause any health problems to hypotension patients. Husain et al. [28] who demonstrated that liquorice root contains potassium (0.66%), calcium (1.87%) and magnesium (0.17%).
Similar findings are illustrated by Desoky et al. [29] who examined that the mineral composition of liquorice root extract contains potassium 47.2g kg-1, calcium 2.20 and magnesium 3.80, respectively. Badr et al. [30] also showed that liquorice roots are not only excellent sources of calcium, potassium and magnesium but also contains copper in minute amounts. The difference in findings may be due to temperature, humidity, soil composition, extraction technique and genetic variation which affects the storage of mineral content in liquorice root powder (Table 2).
The obtained results showed that liquorice root contains considerable amount of minerals. Similar results showed by Hamad et al. [31] that the primary component of the liquorice root extract (112.2 g/100g) was calcium. The quantity of iron in liquorice extract was determined to be rather low, at 1.70 g/100g. These results are in line with those reported by Singh and Singh [32], who analysed the mineral composition of a few therapeutic herbs that are primarily used as conventional condiments and find that fennel seed has a considerably higher K content than other seeds., 1030.1, 11.6 4.52, 3.81, 20192 and 1.54mg/100g of calcium, iron, sodium, manganese, potassium and zinc were the mineral contents of fennel seed. The study by Bukhari et al. [33], who examined the significant amounts of minerals found in Foeniculum vulgare, particularly (mg/100g) manganese 211.35±7.40, sodium 16.21±0.65, potassium 852.45±33.25 and iron 9.72±0.38 is also like these results.
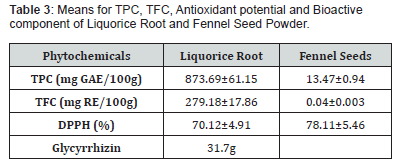
Total phenolic content
Means with standard deviation of liquorice root powder and fennel seed has been shown in Table 2. The results clearly demonstrated that total phenolic content mean values in the liquorice root powder is (873.68±61.15mg GAE/100g) DW while in fennel seed powder is (13.43±0.4 mg GAE/100g) DW. According to Esmaeili et al. [34], In the Bajgah community, TPC (mg GAE/g DW) varied from 6.18 ± 0.33 to 14.91 ± 1.17. Following the Ilam population, the greatest TPC was assigned to the Kashmar (12.67 ± 0.67) and Marvest (11.70 ± 0.30) populations. A plant’s total phenolic content is highly variable depending on its genotype, growth environment, harvesting date and processing method.
According to Asan and Karakoca [35], the phenolic component of the DCM (dichloromethane) extract was found to be greater (214.36mg GAE/g extract) in the liquorice root extracts than the n-hexane extract (86.86mg GAE/g extract). Our results showed that the DCM root extract had a greater overall phenolic content than the Turkish liquorice root aqueous and ethanol extracts (Table 3).
Mean ± Standard Deviation
Mean ± Standard Deviation
The phenolic content of the methanolic root extract from G. Echinata L. was found to be lower in Cakmak et al. [36] research (114.33mg GAE/g) than in DCM extract. Nevertheless, the ethanolic extract’s phenolic content was discovered to be larger in their investigation than the n-hexane extract in this study. In comparison to the DCM extract used in our investigation, Pratibha et al. [37] found that the DCM extract of Glycyrrhiza glabra root had a lower phenolic concentration (73.51 mg GAE/g).
Phenolic substances are the secondary metabolites of plants that are widely distributed from roots to leaves of the plants in plant kingdom. They provide protection to the plants against different environmental conditions and insect attacks. About more than 800 phenolic structures are known. Their structure comprises of single aromatic ring such as simple phenolic acids to highly complex polymeric structure that are lignin and tannins. They are water soluble and may occur as sugar substitutes for glycosides. They are the main functional components of plants that provide health benefits to the human body and reduce the incidence of evolving metabolic diseases. They have been recognized to have anti-inflammatory and anti-allergic activities. These compounds terminate free radical chain reaction and interfere with lipid peroxidation.
Total phenol concentration in seed extracts from three artificial cultivars and seven fennel genotypes was reported by Akbari et al. [38]. The phenolic content of the genotypes varied greatly. The TPC for these genotypes varies from 26.19 to 88.09mg GAE/g. The late maturity synthetic genotype had the greatest TPC (88.09mg GAE/g), which was noticeably greater than that of the other genotypes. Furthermore, the most TPC levels were found after late maturity synthetic genotype is in the Fozveh and Early maturity synthetic (76.04mg GAE/g and 74.92mg GAE/g, respectively). Hajiabad has the lowest reported levels (26.19mg GAE/g). Sayed et al. [39] showed that the TPC of the bread increased when the fennel level increased in both cases. Bread having fennel cake seed had a TPC of 113.73 to 187.49 and 196.05mg GAE 1100g-1 DW, respectively, when the amount of fennel substitute was raised from 0 to 6%. Additionally, at a given dose of fennel, the TPC of bread containing fennel seeds was greater than that of fennel cake. Fortified crackers with fennel seed added significantly increased the TPC, according to Anayatullah et al. [40]. T2 (37.10 ± 0.29), where 20% fennel seed powder was added, had the greatest value, followed by T1 (34.23 ± 0.90), while T0 (19.34 ± 0.11), where no fennel seed powder was applied, had the lowest value. Repaji et al. [41] found that the extractions carried out using pressured liquid extraction exhibited lower TPC values (145.27-474.21mg GAE/100g). Higher TPC was seen in methanolic and ethanolic fennel seed extracts from Pakistan (627.21 ± 18.36– 967.50 ± 35.51mg GAE/100g) and Iran (14 ± 0.18–262 ± 0.91mg/g of dry weight). The TPC values of MAE extracts varied between 237.3 ± 10.5 and 706.2 ± 19.0mg GAE/100g [42]. Bano et al. [43] showed that the fennel methanol extract’s total phenolic content was 3.48 ± 4.2 (mg GAE/g DM). These outcomes matched those that were published by [44].
Total flavonoid content
Means with standard deviation of liquorice root and fennel seed powder has been shown in Table 3. The results revealed that total flavonoid content mean values in the liquorice root powder is (279.18±17.86mg CE/100g) DW and in the fennel seed powder is (0.04±0.003mg CE/100g) DW. The present study’s findings are in line with those of Asan-zusaglam and Karakoca [35], who studied Turkish liquorice root’s antioxidant capacity and concluded that the root’s flavonoids could have antioxidant qualities. In their investigation, n-hexane was extracted from dried liquorice root using a Soxhlet device and left for 24 hours. The obtained extract’s total flavonoid concentration is 392 mg QE/100g. In another study, Tohma and Gulcin [45] examined the differences in the antioxidant and radical-scavenging properties of liquorice’s root and aerial portions. The total flavonoid concentration of the liquorice root and areal sections was found to be 420 and 440 mg QE /100g, respectively, based on their findings. Di Mambro et al. [46] previously examined the flavonoid content and antioxidant capacity of several medicinal plants and discovered that liquorice root extract had the highest flavonoid concentration of any plant extract studied, at 88 mg QE/100g.
The most significant class of polyphenols found in a wide range of plants are flavonoids. These are a set of 4000 naturally occurring substances with a range of health advantages. They are composed of a single benzene ring. In certain plants, they are frequently found as pigments. They are regarded as an essential part of the nutrition of humans. You need to eat about 1g of flavonoids every day. Their main purposes include inhibiting hydrolytic and oxidative enzymes, scavenging free radicals, immunomodulation and anti-inflammatory properties. Through a variety of mechanisms, including a rise in mucosal prostaglandins, a decrease in histamine production and a suppression of H. pylori development and have gastroprotective effect as well.
Flavonoids are important secondary metabolites of plants that perform a variety of essential roles, including UV filtering, cell cycle suppression, floral coloring (yellow or red/blue coloration) and physiological function modulation. Among the most powerful naturally occurring antioxidant components are liquorice flavonoids. They adopt a variety of strategies, including hydrogen donation, metal chelating and free radical scavenging [47]. Different solvents may be used to vary the extraction process of flavonoids and the concentration of each solvent was evaluated to maximise flavonoid recovery from liquorice root.
Liquorice root’s total flavonoid concentration is greatly impacted by its maturation stage, when it is harvested and the weather. In a similar study, Cheel et al. [48] found that the total flavonoid content of liquorice obtained at various periods varied significantly. Their findings showed that from February (mg/g extract) (18.42±0.49)) to August (44.20±0.64) total flavonoid increased with time. However, as the value for this feature sharply dropped from August to November, greater maturation of liquorice had a negative impact on the overall flavonoid content (35.03±0.65 mg/g extract). Ben Abdesslem et al. [49] said showing the TFC for E4 (Chrahil farm) (9.06 ± 0.20mg QE/g DW) and E6 (Commercial Indian fennel) (17.48 ± 0.80mg QE/g DW) in the organic fennel ethanolic extract was the greatest and the lowest, respectively. Khammassi et al. [50] examined the metabolite profiles and possible antioxidant capacity of sixteen wild populations of fennel (Foeniculum vulgare Mill.) in Tunisia and discovered notable differences in TFC across these populations. TFC concentrations varied from 5.17 to 16.42mg QE/gDE. Extracts from the Chnenfa and Utique populations had the highest TFC, which was followed by sites in BeniAyech (14.45mg QE/gDE), OuedBzigh (15.73mg QE/gDE) and Takelssa (15.19mg QE/gDE). However, the Aousja sample (5.17mg QE/gDE) had the fewest flavonoids.
Antioxidant analysis
Means with standard deviation of liquorice root and fennel seed powder has been shown in Table. The results clearly illustrated that the mean values of DPPH in the liquorice root powder is (70.12±4.91279 %) and in fennel seed powder is (78.11±5.46). To assess the capacity to scavenge free radicals, the DPPH test is often employed in the fields of food science, nutrition, phytochemistry and pharmacology. By taking on an electron or a hydrogen radical, the free radical DPPH can be quickly transformed into a stable molecule. With a quick reaction time, this approach can process relatively large volumes of samples and is sensitive to detect the antioxidant activity in samples with lower analyte concentrations [51]. This study’s findings are in line Di-Mambro et al. [46], which used the DPPH free radical test to assess the antioxidant potential of different mechanical plants, including liquorice. Even at low concentrations (1Ul/mL), liquorice extract significantly inhibited DPPH radicals, as evidenced by an 88% drop in absorbance at 517 nm. The current results of liquorice solvent extract’s DPPH free radical scavenging capabilities are also in line with Gabriele et al. [52]. They evaluated the ability of the liquorice cortex and inner yellowish part extracts, which were extracted using various solvents, to scavenge radicals. They found that the cortex extracts had a higher capacity for scavenging than the inner part of the root. In ethanolic extracts, the reported values of cortical extracts’ free radical scavenging activity varied from 90 to 98%. On the other hand, the methanolic extracts showed much lower levels of free radical scavenging activity (67-92%). Furthermore, ethanolic extracts of the inner, yellowish portion of liquorice roots demonstrated 75-86% radical scavenging capabilities, but methanolic extracts only shown 28–81% capability for free radical scavenging.
Likewise, lateef et al. [53] examined the antiradical properties of liquorice methanolic extract and its subfractions that were made in chloroform, n-butanol and ethyl acetate. The study’s findings demonstrated that methanolic extract had the highest capacity to scavenge free radicals (91.3%) and that this potential grew in a dose-dependent manner. Chloroform extract found that the greatest DPPH free radical scavenging capability among the sub fractions was 87% inhibition. Earlier, Jo et al. [54] examined the liquorice ethanolic extract’s capacity to donate electrons using the DPPH free radical scavenging technique. The reported scavenging efficiency of liquorice extract against free radicals was 70.44%.
Bano et al. [43] reveals the fennel extract’s dose-dependent DPPH radical scavenging activity curve. The graph was used to compute the IC50 value, or the concentration of fennel extract required to reduce the starting concentration of DPPH free radicals by 50%. This value came out to be 31 μg/ml. The stronger the antioxidant capacity, the lower the IC50 value. Lee And Kim [55] investigating the DPPH radical scavenging ability of Fennel seed ethanol extract, as the concentration of the FSEE sample increases, the radical scavenging ability increases, reaching 0.831mgAAE/mL at a concentration of 0.25mg/mL, 1.423mgAAE/mL at a concentration of 0.5mg/mL, 2.342mgAAE/ mL at a concentration of 1 mg/mL and 3.129 at a concentration of 2mg/mL. Measured as mgAAE/mL. Anwar et al. [56] reported the IC 50 of DPPH radical scavenging activity of fennel seed extract in 80% ethanol as 23.61μg/mL, but in this study, it was measured as 9.57mg/mL. This is due to the difference in extraction method and origin of fennel and is believed to be due to the higher extraction yield by repeatedly extracting fennel seed powder three times at room temperature for 8 hours.
These results are in line with those by Khammassi et al. [50] who studied the DPPH radical scavenging activity and potential antioxidant activity of sixteen wild populations of fennel (Foeniculum vulgare Mill.) growing in Tunisia. Significant variance was observed across the populations. BeniAyech, on the other hand, was very different from the other samples and had the maximum antioxidant capacity (IC50=23.66μg/mL). El Alia and Takelssa extracts came in second and third, with IC50 values of 64.66 and 175.66μg/mL, respectively. However, the Teborsok and Zouarine populations had the lowest scavenging activity for free radicals (IC50 values of 977.33 and 975.66μg/mL) respectively. Usha and Meena [57] investigate the Comparative study on antioxidant potential and phytochemical composition of cumin and fennel and mention that the ethanol extract of fennel seed and give DPPH Scavenging results by% concentration of methanol extract (μg.ml−1) 25% (15.9 ± 2.45) 50% (28.6 ± 3.58) 75% (51.7 ± 2.69) 100% (71.55 ± 2.8).
Bioactive component
Means with standard deviation of total glycyrrhizin was described in the (Table 3,4). It shows that total glycyrrhizin in liquorice root powder is 31.7/100g of the sample. The results are in line with those reported by Tian et al. [20] who studied at how various solvents affected the amount of glychrrhizin that could be extracted from liquorice root. The water extract had the greatest recovery of glycyrrhizin (2.44mg/g liquorice), followed by methanol and ethanol. Moreover, when the percentage of ethanol was increased from 10% to 90%, the content of glycyrrhizin decreased progressively from 2.44mg/g to 1.09mg/g.
One of the most advanced methods used to identify and characterise physiologically active components is high performance liquid chromatography. Liquorice primary bioactive ingredient, glycyrrhizins, is responsible for its antioxidant capacity as well as other medicinal qualities. It is crucial to precisely measure the concentration of these biomolecules in liquorice extract to determine the appropriate dosage. To ensure precise glycyrrhizin quantification, HPLC analysis was performed on all the solvents.
Recently Deyab [58] assessed how the quantity of the solvent affected the amount of glycyrrhizin that was recovered from liquorice. Liquorice was intentionally extracted using 10-90% ethanol. As a result of reducing the ethanol concentration from 90% to 10% and raising the water consideration in the same way, the glycyrrhizin content of liquorice extract rose from 0.75mg/g to 2.25mg/g. The current investigation are also in line with the study of Ahn et al. [59] who investigated the process of separating liquorice’s bioactive ingredient utilising conventional solvent extraction and ultra critical fluid extraction. HPLC analysis revealed that the bioactive component concentration of the liquorice supercritical fluid extract was substantially greater. Earlier, Wang and yang [60] observed that the glycyrrhizin concentration of one-year-old liquorice root ranged from 1.212±0.054 to 7.881±0.141mg/g. They found that the amount of glycyrrhizin in liquorice rises as the plant ages.
Recently, Hedayati and Ghoreishi [61] examined the effects of various process parameters on the liquorice-derived glycyrrhizin recovery. According to their findings, elevated pressure promoted glycyrrhizin recovery via raising supercritical CO2 density. More glycyrrhizin was soluble as density rose, which improved the extraction yield. Furthermore, methanol was shown to recover glycyrrhizin more effectively than ethanol and using methanol and water in a 50:50 v/v ratio improves recovery rates even more. Muhammadiev et al. [62] further shown that due to their polar character, the extraction of flavonoids and phenolics from liquorice enhanced under high pressure.
In another study, statistical analysis of HPLC-quantified liquorice bioactive components glycyrrhizin and glabridin contents of conventional solvent and SFE varied significantly depending on the treatments. The maximum recovery of glycyrrhizin (2.41 ± 0.027mg/g liquorice) was found in 25% methanolic extract, while the highest concentration of glabridin (1.13 ± 0.010mg/g liquorice) was found in 75% ethanolic extract, according to the average effects of different solvents and their ratios. Glabridin recovery was often greatest in ethanolic extracts and increased with increasing solvent concentration. But methanol worked better as a solvent to recover glycyrrhizin. The data clearly show that glycyrrhizin recovery was enhanced by increasing water content. In contrast, ethyl acetate was least successful in recovering either component. The maximum recovery of glabridin (2.97 ± 0.012mg/g liquorice) and glycyrrhizin (5.02 ± 0.031mg/g liquorice) was identified in TSC3 (5,500 psi, 40°C), followed by TSC2 and TSC1. Among SFEs, the recovery of both bioactive moieties increased with increase in pressure [63].
In another study, Glycyrrhizic acid concentration (mg/g dried plant material) in Glycyrrhiza glabra extracts from various geographic areas was quantitatively analyzed by LC-ESI/MS/MS. The results show that the highest concentrations were found in Chinese Glycyrrhiza glabra roots, at 53.26±2.15, followed by those from Italy at 51.91±1.21, Turkey at 33.45±1.85 and Iran at 32.21±1.02 [64] Glycyrrhizin content in Glycyrrhiza glabra roots varied from 0.177 to 0.688% w/w of dried materials in a different investigation. This result is in line with previously published data that indicated the percentage level of glycyrrhizin was between 0.12 and 2.24% w/w of dry material, depending on the species and growing area. The samples from Dagestan (16.997 ± 0.186) and Uzbekistan (17.047 ± 0.122) had the largest concentrations of glycyrrhizin among the extracts, while one of two samples from young plants gathered in Italy (4.380 ± 0.058) had the lowest amounts of the compound [65].
Liquorice is a plant with a long history that dates from ancient times. Glycyrrhizin, the main ingredient in liquorice extracts, is widely used in tobacco, culinary products and herbal and conventional medicine. As a result, glycyrrhizin and liquorice are widely used in the US; daily intake is reported to be between 0.027 and 3.6mg/kg. The majority of national According to biochemical research, glycyrrhizinates block the activity of 11β-hydroxysteroid dehydrogenase, which is the enzyme that keeps cortisol inactive. Therefore, chronic high-level exposure to glycyrrhizin molecules can have consequences in humans and animals that are like hyper miner lactone. Liquorice or glycyrrhizin removal will reverse these effects. Glycyrrhizin and liquorice ingestion has been shown to have positive benefits in several in vivo and clinical trials, including hepatoprotective, antiviral and anti-ulcer properties. According to several genotoxic studies, glycyrrhizin is neither mutagenic nor teratogenic and in certain cases, may even have anti-genotoxic qualities. Its bioavailability is decreased when ingested as liquorice, according to the pharmacokinetics of glycyrrhizin, which has complicated efforts to determine precise dose-effect values in both people and animals. We suggest an adequate daily dosage of 0.015-0.229mg glycyrrhizin/kg body weight/day based on the in vivo and clinical findings [66].
Analysis of Herbal Teamix Mineral analysis
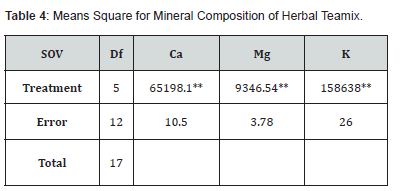
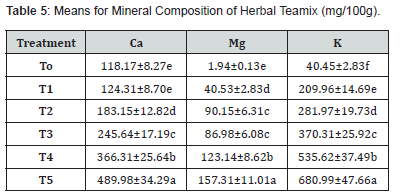
Means square for the mineral composition (calcium magnesium and potassium) of herbal teamix indicated a significant difference as mentioned in (Table 4). The mean value of calcium demonstrated that the average calcium increased significantly. Calcium content ranged from 118.17 to 489.98mg/100g. results indicated that T0 has the lowest level of calcium (118.17) and T5 has the highest calcium content (489.98) among other treatments. The mean value of calcium content increased in order T0 (118.17), T1 (124.31), T2 (183.15), T3 (245.61), T4 (366.31) and T5 (489.98) while mean values of magnesium content ranged from (1.94 to 157.31mg/100g). Result indicated that T5 has the highest level of magnesium content (157.31mg) and T0 has the lowest level of magnesium content (1.94) mg among other treatments. The magnesium content gradually varies in order T0(1.94), T1 (40.53), T2 (90.15), T3(86.98), T4 (123.14) and T5 (157.31) and mean values for potassium content ranged from 40.45 to 680.99mg/100g. It is demonstrated that T0 (40.45) has the lowest level of potassium content and T5 (689.99) has the highest level of potassium among other treatments. The potassium content varied in all treatments. T0 (40.45), T1 (209.96), T2 (281.97), T3 (370.31), T4 (535.62) and T5 (680.99). Processing conditions and variations in the quantity of fennel seeds and liquorice root were associated to variations in the mineral level in all treatments of the generated product.
In a research, it is documented the effects of foliar application of liquorice root extract at four levels (0 (Li0), 2.5g.L-1 (Li2.5), 5g.L-1 (Li5), 10g.L-1 (Li10) and garlic extract at four levels (0 (GA0), 5ml.L-1 (GA5), 10ml.L-1 (GA10) and 20ml.L-1 (GA20) was carried out during the growing season 2015-2016. Using RCBD, a factorial experiment with three replications of each treatment was conducted. There were 96 trees employed. The findings demonstrated how spraying 20ml of garlic extract interacted with it. L-1 mixed with 10g of liquorice root extract. L-1 (GA20Li10) produced high values for the nitrogen and potassium contents of the leaves (1.35% and 1.83%, 2.410 and 2.592% and 210.3 and 234.5mg, respectively) [67].
Liquorice root residues (LRR) are created in significant quantities during industrial liquorice manufacture. Utilizing these wastes as a production input in agriculture may be advantageous for both the environment and sustainable economic growth. Therefore, we evaluated the application of LRR to a few growth media at 50% soil + 50% LRR (SL), 50% perlite + 50% LRR (PL), 50% cocopeat + 50% LRR (CL)and 25% soil + 25% perlite + 25% cocopeat + 25% LRR (SPCL) are the percentages of soil (v/v) that make up each of these combinations. In addition to humic acid (0, 5, 10 or 15gl−1) when growing bell peppers. The mineral content of vegetative and reproductive development of pepper were found to be greatly enhanced by the combination of LRR treatment with HA fertigation. The highest fresh weight (branch and root), plant height, root length, number of leaves and chlorophyll content were observed in plants treated with SPCL + 15gl−1 HA. The peppers produced under SPCL had the most flowers (18.08), fruits per plant, fruit length and diameter, fruit fresh weight and yield. In the S treatment, the number of malformed fruits increased dramatically, but in the SPCL treatment, no damaged fruits were found. The medium culture with 25% LRR (SPCL) + 15gl−1 HA had the greatest leaf K, Ca, Fe and Zn levels. It was discovered that the optimal circumstances for bell pepper fruit development could be achieved by utilizing a fertigation of SPCL + 15gl−1 HA [68].
In another study, the antioxidants and bioactive compounds in liquorice root extract potentially contribute to improving growth, bulb quality and yield of onion (Allium cepa) was studied and illustrated that how the K and Ca2 contents of LRE1 Shandaweel bulbs grew by 31.03 and 78.61%, respectively and how the LRE2 Shandaweel bulbs’ contents climbed by 43.68 and 57.50%? The K and Ca2 levels of the LRE1 and LRE2 Giza 20 bulbs, respectively, showed a significant improvement over the corresponding control values, increasing by 64.58 and 87.50% and 33.16 and 51.95%, respectively. In contrast to the corresponding control, the magnesium ion (Mg2) levels in the LRE1 and LRE2 Shandaweel and LRE1 and LRE2 Giza 20 bulbs did not differ significantly [69].
Sea fennel is said to be an excellent source of minerals, particularly Ca, Mg and K of these minerals, calcium and magnesium are crucial for human nutrition, particularly for cellular metabolism and bone growth [70]. In detail, a mean leaf content of Ca ranging from 2.2 to 3.7 g 100g−1 DW was reported by Martins Noguerol et al. [71], mean levels for Mg and K that ranged from 0.4 to 0.6 g 100g−1 DW and from 1.8 to 6.0g 100g−1 DW. According to reports, sea fennel is a superior source of calcium than broccoli, which is among the vegetable sources that contain the maximum amount of this mineral. With regard to microelements, data gathered by showed Fe, Mn, Zn, Cu and Cr may be found in sea fennel, with mean concentrations ranging from 191.7 to 58.4mg kg−1 DW, 80.3 to 37.2mg kg−1 DW, 23.5 to 41.3mg kg−1 DW, 2.8 to 7.3mg kg−1 DW and 0.5 to 2.7mg kg−1 DW, respectively, depending on the type of habitat and edaphic circumstances.
Phytochemical Analysis Total phenolic content
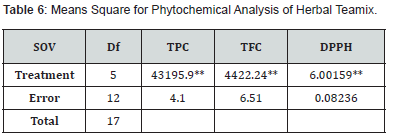
Means square for TPC and TFC of herbal teamix formulation showed significant differences among the treatments as shown in (Table 4,5). The means square for the DPPH of various treatments reveals that herbal teamix had highly significant effect on DPPH Value. Means result of the study are shown in (Table 4,6) that TPC in herbal teamix ranged from 853.05 to 516.73 mg/GAE/100g of dry matter. T5 has the lowest variety of phenolics (516.73). The highest phenolic concentration was identified in T0 (853.05). Other treatments also range greatly in the amount of phenolic content. The table showed random trend in the phenolic content of the herbal teamix from T0 to T5. The phenolic content decreased in the following order: T0(853.05) ≥ T1(770.40) ≥ T2(727.40) ≥ T3(685.06) ≥ T4(603.06) ≥ T5(516.73). The amount of powder has been shown to be closely correlated with its phenolic content. Different amounts of herbal teamix powder are the cause of the variations in the phenol levels amongst the treatments.
The results are in line with Singh et al. [72] who examined the TPCs (mg GAE/g of dw) of the various LREs and found a positive association between TPCs and extract concentration. The ethanol sample has the highest concentration of TPCs in LREs (5.13±0.12mgGAE/g of dw), whereas the water sample has the lowest concentration (1.96±0.04mg GAE/g of dw) at 15.63mg/ mL. Vlaisavljević et al. [73] evaluated content of total phenolic compounds (TPC) in the liquorice root. The fresh root extracts L1 (37.27 ± 0.55mg GA eq/g of dw), L2 (31 ± 0.27), L3 (16.5 ± 0.78) and L4 (13.23 ± 0.36) had the highest TPC concentration among the Glycyrrhiza glabra extracts from Fruska Gora. Numerous factors, including temperature and the vulnerability of phenolic compounds to air oxidation, might be the cause of the discrepancy in the results.
Similar findings were documented by Ahmad et al. [74] who observed that agro-industrial by-products may be used as a source of phenolic compounds and are an excellent source of natural antioxidants. They also claimed that phenolic compounds are the primary classes of chemicals with various therapeutic and protective effects on human health [75]. Consequently, the overall phenol content of the seed cakes following the extraction of vegetable and essential oils as well as the remaining water from the hydro-distillation of Foeniculum vulgare seeds were assessed. The type of residue and the seed’s origin affects the phenolic concentration of our samples. Overall, the hydro distillation residue meal had the lowest total phenolic content and aromatic water had the greatest level.
These results are in line with those obtained by Chatterjee et al. [76] They discovered that the fennel seed aqueous extract had the greatest phenol content. Conversely, the variance in contents among various sources can be linked to other factors, including the stage of seed maturity, climate and culture conditions. Faudale et al. [77] found that the overall amount of phenolic chemicals in fennel seeds from various Mediterranean nations varied significantly.
Total Flavonoid Content
Means square for the Total Flavonoid Content (TFC) of different treatments are shown in (Table 4,5). It is evident that the supplementation had a considerable impact on the TFC. (Table 4,6) makes it evident that TFC means have varied from 268.24 to 160.74 RE/g of dry stuff. The control group, which contains 100% liquorice root powder has the highest range, whereas the lowest range has been seen in T5. There are also notable differences in the flavonoid content of other therapies. The flavonoid content drops in the following sequence for each treatment: T0 (268.24) ≥ T1(241.94) ≥ T2(228.80) ≥ T3(216.99) ≥ T4(188.36) ≥ T5(160.74). These findings are in line with the findings of Dong et al. [78] who said that flavonoids, particularly in Glycyrrhiza glabra L. are among the most significant bioactive components of plants. TFC is therefore a crucial indication for assessing the chemical composition of Glycyrrhiza glabra L.’s leaves and roots. TFC in Glycyrrhiza glabra L. leaf extract was much greater than that in root extract, at 384.75 ± 4.11. 91.75 ± 6.61. The technique based on sodium borohydride and chloranil was selected to quantify TFC in the extracts of leaves and roots. Nearly all forms of flavonoids, such as flavonols, flavones, flavanols, flavononols, isoflavonoids, flavonones and anthocyanins, may be measured with this test [79].
Similar findings are illustrated by Hussain and Iqbal [80] who presented the nutritional and medicinal qualities of the roots of Glycyrrhiza glabra L. and reported that the maximum flavonoid contents (38.96 ± 0.017mg CE) were shown using the chloroform extract. It was discovered that the TFC values for the remaining fractions were as follows: water > n-butanol > ethyl acetate > methanol > ethanol > nhexane. Glycyrrhiza glabra fractions showed TFC in the range of 5.13–38.96mg CE in the current investigation. In contrast to current findings, a previous investigation detailed the 2.6-5.1μg quercitin flavonoid concentrations [45]. Variation among results is due to use of diverse TFC units. Similar results were cited by Basist et al. [81] who determined TFC to be 8.24 ± 0.07mg of rutin equivalent/g dry weight of the extract using the rutin calibration curve, R2 = 0.994. Beyazen et al. [82] Based on various solvent systems, the quantities of TFC extracted from fennel seeds were 1.09 ± 0.48 to 22.93 ± 7.17 mgGAE/g and 2.404 ± 1.05 to 8.581 ± 1.22mg CE/g, respectively.
Antioxidant potential
Antioxidant potential of liquorice root and fennel seed powder based herbal tea mix was analysed by DPPH method. The means square shown in (Table 4,5) for the DPPH of various treatments reveals highly significant effect on DPPH value. The ability of a substance to prevent or restrict the oxidation of other molecules and cellular structures found in live cells is known as antioxidant potential. Compounds with antioxidant activity can either directly or indirectly scavenge free radicals and shield the cell structure against abnormalities or deformities of any type. Table 4,6 makes it evident that the range of DPPH means has been between 68.46 to 72.27 %. T5 has the maximum potential (72.27). In T0, there was the minimum potential (68.46). By adding fennel seed powder to the herbal tea blend, the total accident possibility increases from T0 to T5. The antioxidant potential increases to T0(68.46) ≤ T1(69.35) ≤ T2(69.71) ≤ T3 (70.49) ≤ T4 (71.50) ≤ T5 (72.27) respectively.
Similar findings are reported by Rozi et al. [83] who documented the bioactivities, characterizations and isolations of polysaccharides from the seeds of three different species of Glycyrrhiza. It was found that within the tested range, Glycyrrhiza polysaccharides exhibited strong dose dependent DPPH radical scavenging activity and that this activity was greater than that of the positive control, BHT. For GUP, GIP and GGP, the corresponding half-inhibition rate (IC50) was 59.94, 70.74 and 75.50μg/mL. The polysaccharide derived from Glycyrrhiza uralens exhibited the most potent DPPH radical scavenging ability among them. According to research, proteins that bind to polysaccharides may increase the negative charge of molecules, increase the likelihood of stabilising radicals and prevent the start and spread of additional oxidative processes. This may have to do with GUP having more protein than GIP and GGP. GUPs-1, GUPs-2 and GUPs- 3 from Glycyrrhiza uralensis roots have a DPPH radical scavenging capacity of less than 80%. At 4.0mg/mL, the maximum DPPH radical scavenging ability of GPs1 (68.23%) was noted. As for L4, the ethyl acetate extract of liquorice leaves from the Fruska Gora locale, with an IC50 value of 206.52μg/mL, was found to be less potent than polysaccharides from the seeds of three other Glycyrrhiza species.
In another research, Babich et al. [84] who depicted that the glycyrrhiza glabra’s antioxidant potential activity improved when utilising the (Methanol) Soxhlet technique. The antioxidant capacity (58.16 ± 3.90) of methanol was significantly affected by the Soxhlet technique These results are also in line with Amer et al. [85] who illustrated the effects ecologically friendly substances such as glycyrrhizic, salicylic and humic acids as well as their nanocomposites on French basil and found that the DPPH values of the various samples utilised ranged from 70.63 to 74.93%, showing a substantial increase when compared to the untreated plants. The plants that were foliar sprayed with HA NPs had the highest DPPH scavenging activity, measuring 74.93% with an IC50 of 42.2μg/ml. These plants were followed by those treated with SA NPs and GA NPs, which demonstrated 73.89% and 72.74% with an IC50 of 45.5 and 49.6μg/ml, respectively, in comparison to the control plants, which showed a DPPH scavenging activity of 70.63% with an IC50 of 55.3μg/ml). The other treatments showed slightly increased DPPH. The processing conditions had an impact on the antioxidant capabilities of phytochemicals, which is why the results differed.
These results are in line with those depicted by Tariq et al. [86] who discovered the fennel seeds antioxidant activity by utilising a DPPH assay and a variety of solvents, such as ethanol, methanol solutions, chloroform and distilled water. Methanol has the greatest extraction yield (86.21%) of all the solvents examined, followed by water (4.430%), ethanol (41.35%) and chloroform (65.89%). This study is in with strong argument with the study presented by Sultana et al. [87] that antioxidant components can be extracted in higher amounts in methanol compared with ethanol and water.
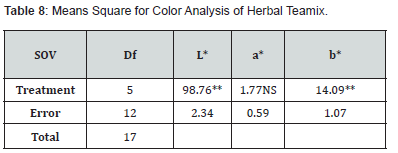
Color analysis
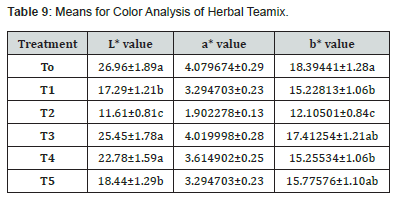
Means square of color (L*, a*and b* value) displays the major variations across different treatments are shown in (Table 4,7). Table 4,8 presented the study’s findings, which presented that the Herbal teamix’s average L* values vary from 26.94 to 18.44. Results indicated that T0 has highest L* value (26.94) while T5 has the lowest L* (18.44) value. L* values varied from T0 to T5 in order to that T0 (26.94) T1 (15.96) T2 (11.91) T3(26.76) T4 (24.93) and T5 (18.44). The results demonstrated that the average values of a* ranged from 4.11 to 2.05. T3 had a maximum a* value (4.11) and T2 had the lowest a* value (2.05) followed by T0, T1, T4 and T5 that is (4.07), (3.01), (3.66), (3.01) respectively. The study’s findings (Table 4,8) showed that the average b* values varied from 17.48 to 12.11. To has the highest value of b* (17.48) while T2 has the lowest value of b* (12.11) followed by T1, T3, T4 and T5, that is (14.29), (17.45), (16.45), (14.84) respectively.
The final product’s acceptance and quality are greatly impacted by color and may be quantified using a variety of tools and methods in addition to senses. The colorimeter was employed in the current investigation. (CIB_LAB space, Color Tech, USA) to evaluate color of herbal teamix. Colorimeter provides reading of three attributes L* (lightness) a* (+ redness, -greenness) and b*(+ yellowness, -blueness). The L*, a*and b* values of liquorice root samples were found to be 12.271, 10.648 and 15.896, respectively, prior to the drying process (raw materials). Following various drying techniques, the liquorice root samples’ L*, a*and b* values ranged from 32.46 to 38.60, 12.02 to 12.15 and 26.09 to 29.48 respectively and seen that different drying methods on L*, a* and b* values of the liquorice samples had statistically significant difference.
Similar results are reported by Sarabi-Aghdam et al. [88] who examined the color properties of both the free and LE-loaded liquorice extract and found that the brightness L* (40.333 ± 2.066) in the LE-loaded microparticles was noticeably higher than that of the free extract (23.83 ± 2.48). The microparticles loaded with LE had a lower a*(9.667 ± 2.257) index than the free extract (18.167 ± 1.322), indicating a shift in the powders’ color from red to green. This is because the type and color of the wall components that were utilised match the outcomes of Ghasemi and Khomeiri [89]. By using WPC (4%) to raise the lightness (L*) from 60 to 76, they were able to mask the orange-yet-yellow color of d-limonene at pH 3. As wall materials, pectin (1%) complex coacervates. Additionally, the LE-loaded microparticles’ b* index increased from 30.167 ± 2.137 to 37.167 ± 1.722, indicating a rise in yellow color. In general, the kind, concentration and color of the carrier all affect the final color of the particles.
Sensory Evaluation
A sensory study was carried out to learn about the behaviour
of consumers about accepting or rejecting a product. A sensory
panel adopted a 9-point hedonic scale to assess the sensory
qualities of herbal Teamix. The detailed results of all sensory
parameters for the herbal teamix are discussed below:
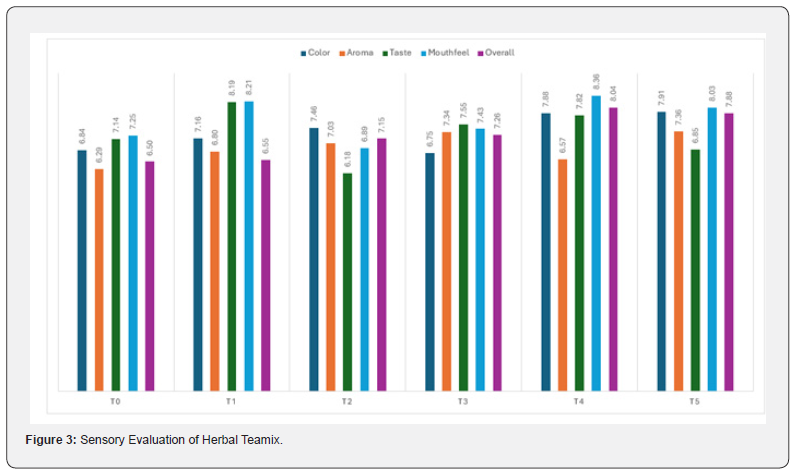
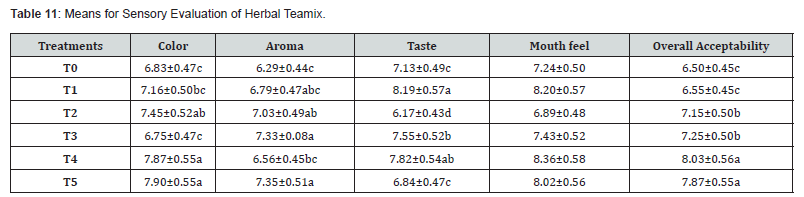
Means square for the sensory attributes (color, aroma, taste,
mouth feel and overall acceptability) is described in (Table 4,9).
They showed a highly significant difference between herbal
teamix prepared with different formulations of liquorice root and
fennel seed. Means of color described in (Table 4,10) have ranged
from 6.75 to 7.90. A maximum score was given to the T5 (7.90)
with 70% of liquorice root and 30% of fennel seed & minimum
score was given to the T3 (6.75) with 80% of liquorice root and
20% of fennel seed (Table 11). The trend of scores given by a
panel of judges was T1 (6.83), < T2 (7.16), < T4 (7.87) and < T0
(6.83). Means of taste are described in (Table 4,10). have ranged
from 6.17 to 8.19. A maximum score was given to the T1 (8.1)
with 90% of liquorice root and 10% of fennel seed and minimum
score was given to the T2 (6.17) with 85% of liquorice root and
15% of fennel seed. The trend of scores given by a panel of judges
was T0 (7.13), < T3 (7.55), < T4 (7.82) and < T5 (6.84). The means
of aroma & mouthfeel are described in (Table 4,10). Means of
aroma ranged from 6.29 to 7.35. Means of mouthfeel ranged
from 6.89 to 8.36. A maximum score was given to the T4 (8.36)
with 70% of liquorice root and 30% of fennel seed and minimum
score was given to the T2 (6.89) with 85% of liquorice root and
15% of fennel seed (Figure 3). The trend of scores given by a panel
of judges was T1 (8.20), < T3 (7.43), < T0 (7.24) and < T5 (8.02)
respectively. The means of overall acceptability are described in
(Table 4,10). Means of overall acceptability ranged from 6.50 to
8.03. A maximum score was given to the T4 (8.03) with 70% of
liquorice root and 30% of fennel seed and minimum score was
given to the T0 (6.50) with 100% of liquorice root. The trend of
scores given by a panel of judges was T1 (6.55), < T3 (7.25), < T2
(7.15) and < T5 (7.87) respectively.
**= highly significant
**= Highly significant

**= Highly significant, NS= Non-significant (P> 0.05)
Shabani et al. [90] claimed that the flavonoids are primarily responsible for the liquorice’s and its extract’s yellowish shade. The results of in vitro testing and HPLC quantification of liquorice extracts clearly show that supercritical fluid extracts include greater levels of glycyrrhizin and total flavonoids. This clarifies the cause of the beverages’ brighter, yellowish color. Additionally, as time went on, scores for the taste, flavour, sweetness and mouthfeel of liquorice beverages declined due to changes in the drinks’ pH and acidity. The findings of this investigation into the sensory qualities of functional beverages made with liquorice are in line with the findings of Kausar et al. [91]. During a fourmonth sensory study, the research team evaluated the qualities of a functional drink made of cucumbers and melons at 15 days. The results showed that, over the course of 120 days, the flavour score decreased from 7.68 to 6.68 and the sensory color score decreased from 7.52 to 6.52. Additionally, the ratings for flavour and general acceptability dropped from 7.40 to 6.44 and 7.48 to 6.48, respectively, in a manner that was comparable. The results of present study are further supported by the work of Murtaza et al. [92] who assessed the sensory qualities and storage durability of strawberry juice kept for three months at various temperatures. Their study’s findings showed that, throughout the course of a 90- day storage trial, ratings for several sensory qualities, including as taste, color and flavour, varied [93-99]. They found that at high temperatures, reducing sugars and amino acids interacted, resulting in non-enzymatic browning and color variations. Additionally, a rise in acidity was cited as the primary cause of the decline in flavor and color sensory ratings.
In summary, the use of liquorice extracts did not negatively impact the beverages’ sensory assessment or storage. Additionally, all the panelists’ values for the various features were within an acceptable range, indicating a good sensory response. The phytochemical content and antioxidant capability both significantly increased with the addition of liquorice extra [100- 101].
Summary
Poor eating habits and harmful environmental factors are responsible for multiple morbidities that can lead to serious health consequences. Across the globe, the mortality rate owing to diseases is on the rise. In present time, millions of people are following unhealthy living patterns. The presence of bioactive components in the diet that stand within the economic standards of people becomes imaginary during a specific period. Consequently, the search for a balanced diet has been emphasized worldwide. Recent research depicts that herbs and seeds are not only abundant in nutrients but an excellent source of antioxidants. Plant-based food is considered an essential part of a balanced diet due to its vitamins, minerals. Many industries are using extracts as well as powder from plants to develop healthy products. Vegetables are already in use of people as source of food but now with the discovery of presence of health promoting and disease preventing bioactive components in leaves, roots, stem and flesh these parts are gaining importance to be used in food as well as nutraceutical products.

** = highly significant (P≤0.01), NS= non-significant (P> 0.05) ++
Mean ± Standard deviation
Liquorice root and fennel seed, individually cherished for their distinct flavors and health benefits, combine synergistically to offer a potent blend of wellness. Liquorice root, with its naturally sweet taste and therapeutic properties, complements the earthy, slightly sweet aroma of fennel seeds. Together, they create a harmonious partnership that extends beyond culinary delights. Known for their digestive properties, this duo aids in soothing gastrointestinal discomfort and promoting healthy digestion. Moreover, liquorice root’s anti-inflammatory and antioxidant properties, combined with fennel seed’s ability to alleviate bloating and gas, make them a dynamic team for digestive wellness. Additionally, both ingredients boast antimicrobial properties, potentially aiding in immune support and fighting off infections. Whether enjoyed in teas, tinctures, or culinary creations, liquorice root and fennel seed offer a holistic approach to health and flavor, promising a brighter future for those seeking natural remedies and culinary delights alike. Tea is one of the most popular drinks around the globe and equally liked by everyone. It is a major part of snack time especially in Asia. It is made by dipping powder in hot water to get an extract. The production and consumption of tea is increasing dramatically. The extract of the liquorice compound glycyrrhizin relieves symptoms of asthma. Both of these compounds have many therapeutic uses because of their phytochemicals profile and it is preferable for people to drink in asthma. There is no source that provides as quick relief as my product does. The goal is to provide patients with a source that provides immediate relief from that issue.
The current study was designed for utilization of liquorice root and fennel seed based herbal teamix for the relief of asthma. For this purpose, after preparation of powder, it was subjected for mineral analysis, total phenolic content, total flavonoid content, antioxidant analysis that include DPPH. The mineral composition of these powders depicted that liquorice root holds (100g) 3.03mg calcium, 37.9mg potassium, 2.12mg magnesium and fennel seed holds (100g) 1200mg calcium, 1700mg potassium, 370mg magnesium The Phytochemical analysis has exhibited that liquorice root powder possesses total phenolic content (850.24mg GAE/100g) and total flavonoid content (279.18mg RE/100g). Antioxidant (DPPH) value was (67%) and antioxidant (31.7g/100g) and fennel seed powder possesses total phenolic content (13mg GAE/100g) and total flavonoid content (0.047mg RE/100g). Antioxidant (DPPH) value was (78%), Afterwards, herbal teamix were made by using the liquorice root and fennel seed powder with different combinations. The developed product was analyzed for mineral, TPC, TFC, antioxidant potential. All the formulations were also analyzed for the color and sensory evaluation. The results of mineral analysis of herbal teamix showed varied range of calcium (118.17 to 489.98mg/100g), potassium (1.94 to 157.31mg/100g) and magnesium (40.45 to 680.99mg/100g). The phytochemical and antioxidant analysis has shown that herbal teamix had a significant effect on TPC, TFC and DPPH. The TPC results illustrated that the highest content of phenolics (853.05mg GAE/g DW) was found in T0 which is 100% liquorice root powder. The minimum phenolics (516.73mg GAE/g DW) were present in T5 which is 60% liquorice root powder and 40% of fennel seed powder. Antioxidant activity ranged from minimum value of 68.46% for T0 maximum value of 72.27% for T5.
The color analysis of functional muffins demonstrated that L values of herbal teamix for all treatment values varied ranged from 26.94 to 11.91 L value. By increasing the liquorice root powder, a decline in a* values (4.07 to 2.05) respectively were noticed for all treatments. The sensory evaluation was performed according to consumer likeness and acceptability. The preferred and most liked herbal teamix formulation was T4 which is 70% liquorice root powder and 30% fennel seed powder due to attractive light red color, aroma, taste, mouthfeel and overall acceptability. There is always a need for the introduction of innovative products in the market whether the food items encouraged or discouraged by consumers. For asthma, teabags can be favorable and use as a quick source to manage asthma symptoms. Herbal teamix with easy packaging can be produced as innovative value-added product for every age individual. It is recommended that herbal teamix are highly nutritious, easy to make and inexpensive.
References
- Habib N, Pasha MA, Tang DD (2022) Current understanding of asthma pathogenesis and biomarkers. Cells 11: 1-17.
- Rodrigues M, Mendes FDC, Delgado L, Padrao P, Paciencia L, et al. (2023) Diet and asthma: A narrative review. Applied Sciences 13: 1-27.
- Murray MT (2020) Glycyrrhiza glabra (liquorice). Journal of Natural Medicine 4: 641-647.
- Dastagir G, Rizvi MA (2016) Glycyrrhiza glabra (Liquorice). Pakistan Journal of Pharmaceutical Sciences 5: 1727-1733.
- Wahab S, Annadurai S, Abullais SS, Das G, Ahmad W, et al. (2021) Glycyrrhiza glabra (Liquorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plant Journal 10: 1-36.
- Schindler C, Heral E, Drinkwate E, Timoshyna A, Muir G, et al. (2022) Wild check assessing risks and opportunities of trade in wild plant ingredients. FAO 7: 83-90.
- Pandey DK, Ayangla NW (2018) Biotechnological aspects of the production of natural sweetener glycyrrhizin from Glycyrrhiza sp. Phytochemistry Reviews 17: 397-430.
- Kwon YJ, Son DH, Chung TH, Lee YJ (2020) A review of the pharmacological efficacy and safety of Liquorice root from corroborative clinical trial findings. Journal of Medicinal Food 23: 12-20.
- Smruti P (2021) A review on natural remedies used for the treatment of respiratory disorders. International Journal of Pharmacology 8: 104-111.
- Rather MA, Dar BA, Sofi SN, Bhat BA, Qurishi MA (2016) Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology and safety. Arabian Journal of Chemistry 9: 1574-1583.
- Dheebisha C, Vishwanath YC (2020) Advances in cultivation of fennel. Journal of Pharmacognosy and Phytochemistry 9: 1295-1300.
- Rahman A, Akbar D, Timilsina S, Trotter T, Thomson M, et al. (2019) Market analysis of fennel seed. Commonwealth Register of Institutions and Courses for Overseas Students 3: 1-15.
- Mehra N, Tamta G, Nand V (2021) A review on nutritional value, phytochemical and pharmacological attributes of Foeniculum vulgare Mill. Journal of Pharmacognosy and Phytochemistry 10:1 255-1263.
- Nowroozinia F, Kargar S, Akhlaghi A, Fard FR, Moghaddam MB, et al. (2022) Feeding fennel (Foeniculum vulgare) seed as a potential appetite stimulant for holstein dairy calves: Effects on growth performance and health. Journal of Dairy Sciences 105: 654-664.
- AOAC (Association of Official Analytical Chemists) (2023) Official Methods of Analysis of AOAC International, 22nd Oxford University Press Oxford UK.
- Naz S, Hanif MA, Bhatti HN, Shahid M (2016) Partition, fractionation, antioxidant potential and phenolics profiling of Cannabis sativa growing in Pakistan. Oxidation Communications 39: 2946-2960.
- Rehman F, Adeel S, Shahid M, Bhatti IA, Nasir F, et al. (2013) Dyeing of γ-irradiated cotton with natural flavonoid dye extracted from irradiated onion shells (Allium cepa) powder. Radiation Physics and Chemistry 92: 71-75.
- Shahid CSA, Hussain AI, Asad R, Majeed M, Aslam N (2014) Bioactive components and antioxidant properties of Terminalia arjuna L. Journal of Food Processing Technology 5: 294-298.
- Naseem Z, Zahid M, Hanif MA, Shahid M (2020) Environmentally friendly extraction of bioactive compounds from Mentha arvensis using deep eutectic solvent as green extraction media. Polish Journal of Environmental Studies 29: 3749-3757.
- Tian M, Yan H, Row KH (2008) Extraction of glycyrrhizic acid and glabridin from liquorice. International journal of molecular sciences 9: 571-577.
- Xu J, Wang M, Zhao J, Wang JH, Tang Q, et al. (2018) Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food research international 107: 567-577.
- Velvizhi S, Annapurani S (2018) Estimation of total flavonoid, phenolic contentand free radical scavenging potential of Glycyrrhiza glabra root extract. Asian Journal of Pharmaceutical and Clinical Research 11: 231-235.
- Alfauomy, GA, Seleem HA, Ali MMA (2020) Evaluation of pies containing liquorice roots (Glycyrrhiza glabra L). Middle East. Journal of Agriculture Research 3: 545-557.
- Quintana SE, Llalla O, Zapateiro LAG, Risco MRG et al. (2020) Preparation and characterization of Liquoricechitosan coatings for postharvest treatment of fresh strawberries. Applied Science 10: 1-23.
- Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP (2018) Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytotherapy research 32: 2323-2339.
- Garcia-Gomez B, Fernandez-Canto N, Vazquez-Oderiz ML, Quiroga-Garcia M, Munoz-Ferreiro N, et al. (2022) Sensory descriptive analysis and hedonic consumer test for Galician type breads. Food Control 134: 1-9.
- Montgomery DC (2019) Design and Analysis of Experiments. 10th Ed John Wiley and Sons Inc Hoboken NJ USA.
- Husain I, Bala K, Khan IA, Khan SI (2021) A review on phytochemicals, pharmacological activities, drug interactions and associated toxicities of Liquorice (Glycyrrhiza sp.). Food Frontiers 2: 449-485.
- Desoky EM, Elrys AS, Rady MM (2019) Liquorice root extract boosts Capsicum annuum production and reduces fruit contamination on heavy metals-contaminated saline soil. International Letters of Natural Sciences 3: s73-79.
- Badr SE, Sakr DM, Mahfouz SA, Abdelfattah MS (2013) Liquorice (Glycyrrhiza glabra ): chemical composition and biological impacts. Research Journal of Pharmaceutical Biological and Chemical Sciences 4: 606-621.
- Hamad G, Elaziz A, Hassan S, Shalaby M, Mohdaly AAAA (2020) Chemical composition, antioxidant, antimicrobial and anticancer activities of Liquorice (Glycyrrhiza glabra ) root and its application in functional yoghurt. Journal of Food and Nutrition Research 8: 707-715.
- Singh S, Singh H (2020) Generic information on fennel to combat cough and cold in COVID19 era. Pharma Innovation Journal 9: 114-116.
- Bukhari H, Shehzad A, Saeed K, Sadiq BM, Tanveer S, et al. (2014) Compositional profiling of fennel seed. Pakistan Journal of Food Science. 24: 132-36.
- Esmaeili H, Mirjalili MH, Zandi F (2022) Antimicrobial and antioxidant activities of the leaf extract of some cultivated Iranian Liquorice populations. Journal of Medicinal Plants 21: 65-74.
- Asan-Ozusaglam M, Karakoca K (2014) Evaluation of biological activity and antioxidant capacity of Turkish Liquorice root extracts. Romanian Biotechnological Letters 19: 8994-9005.
- Çakmak YS, Aktumsek A, Duran A (2012) Studies on antioxidant activity, volatile compound and fatty acid composition of different parts of Glycyrrhiza echinata L Excli Journal 11: 178.
- Pratibha N, Sushma D, Rajinder G (2012) Screening for antioxidant and antibacterial potential of common medicinal plants in the treatment of acne. International Journal of Drug Development and Research 4: 65-71.
- Akbari A, Izadi-Darbandi A, Bahmani K, Farhadpour M, Ebrahimi M, et al. (2023) Assessment of phenolic profile and antioxidant activity in developed breeding populations of fennel (Foeniculum vulgare Mill). Biocatalysis and Agricultural Biotechnology 48: 1-9.
- Sayed-Ahmad B, Straumīte E, Šabovics M, Krūma Z, Merah O, et al. (2017) Effect of addition of fennel (L.) on the quality of protein bread. Proceedings of the Latvian Academy of Sciences Section B. Natural Exact and Applied Sciences 71: 509-514.
- Anayatullah S, Rabail R, Arif S, Hussain S, Abdi G, et al. (2023) Effect of fennel seeds fortified crackers on various obesity biomarkers. Journal of Agriculture and Food Research 14:
- Repajic M, Ekic S, Kruk V, Dragovic-Uzelac V (2020) Effect of accelerated solvent extraction conditions on the isolation of bioactive compounds from fennel (Foeniculum vulgare Mill.) seeds. Hrvatski časopis za prehrambenu tehnologiju, biotehnologiju i nutricionizam 15: 102-106.
- Malin V, Garofulić IE, Repajić M, Zorić Z, Pedisić S, et al. (2022) Phenolic characterization and bioactivity of fennel seed (Foeniculum vulgare Mill.) extracts isolated by microwave-assisted and conventional extraction. Processes 10: 510.
- Bano S, Ahmad N, Sharma AK (2016) Phytochemical investigation and evaluation of anti-microbial and antioxidant activity of Foeniculum vulgare (fennel). International Journal of Pharma Sciences and Research 7: 310-314.
- Hernández-Hernández E, Ponce-Alquicira E, Jaramillo-Flores ME, Legarreta IE (2009) Antioxidant effect rosemary (Rosmarinus officinalis) and oregano (Origanum vulgare L.) extracts on TBARS and color of model raw pork batters. Meat Sciences 8: 410-417.
- Tohma HS, Gulçin I (2010) Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra ). International Journal of Food Properties 13: 657-671.
- Mambro DVM, Fonseca MJ (2005) Assays of physical stability and antioxidant activity of a topical formulation added with different plant extracts. Journal of pharmaceutical and biomedical analysis 37: 287-295.
- Visavadiya NP, Soni B, Dalwadi N (2009) Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. International journal of food sciences and nutrition 60: 135-149.
- Cheel J, Tůmová L, Areche C, Antwerpen PV, Nève J, et al. (2013) Variations in the chemical profile and biological activities of Liquorice (Glycyrrhiza glabra ), as influenced by harvest times. Acta Physiologiae Plantarum 35: 1337-1349.
- Ben Abdesslem S, Boulares M, Elbaz M, Moussa OB, St‐Gelais A, et al. (2021) Chemical composition and biological activities of fennel (Foeniculum vulgare Mill.) essential oils and ethanolic extracts of conventional and organic seeds. Journal of Food Processing and Preservation 45: 1-13.
- Khammassi M, Mighri H, Mansour MB, Amri I, Jamoussi B, et al. (2022) Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) populations growing wild in Tunisia. South African Journal of Botany 148: 407-414.
- Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, et al. (1998) Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2 picrylhydrazyl radical. Biochemical pharmacology 56: 213-22.
- Gabriele B, Fazio A, Carchedi M, Plastina P (2012) In vitro antioxidant activity of extracts of Sybaris liquorice roots from Southern Italy. Natural product research 26: 2176-2181.
- Lateef M, Iqbal L, Fatima N, Siddiqui K, Afza N, et al. (2012) Evaluation of antioxidant and urease inhibition activities of roots of Glycyrrhiza glabra. Pakistan Journal of Pharmacological Sciences 25: 99-102.
- Jo C, Son JH, Shin MG, Byun MW (2003) Irradiation effects on color and functional properties of persimmon (Diospyros kaki L. folium) leaf extract and Liquorice (Glycyrrhiza Uralensis Fischer) root extract during storage. Radiation Physics and Chemistry 67: 143-148.
- Lee JY, Kim CY (2020) Anti browning and antioxidant properties of Foeniculum vulgare seed extracts. Food Science and Preservation 27: 188-196.
- Anwar F, Ali M, Hussain AI, Shahid M (2009) Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare) seeds from Pakistan. Flavour and Fragrance Journal 24: 170-176.
- Usha RM, Meena R (2014) Comparative study on antioxidant potential and phytochemical composition of cumin and fennel. Journal of herbs, spices and medicinal plants 20: 245-255.
- Deyab MA (2015) Egyptian Liquorice extract as a green corrosion inhibitor for copper in hydrochloric acid solution. Journal of Industrial and Engineering Chemistry 22: 384-389.
- Ahn J, Choi W, Kim S, Ha T (2011) Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on Streptozotocin-induced diabetic mice. Food science and biotechnology 20: 251-254.
- Wang YC, Yang YS (2007) Simultaneous quantification of flavonoids and triterpenoids in Liquorice using HPLC. Journal of Chromatography 850: 392-399.
- Hedayati A, Ghoreishi SM (2015) Supercritical carbon dioxide extraction of glycyrrhizic acid from Liquorice plant root using binary entrainer: experimental optimization via response surface methodology. The Journal of Supercritical Fluids 100: 209-217.
- Muhammadiev BT, Gafurov KK, Mirzaeva SU (2017) Supercritical [SC]-CO2 extraction glycyrrhizic acid from Liquorice roots. Butlerov Communications 49: 108-114.
- Sohail M, Rakha A, Butt MS, Asghar M (2018) Investigating the antioxidant potential of Liquorice extracts obtained through different extraction modes. Journal of Food Biochemistry 42: 1-8.
- Montoro P, Maldini M, Russo M, Postorino S, Piacente S, et al. (2011) Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. Journal of pharmaceutical and biomedical analysis 54: 535-544.
- Basar N, Talukdar AD, Nahar L, Stafford A, Kushiev H, et al. (2014) A simple semi‐preparative reversed‐phase HPLC/PDA method for separation and quantification of glycyrrhizin in nine samples of Glycyrrhiza glabra root collected from different geographical origins. Phytochemical analysis 25: 399-404.
- Isbrucker RA, Burdock GA (2006) Risk and safety assessment on the consumption of Liquorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory Toxicology and Pharmacology 46: 167-192.
- Shakir MA, Al-Rawi WAA (2017) Effect of garlic and Liquorice root extract on leaves mineral and hormonal content of pear transplants. Iraqi journal of agricultural science 48: 138.
- Hosseinifarahi M, Yousefi A, Kamyab F, Jowkar MM (2024) Effects of organic amendment with Liquorice (Glycyrrhiza glabra) root residue and humic acid on the vegetative growth, fruit yield and mineral absorption of bell pepper (Capsicum annum). Journal of Plant Nutrition 3: 1-11.
- Younes NA, Rahman MM, Wardany AA, Dawood MF, Mostofa MG, et al. (2021) Antioxidants and bioactive compounds in Liquorice root extract potentially contribute to improving growth, bulb quality and yield of onion (Allium cepa). Molecules 26: 2633.
- Kraouia M, Nartea A, Maoloni A, Osimani A, Garofalo C, et al. (2023) Sea Fennel (Crithmum maritimum) as an emerging crop for the manufacturing of innovative foods and nutraceuticals. Molecules 28: 4741.
- Martins-Noguerol R, Matías L, Pérez-Ramos IM, Moreira X, S. Muñoz-Vallés, et al. (2022) Differences in Nutrient Composition of Sea Fennel (Crithmum maritimum) Grown in Different Habitats and Optimally Controlled Growing Conditions. Journal of Food Composition and Analysis 106: 104266.
- Singh TP, Agrawal RK, Mendiratta SK, Chauhan G (2021) Preparation and characterization of Liquorice root extract infused bio-composite film and their application on storage stability of chhana balls-a Sandesh like product. Food Control 125: 1-7.
- Vlaisavljević S, Šibul F, Sinka I, Zupko I, Ocsovszki I, et al. (2018) Chemical composition, antioxidant and anticancer activity of Liquorice from Fruska Gora locality. Industrial crops and products 112: 217-224.
- Ahmad BS, Talou T, Saad Z, Hijazi A, Cerny M, et al. (2018) Fennel oil and by-products seed characterization and their potential applications. Industrial crops and products 111: 92-98.
- Teixeira A, Baenas NR, Dominguez-Perles A, Barros ER, Moreno DA, et al. (2014) Natural bioactive compounds from winery by-products as health promoters: A review. International journal of molecular sciences 15: 15638-15678.
- Chatterjee S, Goswami N, Bhatnagar P (2012) Estimation of Phenolic Components and in vitro Antioxidant Activity of Fennel (Foeniculum vulgare) and Ajwain (Trachyspermum ammi) seeds. Adv Biores 3: 109-118.
- Faudale M, Viladomat F, Bastida J, Poli F, Codina C (2008) Antioxidant activity and phenolic composition of wild, edible and medicinal fennel from different Mediterranean countries. Journal of agricultural and food chemistry 56: 1912-1920.
- Dong Y, Zhao M, Zhao T, Feng M, Chen H, et al. (2014) Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on H2O2-injured PC12 cells of Glycyrrhiza glabra leaf and root extracts. Molecules 19: 9101-9113.
- He X, Liu D, Liu RH (2008) Sodium borohydride/chloranil-based assay for quantifying total flavonoids. Journal of Agricultural and Food Chemistry 56: 9337-9344.
- Hussain F, Iqbal I (2020) Nutritional and therapeutic potential of Glycyrrhiza glabra roots. Asian Journal of Medical and Biological Research 5: 265-270.
- Basist P, Zahiruddin S, Khan MU, Gautam G, Jan B, et al. (2022) Metabolite profiling and nephroprotective potential of Glycyrrhiza glabra roots against cisplatin-induced nephrotoxicity in vitro and in vivo. Iranian Journal of Basic Medical Sciences 25: 1286.
- Beyazen A, Dessalegn E Mamo W (2017) Phytochemical screening, antioxidant and antimicrobial activities of seeds of Foeniculum vulgare (ensilal). World Journal of Pharmaceutical Sciences 2: 198-208.
- Rozi P, Abuduwaili A, Ma S, Bao X, Xu H, et al. (2020) Isolations, characterizations and bioactivities of polysaccharides from the seeds of three species Glycyrrhiza. International Journal of biological macromolecules 145: 364-371.
- Babich O, Ivanova S, Ulrikh E, Popov A, Larina V, et al. (2022) Study of the Chemical Composition and Biologically Active Properties of Glycyrrhiza glabra Life 12: 1772.
- Amer A, Ghoneim M, Shoala T, Mohamed HI (2021) Comparative studies of eco-friendly compounds like humic acid, salicylicand glycyrrhizic acids and their nanocomposites on French basil (Ocimum basilicum cv. Grand verde). Environmental Science and Pollution Research. 28: 47196-47212.
- Tariq M, Akhtar A, Khalid N (2022) Formulation and evaluation of functional candies fortified with carrot juice and fennel seed extract: nutritional and sensory properties. Pakistan Journal of Agricultural Research 35: 468-476.
- Sultana B, Anwar F, Ashraf M (2009) Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecule 14: 2167-2180.
- Sarabi-Aghdam V, Mousavi M, Hamishehkar H, Kiani H, Emam-Djomeh Z, et al. (2021) Utilization of chickpea protein isolate and Persian gum for microencapsulation of Liquorice root extract towards its incorporation into functional foods. Food Chemistry 362: 1-11.
- Ghasemi S, Jafari SM, Assadpour E, Khomeiri M (2018) Nanoencapsulation of d-limonene within nanocarriers produced by pectin-whey protein complexes. Food Hydrocolloids 77: 152-162.
- Shabani L, Ehsanpour AA, Asghari G, Emami J (2009) Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russian Journal of Plant Physiology 56: 621-626.
- Kausar H, Saeed S, Ahmad MM, Salam A (2012) Studies on the development and storage stability of cucumber-melon functional drink. Journal Agricultural Research 50: 239-248.
- Murtaza MA, Huma N, Javaid J, Shabbir MA, Din DMU, et al. (2004) Studies on stability of strawberry drink stored at different temperatures. International Journal of Agriculture and Biology 6: 58-60.
- Hosseini MS, Samsampour D, Ebrahimi M, Abadia J, Sobhani Najafabadi A, et al. (2020) Evaluation of glycyrrhizin contents in Liquorice (Glycyrrhiza glabra L.) under drought and soil salinity conditions using nutrient concentrations and biochemical traits as biomarkers. Acta Physiologiae Plantarum 42: 1-17.
- Khalmatova BT, Tashmatova GT, Xudoyqulov EA, Shakarova MSQ (2021) Influence of ecological factors on the development and progress of bronchial asthma in children. European Journal of Molecular and Clinical Medicine 7: 4374-4377.
- Kanwal H, Sherazi BA (2017) Herbal medicine: Trend of practice, perspective and limitations in Pakistan. Asian Pacific Journal of Health Sciences 4: 6-8.
- Msomi NZ, Simelane MB (2019) Herbal medicine. InTech: Rijeka, Croatia pp.215-227.
- Sharifi-Rad J, Quispe C, Herrera-Bravo J, Belén LH, Kaur R, et al. (2021) Glycyrrhiza Genus: enlightening phytochemical components for pharmacological and health-promoting abilities. Oxidative medicine and cellular longevity 3: 1-20.
- Shuja S, Owais A, Fatima N, Shan M, Khan M, et al. (2022) The use, phytochemical and antimicrobial activity evaluation of Foeniculum vulgare, Cuminum cyminum and Trachyspermum ammi were reviewed for the treatment of gastric diseases as future compounds. Journal of Pharmacy and Pharmacology 10: 273-282.
- Suraya R, Nagano T, Katsurda M, Sekiya R, Kobayashi K, et al. (2021) Molecular mechanism of asthma and its molecular novel target therapeutic agent. Respiratory Investigation 105: 654-664.
- Zaigham M, Ali Ma, Ikram M (2019) Aslussoos (Glycyrrhiza glabra Linn): A root with immense pharmaceutical potential and its utilization in unani system of medicine. International Journal of Herbal Medicine 7: 1-9.
- Zeeshan A, Akram H, Qasim S, Naseer A, Nazar F, et al. (2023) The Healing Touch of Foeniculum vulgare (Fennel): A Review on Its Medicinal Value and Health Benefits. Journal of Health and Rehabilitation Research 3: 793-800.






























