Abstract
This study aimed to enhance the value of mango, a highly perishable fruit from Northern Côte d’Ivoire. To do this, the physicochemical parameters of the fruit, including pH, moisture content, alcohol content, vitamin C, and Brix degree have been determined according to standard methods. The microbiological analyses first focused on isolating and identifying the yeasts in the mango pulp, followed by assessing their fermentative capacity. Finally, the isolates that showed a strong fermentative ability were subjected to the influence of glucose, ethanol, and NaCl. The results of the physicochemical analyses revealed that the fruit possesses an acidic pH, high moisture content, a vitamin C content of 36.35 mg/100g, an alcohol content of 8%, and a high Brix degree of 14. The microbiological analyses led to the obtaining of twenty-one pure yeast isolates. Among these isolates, seven (7) showed a strong fermentative capacity, with a volume of CO₂ greater than 4 cm³. These seven yeast isolates showed good growth under the impact of glucose, ethanol, and NaCl. Therefore, these isolates could be used as potential starters in biotechnological applications to standardise and control certain fermentation processes.
Keywords:Characterization; Selection; Starters; Yeasts; Mango
Introductıon
The mango (Mangifera indica) is a climacteric fruit with high nutritional and economic potential. Its cultivation is suitable for different agro-ecological zones ranging from sub-humid areas to semi-arid zones [1]. Its production accounts for 50% of tropical fruit production [2]. It is native to the Indo-Burmese region, ranking fifth in global fruit production after citrus, grapes, bananas, and apples [3]. Also, the second most important fruit in terms of area and production in the sequence of the following tropical fruits: banana (37.6%), mango (19.6%), pineapple (12.1%), avocado (9.8%), papaya (5.4%), oranges (4.6%), watermelon (4.2%) and passion fruit (3.7%) [4]. It is also appreciated for its sweet taste and its vitamin richness, especially in vitamins A and C and in minerals such as calcium, potassium, phosphorus, and iron [5]. Mango is one of the most produced and exported fruits in the world [6]. The estimated annual global mango production is about 55,026,131 tons and 54,831,104 tons in 2019 and 2020, respectively [7]. Asia is the leading mango production basin globally, accounting for 70.5% of the total in 2019 [6]. Thus, India is the largest mango producer with over 25 million tons produced in 2019, followed far behind by Indonesia with 3.3 million tons. The other two production areas are mostly located on the American continent (mainly Latin America) and in Africa (mainly sub-Saharan Africa). West Africa is ranked the 7th largest producer in the world with a mango production of about 1.5 million tons per year, which represents 3.8% of global production [8]. There are over 1,000 varieties of mango worldwide. Some are considered fleshy varieties (Early, Gold, Keith, Kent, Palmer, Smith, and Springfield) and others as juicy varieties (Julie, Zill) [7]. Ivory Coast is covered by a significant orchard in the North and South with a production estimated at 150,000 tons per year [6]. Thus, more than 40 varieties of mangoes are cultivated in the country. These varieties consist of local and improved varieties. Ivory Coast is the leading African country and the third-largest supplier of mangoes in the European market after Brazil and Peru, with total exports ranging from 10,000 to 14,000 tons [9]. Ivorian exports mainly concern mangoes of the varieties Kent, Keitt, Brooks, and Amélie [9]. Thanks to its production of Kent variety mangoes and its coastal facade, Côte d’Ivoire reports a total export of 10,000 to 14,000 tons [10] and 32,000 tons in 2020 according to Intermangue-Côte d’Ivoire (Ivorian mango producers organization) (2020). The mango trade generates more than 7 billion FCFA (10,670,732 Euros) in revenue and provides producers with about 1 billion FCFA (1,524,390 Euros) in annual income [10]. Mangoes destined for export are primarily produced in the northern half of Côte d’Ivoire [11]. The annual production is estimated at 100,000 tons. However, only 10% of national production is exported to Europe [11]. Moreover, production faces numerous problems including post-harvest spoilage caused by the resurgence of pathogens [12]. Every year, tens of tons of mangoes rot in the orchards. Post-harvest losses are indeed estimated at 30 to 35% of total production, amounting to approximately 3.3 billion FCFA. Several causes are thought to be behind these losses [12]. The major factors that make mango a perishable fruit are transpiration, mechanical injuries, pathological degradation, high respiration, and ethylene production [9]. Furthermore, fungal alterations are one of the main constraints on the quality of fresh fruit in Côte d’Ivoire [9]. Mango contamination by fungal insects can occur in the field or during post-harvest packaging operations, in storage, and sometimes after purchase by the consumer [9].
Post-harvest losses exceed one third of production; therefore, processing would be an alternative for adding value to mangoes. However, to this day, mango processing remains a marginal activity, using less than 2 to 5% of the harvest. Indeed, mangoes are processed into dried mango, juice/nectar, vinegar, jams, etc. This processing is done in artisanal plants, semi-industrial plants, and very few industrialized ones [13]. Despite these efforts, postharvest losses are numerous. Therefore, it is important to find solutions to value the mangoes spoiled from the production, distribution, and processing circuit.
Several strategies are being sought for the valorisation of mango residues. This context prompted this study within a dynamic of characterization and selection of yeast starters from mango residues.
Materials and Methods
Biological material
The biological material used in this study consisted of a variety of mango: Kent (Figure 1). This variety of mango came from a dried mango production plant in Korhogo. These were rotten, defective, or discarded mangoes that were excluded from the processing chain. These mangoes were sent to the biochemistry laboratory of Peleforo GON COULIBALY University (UPGC) for physicochemical and microbiological analyses.

Methods
Determination of physicochemical parameters
Moisture and dry matter content
The method used to determine the moisture and dry matter content is the one described by [14]. It is based on the dehydration by drying in an oven of the samples until a constant weight is obtained. Five (5) grams of mango pulp are weighed in a known mass glass capsule (m0). The capsule containing the sample (total mass m1) is placed in the oven set at 105°C for 24 hours and then placed in a desiccator to cool. The entire assembly (sample + capsule) is weighed (m2) after cooling in the desiccator.
The moisture content (H) expressed as a percentage of the mass of wet samples is determined by the following relationship:

The dry matter (DM) content expressed as a percentage of the mass of the wet sample is determined by the following relationship:

pH
The pH is determined through the use of a pH meter that measures the electromotive force, thanks to its electrode sensitive to hydrogen ions (H+). This determination is carried out according to the AOAC [14] method. A mass of ten (10) grams of fresh mango pulp is ground and homogenized in 100 ml of distilled water, and the glass electrode of the pH meter (HANNA) is immersed in the filtrate. The pH value is displayed on the pH meter screen, which has been previously calibrated.
Titratable acidity
The titratable acidity is determined according to the method suggested by AOAC [14]. The principle of this method involves measuring the titratable acidity of a product with a titrated sodium hydroxide (NaOH) solution in the presence of phenolphthalein used as a colour indicator. Ten (10) grams (me) of fresh mango pulp are crushed and homogenized in 100 ml of distilled water. A volume (Vo) of 10 ml of the filtrate recovered in a flask is then added with three (03) drops of phenolphthalein. A NaOH solution (0.1N) (V1) contained in a burette is then added drop by drop to the homogenized mixture until a pink colour appears. The titratable acidity is expressed in milliequivalents (meq) per 100 g of fresh material by the following relationship:

N: NaOH concentration
V1: NaOH volume solution
me: mass of the sample
V0: volume of the sample
Soluble dry extract and ethanol content
The soluble dry extract expressed in degrees Brix was measured with a digital refractometer from the brand ATC according to the manufacturer’s recommendations. Ten (10) grams of mango pulp were crushed in the extractor. The mash allowed for obtaining the juice of the mango. A drop of this juice was placed on the prism plate of the refractometer. The values are read directly.
Vitamin C content
The method used to determine the vitamin C content was the one described by Pelletier [15]. The principle of this method consists of stabilizing vitamin C with metaphosphoric acid/acetic acid 2, then oxidizing it with 2,6-dichlorophenol indophenol which is reduced. A mass of 10 g of cashew apple puree (me) was solubilized in 40 mL of metaphosphoric acid/acetic acid (2%; w/v). The mixture was centrifuged at 3000 rpm for 20 minutes. The supernatant is collected in a 50 mL flask and filled up to the mark with distilled boiled and cooled water in an air-tight manner. A volume of 10 mL from the flask’s content was taken and introduced into an Erlenmeyer (sample). The sample is titrated with a solution of 2,6-DCPIP (2,6-dichlorophenol indophenol) at 0.5 g/L, until a persistent pink colour is obtained for 30 seconds. The 2,6-DCPIP solution is previously standardized with a solution of vitamin C at 0.5 g/L. Let V (mL) be the volume of 2,6-DCPIP added at the equivalence. The vitamin C content as a percentage of the mass of the fresh sample is determined by the following relationship:

Vo: volume (mL) of 2.6-DCPIP solution used for the blank
Ve: volume (mL) of solution used for the calibration of the 2.6
DCPIP solution.
Vc: volume (mL) of 2,6-DCPIP solution used for the test sample
Isolation and identification of yeasts
The mango pulps (25 g) were diluted in 225 ml of saline peptone solution (0.1% (w/v) bactopeptone and 0.85% (w/v) NaCl). The solution thus prepared constituted the source solution which underwent successive decimal dilutions (10-1 to 10-4) with a tryptone salt solution. A volume of 100 μL of each dilution was inoculated by spreading on MYGP agar (3 g/L yeast extract, 3 g/L malt extract, 5 g/L bactopeptone, and 10 g/L glucose) containing 100 mg/L of chloramphenicol. After seeding, the Petri dishes were incubated and the yeast strains were morphologically identified after 3 days of incubation at 30 °C, then the yeast cells were observed fresh under a precision optical microscope (Zeiss Microlmaging GmbH 37081, Germany) with a ˟100 objective. The presumptive yeast isolates were preserved in cryotubes containing MYGP broth supplemented with 20% glycerol at -20 °C for further testing [16].
Technological properties of yeast isolates from mango
Study of the strong fermentative power of yeasts
The fermentative capacity of yeast strains isolated from the pulp was studied according to the method of [17] with slight modifications. A 24-hour pre-culture (100 μL) with an optical density of 0.7 at 600 nm was inoculated in a test tube containing 10 mL of YPG medium and a hemolysis tube (replacing the Durham tube). The culture was incubated at 30 °C for 6 days, without agitation. The fermentative capacity was determined by measuring gas production in the hemolysis tube. Indeed, under anaerobic conditions, yeasts oxidize sugars into ethanol with the production of CO2 [18]. The volume of CO2 correlated with the fermentative power of the strain is related to the ethanol produced [19].
Influence of glucose, ethanol, and NaCl concentration on the growth of yeast isolates
The influence of glucose and ethanol on the growth of yeast isolates was evaluated using a liquid medium containing 0.05% yeast extract; 0.3% casein peptone with different concentrations of sugar. Glucose was added at 10%, 20%, 30%, and 40%. As for ethanol, it was added at the following concentrations: 0 %, 5 %, 8 %, 10 %, 12 %, 14 %, 16 %, 18 %, 20 % et 22 %. Finally, the NaCl was added to 2,5 %, 5 %, 10 % et 15 %. Ten (10) mL of the liquid medium contained in a test tube was inoculated with 100 μL of yeast pre-culture, OD600 = 0.7. After inoculation, the medium was incubated at 30 °C for 3 days. The growth of yeast isolates was determined by measuring the turbidity of the culture medium at 600 nm using a spectrophotometer [20].
Statistical data analyses
Statistical analyses of the data were performed using Statistica version 7.1 software. The comparison of means was carried out using Tukey’s HSD test with a significance threshold of 5% (p < 0.050).
Results and Discussion
Results
Physicochemical Parameters
The results of the physicochemical parameters of the Kent variety mango are recorded in (Table 1). The analysis of this table indicates that the studied Kent mango variety had a very acidic pH of 3.74 ± 0.03 with a titratable acidity value of 5.07 ± 0.12%. Furthermore, the obtained value for dry matter is low (15.80 ± 0.41%) while the moisture content is high with a value of 84.19 ± 0.41%. The average values obtained for alcohol content and soluble dry extract are respectively 8 ± 0% and 14 ± 0 °B. Finally, the vitamin C content of the mango is high at 36.35 ± 5.87 mg/100g.

Isolation and purification of yeasts from the Kent variety mango
In the MYGP medium, twenty-one (21) presumptive yeast isolates were obtained from mango pulp. The twenty-one (21) isolates, after being re-cultured on fresh medium, yielded pure isolates that were coded so that the first letter corresponds to the initial of the yeast name followed by the initial of the fruit name and finally the number assigned to the strain (YK1 to YK21).
Fermentative capacity of yeasts obtained from mango
Twenty-one (21) yeast isolates were tested for their ability to produce CO2. Based on their fermentation capacity, these twenty-one (21) yeast isolates were classified into three (3) groups according to the volume of CO2 produced (Table 2). The volumes ranged from 0 to 7.5 cm3. Among the twenty-one (21) yeast isolates analyzed for their fermentation capacity, seven (07), or 33.33%, showed a high fermentation capacity. These isolates produced a volume of CO2 greater than 4 cm3. Also, eight (08) isolates, or 38.09%, were considered moderately fermentative due to their CO2 production ranging between 1 and 4 cm3. Finally, six (06) isolates, or 28.57% of the remaining isolates, were classified as weakly fermentative because of their CO2 production of less than 1 cm3.
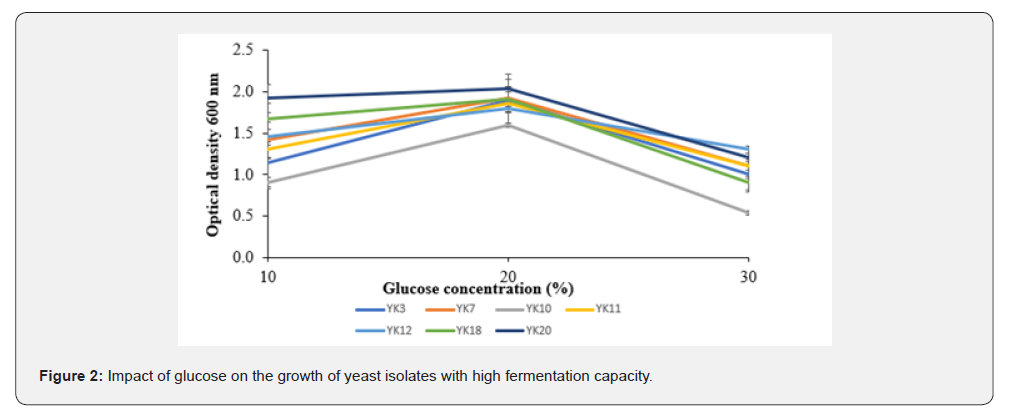
Effect of glucose on the growth of yeast isolates with high fermentation capacity
The impact of glucose on the growth of yeast isolates with high fermentation capacity is presented in (Figure 2). The analysis of variance showed a significant difference (p<0.05) in the growth levels of the selected yeast isolates at different glucose concentrations. The growth of the yeast isolates varies depending on the concentration of glucose. Indeed, from 10 to 20% of glucose, we notice an increase in growth for all isolates, reaching their peak at 20% of glucose. The isolate YK20 achieved the best growth (OD = 2.0), while the isolate YK10 had the lowest growth with an OD = 1.6. Thus, from 20 to 30% glucose there is a decrease in growth for all yeast isolates and the isolate YK13 showed the best growth with an OD = 1.3. Meanwhile, the isolate YK10 recorded the lowest growth with an OD = 0.5.

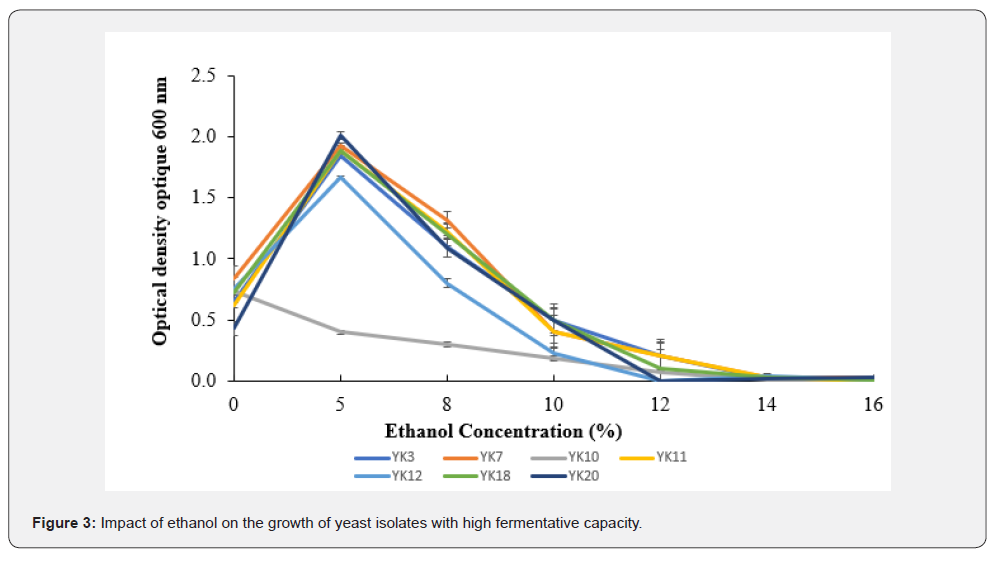
Impact of ethanol on the growth of yeast isolates with high fermentative capacity
Figure 3 shows the impact of ethanol on the growth of yeast isolates with high fermentative capacity. The analysis of variance indicated a significant difference (p<0.05) for all selected yeast isolates at concentrations of 0%, 5%, 10%, and 12% of ethanol, while no significant difference (p>0.05) was observed for all yeast isolates at ethanol concentrations of 8%, 14%, and 16%. For all isolates, three phases (an increasing phase, a decreasing phase, and a constant phase) were observed except for isolate YK10, for which only two phases were observed (a decreasing phase and a constant phase). During the first phase (from 0 to 5%), the growth of isolates YK3, YK7, YK11, YK12, YK18, and YK20 was rampant, reaching their peaks at 5% ethanol. The highest value (OD = 2.0) was obtained with isolate YK20, while the lowest value (OD = 1.7) was obtained for isolate YK12. For the second phase (from 5 to 12 for isolates YK12 and YK20, and from 5 to 14 for isolates YK3, YK7, YK11, and YK18) a gradual decrease was observed. And in the third phase (from 12 to 16% for isolates YK12 and YK20, and from 14 to 16% for isolates YK3, YK7, YK11, and YK18) the evolution occurred consistently. In contrast, in the YK10 isolate from 0 to 14% ethanol (first phase), a gradual decrease in growth was observed, and from 14 to 16% ethanol (second phase), growth remains stable.
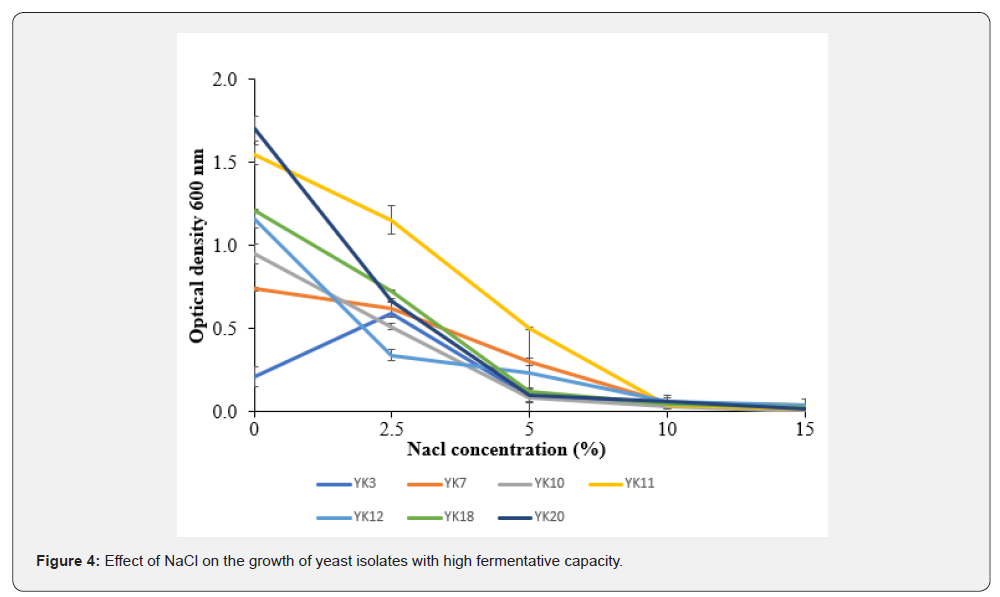
Impact of NaCl on the growth of yeast isolates with high fermentation capacity
Figure 4 shows the influence of NaCl on the growth of yeast isolates with high fermentative capacity. The analysis of variance indicated a significant difference (p<0.05) in the growth of selected yeast isolates at NaCl concentrations of 0%, 2.5%, and 5%, while no significant difference was observed at concentrations beyond 5% (10%, 15%) of NaCl. The growth of the isolates varies according to the concentration of NaCl. From 0 to 10% NaCl, a gradual decrease was noted for all isolates except for isolate YK3, which experienced a growth phase from 0 to 2.5% before reaching its peak (OD = 0.6). From 2.5% to 10% NaCl concentration, a progressive decline in growth was observed. A stabilization (DO = 0.0) of the growth of all isolates was observed at a concentration from 10 to 15% NaCl.
Discussion
The physicochemical parameters were determined in order to assess the activity of the fermentative microflora of mango in general and specifically the yeasts initiating this process. In fact, this study made it possible to determine the physicochemical parameters of the Kent variety mango. These parameters consisted of dry matter, moisture content, pH, alcohol content, sugar content, titratable acidity, and vitamin C. It results in an acidic pH with an average of 3.74 ± 0.03. The pH value obtained in this study is consistent with those found by Elsheshetawy et al. [21] in Egyptian varieties, which had a pH ranging from 3.6 to 5.1. Similarly, Monrose [22] states that the pH of fruits is naturally acidic and ranges between 2 and 6. Indeed, the pH is related to the total acidity of the pulp, due to the presence of organic acids. The level of organic acid may increase due to incomplete oxidation of glucose. Moreover, a high proportion of organic acid can lead to an acidic pH. These acidic pH levels provide an advantage for the development of yeasts and molds over other microorganisms. The average titratable acidity obtained in this study is 5.07 ± 0.12%. This result confirms the statements of Sajib et al. [23] and Elsheshetawy et al. [21] who reported that the titratable acidity in mango is due to the presence of organic acids. The observed value of the soluble dry extract of the Kent variety was 14 ± 00 °B. This result is consistent with those found by Koné et al. [24] for the black plum (Vitex doniana) from Côte d’Ivoire, which remains a criterion for assessing the sweet taste of fruits and promotes yeast development. Indeed, the low rate of soluble dry extract would be due to the hydrolysis of starch during the transformation of fermentable sugars into alcohol by yeast. The obtained humidity rate was 84.19 ± 0.41 %. This high humidity rate in this variety of mango is favourable for the proliferation of microorganisms. These results are similar to those of Liu et al. [25], who found that most fleshy fruits have a relatively high moisture content that can reach 76.19 ± 0.29 % in the Poro region. This high moisture activity could limit the storage duration of these fruits, making them perishable goods. However, the obtained alcohol content is 8 ± 00%. This result is similar to that of Liu et al. [25], who reported that the sugars present in mangoes are transformed by the microorganisms present in the same mango. The pulp of the mango showed an average of 36.35 ± 5.87 mg/100g of vitamin C, this value is similar to that of Ma et al. [26] who reported vitamin C contents ranging between 19.79 ± 3.71 and 34.59 ± 4.41 mg/100 g of fresh pulp in 8 varieties of Chinese mangoes. Indeed, various studies have demonstrated that the vitamin C content decreases during ripening and post-harvest treatment in fruits. Moreover, the production of CO2 varies from one isolate to another. Thus, among the 21 yeast strains tested for their fermentative capacity, seven (07) showed a strong fermentative capacity with a CO2 volume greater than 4 cm3. These results are identical to those of Koffi et al. [27], which showed that among 743 strains, 113 yeast strains were selected for their high fermentative capacity with a CO2 volume greater than 4 cm3. These yeast strains are likely to produce large amounts of ethanol because during alcoholic fermentation, the amount of carbon dioxide (CO2) would correspond to the amount of ethanol produced Dung et al. [19].
Regarding the effect of glucose on the growth of yeast isolates, achieving peak growth with 20% glucose is identical to the results of Soumahoro et al. [28] who obtained the same results. However, Soumahoro et al. [28] and Koffi et al. [27], respectively isolated yeast strains capable of having their best growths with 30% and 50% glucose, while those characterized by Soumahoro et al. [29] had their maximum growth at only 10% glucose. The sensitivity of yeasts to increasing concentrations of NaCl (0 to 10%) shown by a continuous decrease in microbial growths was also observed by Soumahoro et al. [28-29] and Logothetis et al. [30]. The yeast strains isolated by these authors had also observed this same decrease at these concentrations. The 5% concentration of alcohol that gave a microbial growth peak for most strains was the same observed by Soumahoro et al. [28]. Soumahoro et al. [29] obtained similar results with better growths at 5 and 8% alcohol. However, Soumahoro et al. [28] showed that certain yeast strains could be tolerant to 14% alcohol. This type of alcohol resistance was also found in certain yeasts such as those isolated by Hawaz et al. [29] and Tadesse et al. [30]. Priyanka et al. [31] attested that these are responses of yeast towards various environmental stresses.
Conclusion
The objective of this study was to contribute to the valorisation of mango waste from the Kent variety. The physicochemical analyses performed on the pulp showed that it is favourable for the growth and development of yeasts. Thus, 21 yeast strains were isolated and purified. Among these isolated strains, seven (7) exhibited a strong fermentative capacity with CO2 volumes exceeding 4 cm3. These seven isolates also showed good resistance when subjected to the influence of various fermentative parameters and could be used as starters in biotechnological applications. However, further studies should be conducted on the molecular identification of the yeast isolates in order to understand the species isolated from the Kent variety mango.
References
- Grant W, Kadondi E, Mbaka M, Ochieng S (2015) Opportunities for financing the mango value chain: A case study of lower eastern Kenya. FSD Kenya (Nairobi, Kenya) pp.52.
- Jedele S, Hau AM, Von Oppen M (2003) An analysis of the world market for mangos and its importance for developing countries. Deutscher Tropentag 2003 Gö Conference on International Agricultural Research for Development.
- FAO (2020) Major Tropical Fruits: Market Review February 2020 24.
- USAID-Kaves (2014) USAID Kenya horticulture competitiveness project (USAID-KHCP). Final Year Annual Report pp.61.
- Maldonado-Celis ME, Yahia EM, Bedoya R, Landázuri P, Loango N, et al. (2019) Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front Plant Sci 10 : 1073.
- FAO (2021) FAOSTAT. Yeasts characteristic and identification. 2nd Edition Cambridge University Press pp. 1-2.
- FAOSTAT (2022).
- Trade H (2017) Symposium sur la Mangue : accroître les exportations et la compétitivité de la mangue fraîche transformée en Côte d’Ivoire. 6-7 avril 2017 Hôtel Olympe Korhogo, Côte d’Ivoire pp.28.
- Kouamé LM, Kouamé AK, Ouattara L, Kouadio NF, Mireille W, et al. (2020) Contraintes liées à la production et à la commercialisation des mangues (Mangifera indica) en Côte d’Ivoire : cas des variétés exportées vers l’Europe pp.27.
- Kouassi AO (2012) Revue Nationale pour identifier les initiatives de valorisation non alimentaire de la mangue en Côte d’Ivoire. Revue Nationale No12/Coleacp Paepard-01/BNA-12 pp.43.
- Firca (2014) Présentation Des Filières Fruitiè La Filière Du Progrès N°13 Du 1er Trimestre Pp. 4 -5.
- Yaouba A, Mpounze EGP (2017) Isolation and pathogenicity evaluation of postharvest fungal of some fruits in Cameroun. International Journal of Environment Agriculture and Biotechnology (IJEAB) pp. 256-260.
- Kanté TH (2019) Valorisation des variétés de mangue produites au Burkina Faso : aspects biochimiques, biotechnologiques et nutritionnels pp.72.
- AOAC (1990) Official methods of analysis. Association of Official Analytical Chemists Ed Washington DC pp.684.
- Pelletier O (1985) Vitamin C, (L-Ascorbic and Dehydro-L-Ascorbic Acids), Methods of Vitamin Assa (Wiley, New york) pp. 303-347.
- Samagaci L, Ouattara HG, Goualié BG, Niamké SL (2014) Growth capacity of yeasts potential starter strains under cocoa fermentation stress conditions in Ivory Coast. Emirates journal of food and agriculture 26(10): 861-870.
- Dung NTP, Phong XH (2013) Screening thermo- and ethanol tolerant bacteria for ethanol fermentation. American Journal of Microbiological Research 1(2): 25-31.
- Hesclot H, Vladescu B (1994) La levure dans les industries alimentaires Ed Tec & Doc Lavoisier p. 56.
- Dung NTP, Pornthap T, Huynh XP (2012) Screening useful isolated yeasts for ethanol fermentation at high temperature. International Journal of Applied Science & Technology 4: 65-71.
- Soumahoro S, Kouame ML, Yao WK, Toure A, Soro YR (2024) Cashew apples: Physico-chemical analysis and occurrence of yeasts in plantations north of Côte d'Ivoire for processing into bioethanol and fermented beverages. GSC Biological and Pharmaceutical Sciences 29(1): 124-133.
- Elsheshetawy HE, Mossad A, Elhelew WK, Farina V (2016) Comparative study on the quality characteristics of some Egyptian mango cultivars used for food processing. Annals of Agricultural Science 61(1): 49-56.
- Monrose G (2009) Standardisation d’une formulation de confiture de chadèque et évaluation des physico-chimiques, microbiologiques et sensoriels. Mémoire de Master, Université d’Etat d’Haïti, Port-au-Prince (Haïti) pp.60.
- Sajib MAM, Jahan S, Islam MZ, Khan TA, Saha BK (2014) Nutritional evaluation and heavy metals content of selected tropical fruits in Bangladesh. International Food Research Journal 21(2): 609-615.
- Kone KY, Kone HS, Soro D, Akaki KD, Elleingang FE, et al. (2018) Caractérisation Biochimique des fruits du Prumier noir (Vitex Doniana) de la cote d’ivoire. European Scientific journal 14(3) :252-270.
- Liu FX, Fu SF, Bi XF, Chen F, Liao XJ, et al. (2013) Physicochemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chemistry 138: 396-405.
- Ma C, Sun Z, Chen C, Zhang L, Zhu S (2014) Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits 3by HPLC-ELSD. Food Chemistry 145: 784-788.
- Koffi O, Samagaci L, Goualie B, Niamke S (2018) Screening of potential yeast starters with high ethanol production for a small-scale cocoa fermentation in Ivory Coast. Food and Environment Safety 7(2): 113 -130.
- Soumahoro S, Kouame ML, Yao WK, Toure A, Soro YR (2024) Physico-Chemical Analysis and Isolation of Yeasts from Wild Fruits Cola Cordifolia in the North of Côte d’Ivoire: Selection of Potential Starters. Journal of Advances in Biology & Biotechnology 27(10): 104-12.
- Soumahoro S, Kouame ML, Yao WK, Coulibaly GW, Toure A, et al. (2025) Fermentative Potential of Yeasts Isolated from the Fruits of the African Ebony Tree (Diospyros Mespiliformis) In Côte d'Ivoire for Bioethanol Production. Scholar Academic Journal of Bioscience 13(3): 340-348.
- Logothetis S, Nerantzis ET, Gioulioti A, Kanelis T, Panagiotis T, et al. (2010) Influence of sodium chloride on wine yeast fermentation performance. International Journal of Wine Research 2: 35-42.
- Priyanka S, Arun B, Anusha K, Shilpa V (2018) Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Science Direct 72: 1-12.






























