Abstract
The regulatory effect of new synthetic azaheterocyclic compounds, furopyrimidine derivatives on the vegetative growth and adaptation of maize (Zea mays L.) variety Mas 24.C to abiotic stresses such as heat and drought was studied. A comparative analysis of the regulatory effect of these synthetic azaheterocyclic compounds at a concentration of 10-6M with the regulatory effect of phytohormones: auxin IAA (1H-indol-3-yl)acetic acid) and cytokinin Kinetin (N-(2-Furylmethyl)-7H-purin-6-amine), as well as known synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), sodium and potassium salts of methyl-2-mercapto-4-hydroxypyrimidine (Methyur and Kamethur) at a similar concentration on maize growth and adaptation to heat and drought stresses was also carried out. After 3 weeks of maize plant growth, morphological parameters such as average shoot length (mm), average root length (mm), average number of the roots (pcs), average biomass of 10 plants (g), as well as biochemical parameters such as chlorophyll and carotenoid content (mg/g fresh weight) and total soluble protein content (g/100 g FW) of maize plants were measured. The most active synthetic azaheterocyclic compounds, furopyrimidine derivatives that showed auxin- and cytokinin-like regulatory effects on improving maize growth and its adaptation to heat and drought stresses, were selected and their chemical structures were analyzed. The practical application of the most physiologically active synthetic azaheterocyclic compounds, furopyrimidine derivatives, for regulating the vegetative growth of maize and its adaptation to heat and drought stresses is proposed.
Keywords:Zea mays L; Auxin IAA; Cytokinin kinetin; Ivin; Methyur;Kamethur; furopyrimidine derivatives; heat and drought stresses
Introductıon
Maize (Zea mays L.) is one of the most important grain and oilseed crops used in human nutrition due to its high content of nutrients such as proteins, lipids, carbohydrates, dietary fiber, vitamins A and E from grains, as well as a raw material for the biofuel industry and the production of feed for livestock and poultry [1]. Maize can play a role in carbon sequestration by capturing atmospheric carbon dioxide and reducing greenhouse gas emissions, biomass production, and soil conservation in crop rotations [2-5].
Currently, maize production tends to decline due to abiotic stress factors such as rising global average air temperature, falling relative humidity, water deficit, uneven rainfall distribution, increased solar radiation, as well as soil salinization and depletion [6]. Among the abiotic stresses, heat and drought are the most negative factors that reduce plant productivity by disrupting the processes of respiration, photosynthesis and metabolism, reducing the uptake of nutrients from the soil through the root system, declining chlorophyll content and water accumulation in plant leaves, and reducing the reproductive capacity of plants [7-11]. According to FAO and a recent comprehensive study conducted in framework of Inter-Sectoral Impact Model Intercomparison Project (ISI-MIP) and Agricultural Model Intercomparison and Improvement Project (AgMIP) on the impacts of world climate change on agriculture, if current climate change continues, maize yields will decline sharply by 20–45% by 2100 [12].
As is known, the root system plays a key role in the growth and development of above-ground plant organs: shoots, leaves, fruits and seeds in the vegetative and reproductive phases, providing plants with water, micro- and macroelements, as well as organic matter from the soil, and enhances plant adaptation to abiotic stress [13-14]. Phytohormones auxins and cytokinins play a central role in the formation, organization, and maintenance of root and shoot meristems, starting from embryogenesis and continuing with postembryonic development [15-19]. Since homeostasis and metabolism of endogenous auxins and cytokinins change in different phases of growth and development, as well as under the influence of stress factors [20-22], synthetic analogues of these phytohormones, capable of exerting a direct regulatory effect on plant growth or manipulating the biosynthesis and metabolism of endogenous phytohormones, are widely used for exogenous treatment of agricultural crops in order to improve their growth and increase resistance to stress factors [23-30]. Currently, maize cultivation is also based on the use of phytohormones auxins and cytokinins to improve plant growth and enhance photosynthesis in leaves, increase plant productivity and its resistance to biotic and abiotic stresses, among which heat and drought are the most negative stress factors for maize yields [31-35].
Today, the development of new environmentally friendly plant growth regulators based on synthetic low-molecular-weight azaheterocyclic compounds, pyridine and pyrimidine derivatives capable of exerting a regulatory effect on plant growth similar to the phytohormones auxins and cytokinins is a very promising task for modern agriculture [36-41]. Among these classes of synthetic compounds, the most attractive as plant growth regulators are the well-known synthetic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), sodium and potassium salts of 6-methyl- 2-mercapto-4-hydroxypyrimidine (Methyur, Kamethur), as well as new pyrimidine derivatives that exhibit physiological activity at low non-toxic concentrations and are capable of reducing the toxic effects of pesticides, which is of great importance for the environmental ecology and human health [40, 42-52]. The main advantage of using pyridine and pyrimidine derivatives in agriculture is their regulatory effect, similar to the phytohormones auxins and cytokinins, on agricultural crops of various species and varieties at the vegetation stage, starting with seed germination, subsequent formation and growth of roots, shoots, leaves, and reproductive organs of plants, as well as their effect on increasing the adaptation of plants to abiotic stress factors [42-62].This is why the development of new maize plant growth regulators based on pyridine and pyrimidine derivatives has the greatest potential for modern agriculture [42-44].
The main objective of this work is to study the regulatory effect of new synthetic low-molecular-weight azaheterocyclic compounds, furopyrimidine derivatives, in comparison with the regulatory effect of the phytohormones auxin IAA and cytokinin Kinetin, or known synthetic azaheterocyclic compounds such as Ivin, Methyur and Kamethur, on the vegetative growth of maize (Z. mays L.) variety Mas 24.C and its adaptation to heat and drought.
Materials and Methods
Chemical structures of phytohormones and synthetic compounds
Phytohormones auxin IAA and cytokinin Kinetin were produced by Sigma-Aldrich, USA. Synthetic low-molecular-weight azaheterocyclic compounds: Ivin, Methyur, Kamethur, as well as furopyrimidine derivatives (compounds № 1-12) were synthesized at the Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine.
Chemical structures of phytohormones: auxin IAA (1H-indol- 3-yl) acetic acid) and cytokinin Kinetin (N-(2-Furylmethyl)- 7H-purin-6-amine), and synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur, Kamethur), as well as furopyrimidine derivatives (compounds № 1 – 12) are described in (Table 1).

Seed treatment and plant growing conditions
The seeds of maize (Z. mays L.) variety Mas 24.C were sterilized with 1 % KMnO4 solution for 10-15 min., then treated with 96 % ethanol solution for 1 min, after which they were washed three times with sterile distilled water. The sterilized seeds were then placed in the plastic cuvettes each containing 15-20 seeds on the perlite moistened with solutions of phytohormones: auxin IAA (1H-indol-3-yl)acetic acid) or cytokinin Kinetin (N-(2-Furylmethyl)- 7H-purin-6-amine), or synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), or sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur, Kamethur), or furopyrimidine derivatives (compounds № 1 – 12) at a concentration of 10-6M.
Seed germination was carried out in a thermostat in the dark at a temperature of 20-22 °C for 48 hours. Seedling cultivation was carried out in a climate chamber, in which the plants were grown for 3 weeks under a light intensity of 3000 lux, a light/dark regime of 16/8 h., and under conditions of abiotic stresses: heat (at an increased temperature to 35 °C) and drought (with reduced watering by 50%). Control maize plants were germinated from seeds moistened with distilled water and grown under similar conditions of abiotic stress factors: heat and drought.
Comparative analysis of average plant growth parameters (length of the shoots (mm), length of the roots (mm), number of the roots (pcs), and biomass of 10 plants (g)) was performed according to the methodical manual [63]. Plant growth parameters determined at the end of the 3-week period on experimental plants, compared with similar parameters of control plants, were expressed in %.
Determination of chlorophyll and carotenoid content
To extract photosynthetic pigments (chlorophylls and carot enoids) from plant leaves, we homogenized the sample (500 mg) of leaves in the porcelain mortar in a cooled at the temperature 10 °С 96 % ethanol at the ratio of 1:10 (weight:volume) with addition of 0.1-0.2 g CaCO3 (to neutralize the plant acids). The 1 ml of obtained homogenate was centrifuged at 8000 g in a refrigerated centrifuge K24D (MLW, Engelsdorf, Germany) during 5 min at the temperature 4 °С. The obtained precipitate was washed three times, with 1 ml 96 % ethanol and centrifuged at above mentioned conditions. After this procedure, the optical density of chlorophyll a, chlorophyll b and carotenoid in the obtained extract was measured using spectrophotometer Specord M-40 (Carl Zeiss, Germany).
The content of chlorophyll a, chlorophyll b, and carotenoids
(mg/g fresh weight) in plant leaves was calculated in accordance
with formula [64-65]:
Cchl a = 13.36×A664.2 – 5.19×A648.6,
Cchl b = 27.43×A648.6 – 8.12A×664.2,
Cchl (a + b) = 5.24×A664.2 + 22.24×A648.6,
Ccar = (1000×A470 – 2.13×Cchl a – 97.64×Cchlb)/209,
Where, Cchl - concentration of chlorophylls (μg/ml), Cchl a -
concentration of chlorophyll a (μg/ml), Cchl b - concentration of
chlorophyll b (μg/ml), Ccar - concentration of carotenoids (μg/
ml), А – absorbance value at a proper wavelength in nm.
The chlorophyll and carotenoids content per 1g of fresh weight
of extracted from leaves was calculated by the following formula
(separately for chlorophyll a, chlorophyll b and carotenoids):
A1=(C×V)/(1000×a1),
Where, A1 – content of chlorophyll a, chlorophyll b, or carotenoids
(mg/g FW), C - concentration of pigments (μg/ml), V - volume
of extract (ml), a1 - sample of leaves (g). The ratio of chlorophyll
and carotenoid content between experimental and control
plants was expressed in %.
Determination of total soluble protein content
The content of total soluble protein (g/100 g FW) in plant leaves was determined by the Bradford technique [66]. To make the plant extracts, a sample (100 mg) of plant leaves was homogenized in a porcelain mortar in a 0.1 M sodium phosphate buffer (pH 6.0–8.0) at a weight-to-volume ratio of 1:5 at 4 °C for 1 h. The resulting homogenates were centrifuged at 8000 g in a refrigerated centrifuge K24D (manufactured by MLW, Engelsdorf, Germany) at 4°C for 15 min. A volume of 1.5 mL of distilled water and 1.5 mL of Coomassie Brilliant Blue G-250 reagent (manufactured by Bio-Rad, 500-0006) were added to 50 mL of the obtained supernatant. The resulting mixture was stirred for 10 min. The optical density (OD) of total soluble protein was then determined using spectrophotometer Specord M-40 at a wavelength of 595 nm. The total soluble protein content (g of protein per 100 g of fresh weight (FW) of plant material) in the sample was quantified using a calibration curve based on the optical density (OD) measurements of standard samples containing 1.5 mL of bovine serum albumin (BSA) solution and 1.5 mL of Coomassie Brilliant Blue G-250 reagent (manufactured by Bio-Rad, 500-0006). The total soluble protein content in the leaves of experimental plants was calculated relative to that of the control plants and expressed in %.
Statistical processing of the experimental data
Each experiment was performed three times. Statistical processing of the experimental data was carried out using Student’s t-test with a significance level of P≤0.05; mean values ± standard deviation (± SD) [67].
Results and Discussion
Regulatory effect of furopyrimidine derivatives on morphometric parameters of maize plants
The vegetative growth of maize (Z. mays L.) variety Mas 24.C under heat and drought conditions was studied. A comparative analysis of the regulatory effect of phytohormones auxin IAA and cytokinin Kinetin, known and well-studied in various agricultural crops synthetic azaheterocyclic compounds, derivatives of N-oxide- 2,6-dimethylpyridine (Ivin), or sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine (Methyur, Kamethur) [42-51, 53-62], as well as new synthetic azaheterocyclic compounds, furopyrimidine derivatives (compounds № 1-12), applied at a concentration of 10-6M, on the formation and development of roots and shoots of maize plants in the vegetative phase under conditions of heat and drought stress was carried out (Figure 1).
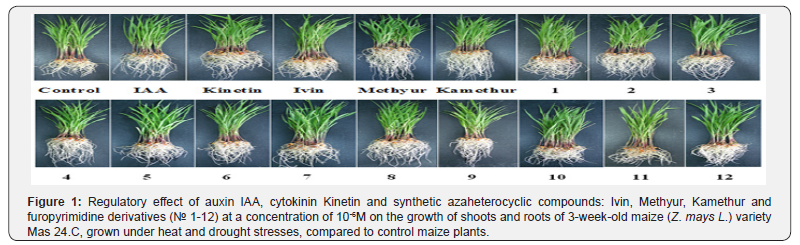
Treatment of maize plants with the synthetic azaheterocyclic compounds such as Ivin, Methyur, Kamethur and furopyrimidine derivatives had a positive effect on the growth and development of roots and shoots, as also increased the resistance of plants to heat and drought stresses, compared to control maize plants. The regulatory effect of synthetic azaheterocyclic compounds on the growth and development of roots and shoots of maize plants under heat and drought stresses was similar to or higher than the effects of phytohormones auxin IAA and cytokinin Kinetin (Figure 1).
Statistical analysis of the morphometric parameters of 3-week-old maize plants, grown under heat and drought stresses, showed that known synthetic azaheterocyclic compounds, derivatives of sodium and potassium salts of 6-methyl-2-mercapto- 4-hydroxypyrimidine (Methyur, Kamethur), as well as new synthetic azaheterocyclic compounds, furopyrimidine derivatives (compounds № 2, 3, 4, 5, 6, 7, 8, 9, 11, 12) revealed the highest regulatory effect, which was similar to or exceeded the effects of auxin IAA and cytokinin Kinetin. Synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin) and furopyrimidine derivatives (compounds № 1 and 10) revealed a lower regulatory effect, which was similar to or lower than the effect of auxin IAA and cytokinin Kinetin.
According to the length of the shoots of maize plants, grown under heat and drought stresses, the highest regulatory effect was demonstrated by synthetic compounds, derivatives of 6-methyl- 2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur) and furopyrimidine derivatives (compounds № 4, 5, 7, 8, 9 and 10), their parameters increased: by 43,92 % – under effect of Methyur, by 38,17 % – under effect of Kamethur, by 39,75–63,25 % – under effect of compounds № 4, 5, 7, 8, 9 and 10, compared to control maize plants (Figure 2)
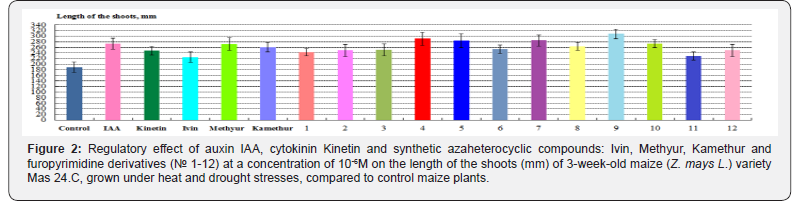
A lower regulatory effect according to the length of the shoots of maize plants, grown under heat and drought stresses, was demonstrated by synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin) and furopyrimidine (compounds № 1, 2, 3, 6, 11 and 12), under the effect of which these parameters increased: by 19,08 % - under effect of Ivin, by 22,08–34,28 %- under effect of compounds № 1, 2, 3, 6, 11 and 12, compared to control maize plants (Figure 2).
Auxin IAA and cytokinin Kinetin showed similar regulatory effects according to the length of the shoots of maize plants, grown under heat and drought stresses, under the effect of which these parameters increased: by 44,52% - under effect of IAA, by 31,1% - under effect of Kinetin, compared to control maize plants (Figure 2).
According to the length of the roots of maize plants, grown under heat and drought stresses, the highest regulatory effect was demonstrated by synthetic compounds, derivatives of 6-methyl- 2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur) and furopyrimidine derivatives (compounds № 2, 3, 4, 6, 8 and 9), under the effect of which these parameters increased: by 253,05 %- under effect of Methyur, by 200% - under effect of Kamethur, by 45,12–254,88% – under effect of compounds № 2, 3, 4, 6, 8 and 9, compared to control maize plants (Figure 3).
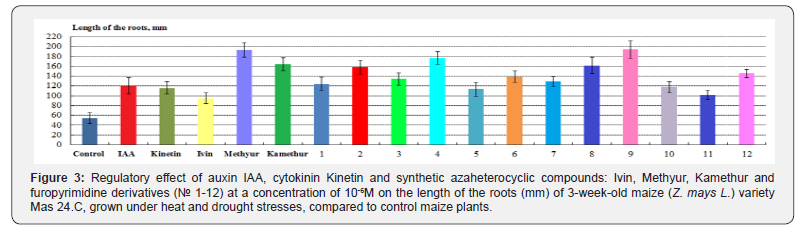
A lower regulatory effect according to the length of the roots of maize plants, grown under heat and drought stresses, was demonstrated by synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin) and furopyrimidine (compounds № 1, 5, 7, 10, 11 and 12), under the effect of which these parameters increased: by 73,17% - under effect of Ivin, by 85,37-135,37% - under effect of compounds № 1, 5, 7, 10, 11 and 12, compared to control maize plants (Figure 3).
Auxin IAA and cytokinin Kinetin showed similar regulatory effects according to the length of the roots of maize plants, grown under heat and drought stresses, under the effect of which these parameters increased: by 119,51% - under effect of IAA, by 112,2 % - under effect of Kinetin, compared to control maize plants (Figure 3).
According to the number of the roots of maize plants, grown under heat and drought stresses, the highest regulatory effect was demonstrated by synthetic compounds, derivatives of 6-methyl- 2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur) and furopyrimidine derivatives (compounds № 2, 4, 5, 6, 7, 8, 9, 11 and 12), under the effect of which these parameters increased: by 86,67% – under effect of Methyur, by 175,56% – under effect of Kamethur, by 40–96,67% – under effect of compounds № 2, 4, 5, 6, 7, 8, 9, 11 and 12, compared to control maize plants (Figure 4).
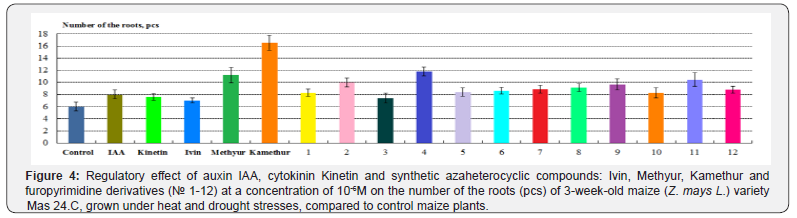
A lower regulatory effect according to the number of the roots of maize plants, grown under heat and drought stresses, was demonstrated by synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin) and furopyrimidine (compounds № 1, 3 and 10), under the effect of which these parameters increased: by 16,67% – under effect of Ivin, by 23,33– 38,89% – under effect of compounds № 1, 3 and 10, compared to control maize plants (Figure 4).
Auxin IAA and cytokinin Kinetin showed similar regulatory effects according to the number of the roots of maize, grown under heat and drought stresses, under the effect of which these parameters increased: by 33,33% – under effect of IAA, by 26,67% – under effect of Kinetin, compared to control maize plants (Figure 4).
According to the biomass of 10 maize plants, grown under heat and drought stresses, the highest regulatory effect was demonstrated by synthetic compounds, derivatives of 6-methyl- 2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur) and furopyrimidine derivatives (compounds № 2, 3, 4, 5 та 6), under the effect of which these parameters increased: by 79,6% – under effect of Methyur, by 69,4% – under effect of Kamethur, by 48,76–71,01% – under effect of compounds № 2, 3, 4, 5 та 6, compared to control maize plants (Figure 5).
A lower regulatory effect according to the biomass of 10 maize plants, grown under heat and drought stresses, was demonstrated by synthetic azaheterocyclic compounds, derivatives of N-oxide- 2,6-dimethylpyridine (Ivin) and furopyrimidine (compounds № 1, 7, 8, 9, 10, 11 and 12), under the effect of which these parameters increased: by 45,59% – under effect of Ivin, by 27,28– 39,96% – under effect of compounds № 1, 3 and 10, compared to control maize plants (Figure 5).
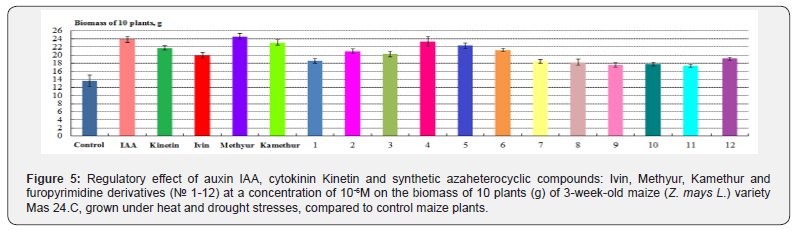
Auxin IAA and cytokinin Kinetin showed similar regulatory effects according to the biomass of 10 maize plants, grown under heat and drought stresses, under the effect of which these parameters increased: by 74,96% – under effect of IAA, by 59,64% – under effect of Kinetin, compared to control maize plants (Figure 5).
Thus, the results obtained confirmed the positive regulatory auxin-like and cytokinin-like effect of known synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), 6-methyl-2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur), and new furopyrimidine derivatives № 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, on increasing the morphometric parameters of 3-week-old maize (Z. mays L.) variety Mas 24.C, grown under heat and drought stresses. Obviously, this fact can be explained by the auxin-like and cytokinin-like regulatory effect of synthetic azaheterocyclic compounds on enhancing the division, elongation and differentiation of root and shoot meristem cells during the growth and development of maize in the vegetative phase, and protecting plants from damage caused by heat and drought stress, as well as preventing wilting and death of plants [15–19].
Regulatory effect of furopyrimidine derivatives on chlorophyll and carotenoid content in maize plants
A comparative analysis of the regulatory effect of auxin IAA, cytokinin Kinetin and synthetic azaheterocyclic compounds: Ivin, Methyur, Kamethur and furopyrimidine derivatives (№ 1-12) at a concentration of 10-6M on chlorophyll and carotenoid content (mg/g fresh weight), which are the main indicators of plant productivity and photoprotection [64, 65, 68-70], in the leaves of 3-week-old maize (Z. mays L.) variety Mas 24.C, grown under heat and drought stresses, was carried out.
The conducted studies showed that the highest regulatory effect on chlorophyll and carotenoid content in maize leaves was found by synthetic compounds, derivatives of 6-methyl-2-mercapto- 4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur), and furopyrimidine derivatives № 1, 2, 3, 4, 6, 7, 8, 9, 10, 11 and 12, compared to control maize plants (Figure 6). The content of chlorophylls a, b, a+b and carotenoids in the leaves of maize, grown under heat and drought stresses, increased under the effect of these compounds as follows: chlorophyll a increased: by 39,58% - under the effect of Methyur, by 28,81% - under the effect of Kamethur, by 22,34– 47,33% - under the effect of furopyrimidine derivatives № 1, 2, 3, 4, 6, 7, 8, 9, 10, 11 and 12; chlorophyll b increased: by 7,7% - under the effect of Methyur, by 7,1% - under the effect of Kamethur, by 6,6–61,78% - under the effect of furopyrimidine derivatives № 1, 2, 3, 4, 6, 7, 8, 9, 10, 11 and 12; chlorophylls a+b increased: by 30,35% - under the effect of Methyur, by 22,52% - under the effect of Kamethur, by 10,73– 51,51% - under the effect of furopyrimidine derivatives № 1, 2, 3, 4, 6, 7, 8, 9, 10, 11 and 12; carotenoids increased: by 51,37% - under the effect of Methyur, by 17,58% - under the effect of Kamethur, by 3,31–43,33% - under the effect of furopyrimidine derivatives № 1, 2, 3, 4, 6, 7, 8, 9, 10, 11 and 12, compared to control maize plants (Figure 6).
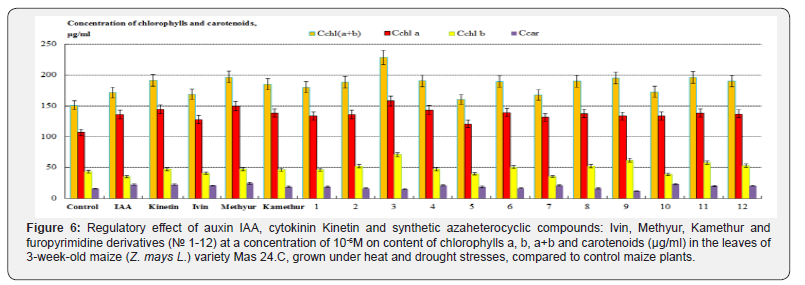
Auxin IAA and cytokinin Kinetin had similar regulatory effects on the content of chlorophylls a, b, a+b and carotenoids in the leaves of maize, grown under heat and drought stresses; these indicators increased under the effect of these compounds as follows: chlorophyll a increased: by 26,79% - under the effect of IAA, by 34,67% - under the effect of Kinetin; chlorophyll b increased: by 8,34 % - under the effect of Kinetin; chlorophylls a+b increased: by 13,75% - under the effect of IAA, by 27,05% - under the effect of Kinetin; carotenoids increased: by 37,31% - under the effect of IAA, by 39,91% - under the effect of Kinetin, compared to control maize plants (Figure 6).
A lower regulatory effect on the content of chlorophylls a, b, a+b and carotenoids in the leaves of maize, grown for 3 weeks under heat and drought stresses, was found by synthetic compound, a derivative of N-oxide-2,6-dimethylpyridine (Ivin) and furopyrimidine derivative № 5, compared to control maize plants (Figure 6). The content of chlorophylls a, b, a+b and carotenoids increased under the effect of these compounds as follows: chlorophyll a increased: by 19,26% - under the effect of Ivin, by 12,35% - under the effect of furopyrimidine derivative № 5; chlorophylls a+b increased: by 11,81% - under the effect of Ivin, by 6,34% - under the effect of furopyrimidine derivative № 5; carotenoids increased: by 27,66% - under the effect of Ivin, by 15,13% - under the effect of furopyrimidine derivative № 5, compared to control maize plants (Figure 6). At the same time, the synthetic compound Ivin and furopyrimidine derivative № 5 did not show a regulatory effect on the content of chlorophyll b, which was not statistically significantly different or was slightly lower than in control maize plants (Figure 6).
Thus, the results obtained confirmed the positive regulatory cytokinin-like effect of known synthetic azaheterocyclic compounds, derivatives of 6-methyl-2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur), and new furopyrimidine derivatives № 1, 2, 3, 4, 6, 7, 8, 9, 10, 11 and 12, on increasing the content of photosynthetic pigments - chlorophylls and carotenoids in the leaves of 3-week-old maize (Z. mays L.) variety Mas 24.C. Obviously, this fact can be explained by the regulatory cytokinin-like effect of synthetic azaheterocyclic compounds on preventing leaf senescence by enhancing synthesis and slowing down degradation of chlorophyll a, b and carotenoids in plant cells [71-73], which plays a key role in photosynthesis of maize plants and ensure their productivity and photoprotection under conditions of heat and drought stresses [68-70].
Regulatory effect of furopyrimidine derivatives on total soluble protein content in maize plants
A comparative analysis of the regulatory effect of auxin IAA, cytokinin Kinetin and synthetic azaheterocyclic compounds: Ivin, Methyur, Kamethur and furopyrimidine derivatives (№ 1-12) at a concentration of 10-6M on total soluble protein content (g/100 g FW), which is the main indicator of plant growth and metabolism [66-74], in the leaves of 3-week-old maize (Z. mays L.) variety Mas 24.C, grown under heat and drought stresses, was carried out.
The conducted studies showed that the highest regulatory effect on total soluble protein content in maize leaves was found by synthetic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), 6-methyl-2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur), and furopyrimidine derivatives № 4, 5, 7, 8, 10, 11 and 12, compared to control maize plants (Figure 7). The total soluble protein content in the leaves of maize plants, grown under heat and drought stresses, increased under the effect of these compounds as follows: by 156,83% - under the effect of Ivin, by 124,75% – under effect of Methyur, by 163,51% – under effect of Kamethur, by 85,78–136,89% – under effect of compounds № 4, 5, 7, 8, 10, 11 and 12, compared to control maize plants (Figure 7).
Auxin IAA and cytokinin Kinetin had similar regulatory effects on total soluble protein content in the leaves of maize plants, grown under heat and drought stresses; this indicator increased under the effect of these phytohormones as follows: by 138,38% - under the effect of IAA, by 153,24 % - under the effect of Kinetin, compared to control maize plants (Figure 7).
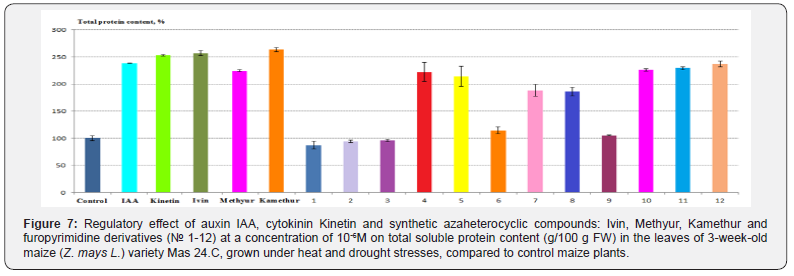
A lower regulatory effect on total soluble protein content in the leaves of maize plants, grown under heat and drought stresses, was found by synthetic compounds, furopyrimidine derivatives № 6 and 9, compared to control maize plants (Figure 7). The total soluble protein content under the effect of these compounds increased by 4,88–13,84 %, compared to control maize plants (Figure 7).
At the same time, synthetic compounds, furopyrimidine derivatives № 1, 2 and 3 did not exert a regulatory effect on total soluble protein content in the leaves of maize plants, grown under heat and drought stresses; this indicator did not differ statistically significantly under the effect of these compounds or was lower than the indicator of control maize plants (Figure 7).
Thus, the results obtained confirmed the positive regulatory auxin-like and cytokinin-like effect of known synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), 6-methyl-2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur), and new furopyrimidine derivatives № 4, 5, 7, 8, 10, 11 and 12 on increasing the content of total soluble proteins in the leaves of maize, which play a key role in plant growth and metabolism [66-74]. Apparently, the increase in the biosynthesis of total soluble proteins may also be associated with the presence in their fraction of a large number of antioxidant enzymes and drought and heat stress-related proteins, which are involved in the processes of sensing, signaling and protection of maize plants in response to drought and heat stresses [75-79]. Thus, stimulation of the activity of antioxidant enzymatic system, as well as drought and heat stress-related proteins in plants using phytohormones auxins and cytokinins, or synthetic azaheterocyclic compounds may be one of the important defense mechanisms to avoid abiotic damage to plants.
Analyzing the relationship between the chemical structure and the highest regulatory auxin-like and cytokinin-like effect of synthetic azaheterocyclic compounds, furopyrimidine derivatives № 2, 3, 4, 5, 6, 7, 8, 9, 11 and 12, which increased the morphometric parameters of maize plants and increased the content of photosynthetic pigments, and some of these compounds № 4, 5, 7, 8, 11 and 12 also increased the content of total soluble proteins in plants, it is possible to suggest that their high activity similar to phytohormones auxins and cytokinins is associated with the presence of substituents in the chemical structures of the compounds: compound № 2 contains a 2-methoxyethyl substituent in position 3, a methyl group in position 6, and a carboxylic acid ethyl ester group in position 5 of the 4-oxofuro[ 2,3-d]pyrimidine ring; compound № 3 contains a furan-2-ylmethyl substituent in position 3, a methyl group in position 6, and a carboxyl group in position 5 of the 4-oxofuro[2,3-d]pyrimidine ring; compound № 4 contains a cyclohexyl substituent in position 3, a methyl group in position 6, and a carboxyl group in position 5 of the 4-oxofuro[2,3-d]pyrimidine ring; compound № 5 contains a cyclohexyl substituent in position 3, a methyl group in position 6, and a N-(naphthalen-1-yl)amide substituent in position 5 of the 4-oxofuro[2,3-d]pyrimidine ring; compound № 6 contains a cyclohexyl substituent in position 3, a methyl group in position 6, and a N-(4-chlorophenyl)amide substituent in position 5 of the 4-oxofuro[ 2,3-d]pyrimidine ring; compound № 7 contains a benzyl substituent in position 3, a methyl group in position 6, and a methyl 4-amidobenzoate substituent in position 5 of the 4-oxofuro[2,3-d] pyrimidine ring; compound № 8 contains a benzyl substituent in position 3, a methyl group in position 6, and a N-(4-chlorophenyl) amide substituent in position 5 of the 4-oxofuro[2,3-d]pyrimidine ring; compound № 9 contains a benzyl group in position 3, a methyl group in position 6, and a 4-ethylphenylamide group in position 5 of the 4-oxofuro[2,3-d]pyrimidine ring; compound № 11 contains a benzyl group in position 3, a methyl group in position 6, and a 4-methylphenylamide group in position 5 of the 4-oxofuro[ 2,3-d]pyrimidine ring; compound № 12 contains a benzyl group in position 3, a methyl group in position 6, and a carboxyl group in position 5 of the 4oxofuro[2,3-d]pyrimidine ring. The decrease of the regulatory auxin-like and cytokinin-like effect of synthetic azaheterocyclic compounds, furopyrimidine derivatives № 1 and 10 can be explained by the presence of substituents in their chemical structures: compound № 1 contains a 2-methoxyethyl substituent in position 3, a methyl group in position 6, and a carboxyl group in position 5 of the 4-oxofuro[2,3-d]pyrimidine ring; compound № 10 contains a benzyl group in position 3, a methyl group in position 6, and a N-(3,4-dimethylphenyl)amide group in position 5 of the 4-oxofuro[2,3-d]pyrimidine ring.
The results obtained in this work indicate the prospects for practical application in agriculture of the most active synthetic azaheterocyclic compounds: Ivin, Methyur, Kamethur and furopyrimidine derivatives № 2, 3, 4, 5, 6, 7, 8, 9, 11 and 12, which improve the growth of shoots and the roots of maize, increase biomass, enhance the biosynthesis of chlorophylls, carotenoids and proteins in maize, as well as improve plant adaptation to abiotic stress factors such as heat and drought.
Conclusion
The regulatory effect of new synthetic azaheterocyclic compounds, furopyrimidine derivatives № 1–12, on the vegetative grown and development of maize (Z. mays L.) variety Mas 24.C and its adaptation to heat and drought stresses was investigated. The conducted study showed that synthetic azaheterocyclic compounds, furopyrimidine derivatives exert an auxin-like and cytokinin-like regulatory effect on the improving morphometric parameters and enhancing the biosynthesis of chlorophylls, carotenoids, and total soluble proteins in 3-week-old maize (Z. mays L.) variety Mas 24. C, grown under heat and drought stresses. The regulatory effect of synthetic azaheterocyclic compounds applied at concentration of 10-6M was equal to or higher than the regulatory effect of auxin IAA and cytokinin Kinetin, or known synthetic azaheterocyclic compounds, derivatives of N-oxide-2,6-dimethylpyridine (Ivin), 6-methyl-2-mercapto-4-hydroxypyrimidine sodium and potassium salts (Methyur and Kamethur), applied at the same concentration of 10-6M. A correlation between the chemical structure and the selectivity of the regulatory effect of synthetic azaheterocyclic compounds, furopyrimidine derivatives, has been demonstrated. Based on the obtained results, a conclusion was made about the prospects for practical application in agriculture of the most active synthetic azaheterocyclic compounds: Ivin, Methyur, Kamethur and furopyrimidine derivatives № 2, 3, 4, 5, 6, 7, 8, 9, 11 and 12 to improve maize growth and increase adaptation to abiotic stress factors such as heat and drought.
Statement of conflict of interest
The authors are declared that they have no conflict with this research article.
References
- Klopfenstein TJ, Erickson GE, Berger LL (2013) Maize is a critically important source of food, feed, energy and forage in the USA. Field Crops Research 153: 5-11.
- Cui J, Sui P, Wright DL, Wang D, Beibei S, et al. (2019) Carbon emission of maize-based cropping systems in the North China Plain. Journal of Cleaner Production 213: 300-308.
- Chaima ELK, Assia H, Hanane O, Tarik B, Abdellah E (2024) Sustainable Maize Production and Carbon Footprint in Arid Land Context: Challenges and Perspectives. Agricultural Sciences. IntechOpen.
- Wang P, Xie W, Ding L, Zhuo Y, Gao Y, et al. (2023) Effects of Maize–Crop Rotation on Soil Physicochemical Properties, Enzyme Activities, Microbial Biomass and Microbial Community Structure in Southwest China. Microorganisms 11(11): 2621.
- Kazula MJ, Lauer JG (2018) The influence of crop rotation on corn total biomass production. Journal of Soil and Water Conservation. 73(5): 541-548.
- Santos GO, Carvalho R, Oliveira AM, Abreu CM, Campos GM (2024) Climate changes and trends and impacts on grain production. Contribuciones a Las Ciencias Sociales 17(6): e7795.
- Lamaoui M, Jemo M, Datla R, Bekkaoui F (2018) Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front Chem 6: 26.
- Cabello GGC, Rodriguez AR, Gondal AH, Areche FO, Flores DDC, et al. (2023) Plant adaptability to climate change and drought stress for crop growth and production. CABI Reviews.
- Tardieu F, Granier C, Muller B (2011) Water deficit and growth. Co-ordinating processes without an orchestrator? Curr Opin Plant Biol 14(3): 283-289.
- Chávez-Arias CC, Ligarreto-Moreno GA, Ramírez-Godoy A, Restrepo-Díaz H (2021) Maize Responses Challenged by Drought, Elevated Daytime Temperature and Arthropod Herbivory Stresses: A Physiological, Biochemical and Molecular View. Front Plant Sci 12: 702841.
- Aslam M, Maqbool MA, Cengiz R (2015) Effects of Drought on Maize. In book: Drought Stress in Maize (Zea mays L.): Effects, Resistance Mechanisms, Global Achievements and Biological Strategies for Improvement. Springer Briefs in Agriculture 1st ed pp.82.
- Alotaibi M (2023) Climate change, its impact on crop production, challenges, and possible solutions. Not Bot Horti Agrobo 51(1): 13020.
- Fageria NK (2012) The Role of Plant Roots in Crop Production. 1st Edition. CRC Press, Taylor @ Francis Group LLC NY pp.467.
- Khan MA, Gemenet DC, Villordon A (2016) Root System Architecture and Abiotic Stress Tolerance: Current Knowledge in Root and Tuber Crops. Front Plant Sci 7: 1584.
- Tsygankova VA (2015) Genetic Control and Phytohormonal Regulation of Plant Embryogenesis. Int J Med Biotechnol Genetics (IJMBG) 3(1): 9-20.
- Schaller GE, Bishopp A, Kieber JJ (2015) The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 27: 44-63.
- Lee ZH, Hirakawa T, Yamaguchi N, Ito T (2019) The Roles of Plant Hormones and Their Interactions with Regulatory Genes in Determining Meristem Activity. Int J Mol Sci 20(16): 4065.
- Sosnowski J, Truba M, Vasileva V (2023) The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 13(3): 724.
- Garay-Arroyo A, De La Paz Sánchez M, García-Ponce B, Azpeitia E, Álvarez-Buylla ER (2012) Hormone symphony during root growth and development. Dev Dyn 241(12): 1867-1885.
- Ljung K (2013) Auxin metabolism and homeostasis during plant development. Development 140(5): 943-950.
- Hu Y, Shani E (2023) Cytokinin activity-transport and homeostasis at the whole plant, cell, and subcellular levels. New Phytol 239: 1603-1608.
- Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52: 89-118.
- Novickienė L, Asakavičiūtė R (2006) Analogues of auxin modifying growth and development of some monocot and dicot plants. Acta Physiol Plant 28(6): 509-515.
- Savaldi-Goldstein S, Baiga TJ, Pojer F, Dabi T, Butterfield C, Parry G, et al. (2008) New auxin analogs with growth-promoting effects in intact plants reveal a chemical strategy to improve hormone delivery. Proc Natl Acad Sci USA 105(39): 15190-5.
- Fukui K, Hayashi K (2018) Manipulation and Sensing of Auxin Metabolism, Transport and Signaling.Plant and Cell Physiol 59(8): 1500-1510.
- Rigal A, Ma Q, Rober S (2014) Unraveling plant hormone signaling through the use of small molecules. Front Plant Sci 5: 273.
- Jameson PE (2023) Zeatin: The 60th anniversary of its identification. Plant Physiology 192(1): 34-55.
- Vylíčilová H, Bryksová M, Matušková V, Doležal K, Plíhalová L, et al. (2020) Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules 10(6): 832.
- Podlešáková K, Zalabák D, Čudejková M, Plíhal O, Szüčová L, et al. (2012) Novel Cytokinin Derivatives Do Not Show Negative Effects on Root Growth and Proliferation in Submicromolar Range. PLoS ONE 7(6): e39293.
- Nowicka B (2022) Modifications of Phytohormone Metabolism Aimed at Stimulation of Plant Growth, Improving Their Productivity and Tolerance to Abiotic and Biotic Stress Factors.Plants 11(24): 3430.
- Kaya C, Tuna AL, Okan AM (2010) Effect of foliar applied kinetin and indole acetic acid on maize plants grown under saline conditions. Turk J Agric For 34(6): 529-538.
- Márquez G, Alarcón MV, Salguero J (2016) Differential responses of primary and lateral roots to indole-3-acetic acid, indole-3-butyric acid, and 1-naphthaleneacetic acid in maize seedlings. Biol Plant 60: 367-375.
- Rivas MÁ, Friero I, Alarcón MV, Salguero J (2022) Auxin-Cytokinin Balance Shapes Maize Root Architecture by Controlling Primary Root Elongation and Lateral Root Development. Front Plant Sci 13: 836592.
- Chávez-Arias CC, Ligarreto-Moreno GA, Ramírez-Godoy A, Restrepo-Díaz H (2021) Maize Responses Challenged by Drought, Elevated Daytime Temperature and Arthropod Herbivory Stresses: A Physiological, Biochemical and Molecular View. Front. Plant Sci 12: 702841.
- Aslam M, Maqbool MA, Cengiz R (2015) Effects of Drought on Maize. In book: Drought Stress in Maize (Zea mays L.): Effects, Resistance Mechanisms, Global Achievements and Biological Strategies for Improvement. Springer Briefs in Agriculture 1st ed pp.82.
- Ota C, Kumata S, Kawaguchi S (2007) Novel herbicides, usage thereof, novel thienopyrimidine derivatives, intermediates of the same, and process for production thereof. Patent US20070010402A1.
- Cansev A, Gülen H, Zengin MK, Ergin S, Cansev M (2014) Use of Pyrimidines in Stimulation of Plant Growth and Development and Enhancement of Stress Tolerance. WIPO Patent WO 2014/129996A1.
- Boussemghoune MA, Whittingham WG, Winn CL, Glithro H, Aspinall MB (2012) Pyrimidine derivatives and their use as herbicides. Patent US20120053053 A1.
- Li JH, Wang Y, Wu YP, Li RH, Liang S, et al. (2012) Synthesis, herbicidal activity study and molecular docking of novel pyrimidine thiourea. Pestic Biochem Physiol 172: 104766.
- Tsygankova VA, Brovarets VS, Yemets AI, Blume YB (2021) Prospects of the development in Ukraine of the newest plant growth regulators based on low molecular heterocyclic compounds of the azole, azine and their condensed derivatives. In: A.I. Vovk (Ed.). Synthesis and bioactivity of functionalized nitrogen-containing heterocycles, Kyiv: Interservice 246-285.
- Tsygankova VA, Andrusevich YaV, Shtompel OI, Solomyanny RM, Hurenko AO, et al. (2022) New Auxin and Cytokinin Related Compounds Based on Synthetic Low Molecular Weight Heterocycles, Chapter 16, In: Aftab T. (Ed.) Auxins, Cytokinins and Gibberellins Signaling in Plants, Signaling and Communication in Plants, Springer Nature Switzerland AG 353-377.
- Rudnytska MV, Palladina TA (2017) Effect of preparations Methyur and Ivine on Са2+-ATPases activity in plasma and vacuolar membrane of corn seedling roots under salt stress conditions. Ukr Biochem J 89(1): 76-81.
- Tsygankova VA, Andrysevich YV, Shtompel OI, Kopich VM, Kluchko SV, et al. (2018) Using Pyrimidine Derivatives - Sodium Salt of Methyur and Potassium Salt of Methyur, to Intensify the Growth of Corn. Patent of Ukraine 130921.
- Tsygankova VA, Andrysevich YV, Shtompel OI, Kopich VM, Kluchko SV, et al. (2021) The method of intensifying the growth of corn plants using Methyur potassium salt. Patent of Ukraine 123222.
- Tsygankova VA, Voloshchuk IV, Pilyo SH, Klyuchko SV, Brovarets VS (2023) Enhancing Sorghum Productivity with Methyur, Kamethur, and Ivin Plant Growth Regulators. Biology and Life Sciences Forum 27(1): 36.
- Tsygankova VA, Voloshchuk IV, Kopich VM, Pilyo SG, Klyuchko SV, et al. (2023) Studying the effect of plant growth regulators Ivin, Methyur and Kamethur on growth and productivity of sunflower. Journal of Advances in Agriculture 14: 17-24.
- Tsygankova VA, Kopich VM, Vasylenko NM, Golovchenko OV, Pilyo SG, et al. (2024) Increasing the productivity of wheat using synthetic plant growth regulators Methyur, Kamethur and Ivin. Znanstvena misel journal 94: 22-26.
- Kovalenko OA, Mikolaychuk VG, Tsygankova VA, Andreev AM, Pilyo SG, et al. (2025) Influence of the plant growth regulator Kamethur on the morphological features and yield of Chinese cowpea (Vigna sinensis L.). Sciences of Europe 166: 3-17.
- Tsygankova VA, Andrusevich YaV, Kopich VM, Voloshchuk IV, Pilyo SG, et al. (2023) Application of pyrimidine and pyridine derivatives for regulation of chickpea (Cicer arietinum L.) growth. International Journal of Innovative Science and Research Technology (IJISRT) 8(6): 19-28.
- Tsygankova VA, Andrusevich YaV, Vasylenko NM, Pilyo SG, Klyuchko SV, et al. (2023) Screening of Auxin-like Substances among Synthetic Compounds, Derivatives of Pyridine and Pyrimidine. J Plant Sci Phytopathol 7: 151-156.
- Tsygankova VA, Kopich VM, Voloshchuk IV, Pilyo SG, Klyuchko SV, et al. (2023) New growth regulators of barley based on pyrimidine and pyridine derivatives. Sciences of Europe 124: 13-23.
- Vasetska O, Zhminko P, Prodanchuk M, Galkin A, Tsygankova V (2022) Perspective for using 2, 6-dimethylpyridine-N-oxide to reduce the toxic effect of xenobiotics in mammals. J Adv Pharm Educ Res 12(1): 21-29.
- Mohilnikova IV, Tsygankova VA, Solomyannyi RM, Brovarets VS, Bilko NМ, et al. (2020) Screening of growth-stimulating activity of synthetic compounds — pyrimidine derivatives. Reports of the National Academy of Sciences of Ukraine 10: 62-70.
- Tsygankova VA, Andrusevich YaV, Vasylenko NM, Kopich VM, Popilnichenko SV, et al. (2024) Auxin-like and cytokinin-like effects of new synthetic pyrimidine derivatives on the growth and photosynthesis of wheat. J Plant Sci Phytopathol 8(1): 15-24.
- Tsygankova VA, Vasylenko NM, Andrusevich YaV, Kopich VM, Solomyannyi RM, et al. (2024) New Wheat Growth Regulators Based On Thioxopyrimidine Derivatives. Int J Med Biotechnol Genetics S1:02:004:23-30.
- Tsygankova VA, Kopich VM, Vasylenko NM, Andrusevich YaV, Pilyo SG, et al. (2024) Phytohormone-like effect of pyrimidine derivatives on the vegetative growth of haricot bean (Phaseolus vulgaris ). Polish Journal of Science 1(71): 6-13.
- Tsygankova VA, Vasylenko NM, Andrusevich YaV, Kopich VM, Kachaeva MV, et al. (2025) Use of Thienopyrimidine Derivatives to Optimize Sorghum Growth and Photosynthesis during the Vegetation Period. Journal of Biomedical Research & Environmental Sciences 6(1): 071-080.
- Tsygankova VA, Vasylenko NM, Andrusevich YaV, Kopich VM, Solomyannyi RM, et al. (2025) Screening of Synthetic Auxin-Like and Cytokinin-Like Compounds, Derivatives of Thioxopyrimidine as New Plant Growth Regulators. Significances Bioeng Biosci 7(2): 000657.
- Tsygankova VA, Andrusevich YaV, Kopich VM, Vasylenko NM, Solomyannyi RM, et al. (2025) New eco-friendly pea growth regulators based on synthetic azaheterocyclic compounds, thioxopyrimidine derivatives. Agri Res & Tech: Open Access J 29(1): 556438.
- Tsygankova VA, Andrusevich YaV, Vasylenko NM, Kopich VM, Solomyannyi RM, et al. (2025) Application of Pyrimidine Derivatives as New Regulators to Enhance Wheat Growth in The Vegetative Phase J Nutrition and Food Processing 8(6): 1-12.
- Tsygankova VA, Vasylenko NM, Andrusevich YaV, Kopich VM, Solomyannyi RM, et al. (2025) Using pyrimidine derivatives to enhance soybean growth under conditions of heat and drought. Agri Res & Tech: Open Access J 29(2): 556445.
- Pidlisnyuk V, Mamirova A, Newton RA, Stefanovska T, Zhukov O, et al. (2022) The role of plant growth regulators in Miscanthus × giganteus utilisation on soils contaminated with trace elements. Agronomy 12(12):
- Voytsehovska OV, Kapustyan AV, Kosik OI (2010) Plant Physiology: Praktykum, Parshikova TV (Ed), Lutsk: Teren pp. 420.
- Lichtenthaler H (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology 148: 331- 382.
- Lichtenthaler HK, Buschmann C (2001) Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy Current Protocols in Food Analytical Chemistry F4.3.1-F4.3.8.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 7(72): 248-254.
- Bang H, Zhou XK, van Epps HL, Mazumdar M (2010) Statistical Methods in Molecular Biology. Series: Methods in molecular biology, New York: Humana press 13(620): 636.
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, et al. (2000) Molecular Cell Biology. Section 16.3, Photosynthetic Stages and Light-Absorbing Pigments. 4th Edtn. New York: WH Freeman and Company.
- Hitchcock A, Proctor MS, Sobotka R (2023) Coordinating plant pigment production: A green role for ORANGE family proteins. Molecular Plant 16 (9): 1366-1369.
- Sun T, Rao S, Zhou X, Li L (2022) Plant carotenoids: recent advances and future perspectives. Mol Horticulture 2(3).
- Hönig M, Plíhalová L, Husičková A, Nisler J, Doležal K (2018) Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int J Mol Sci 19(12): 4045.
- Joshi S, Choukimath A, Isenegger D, Panozzo J, Spangenberg G, et al. (2019) Improved wheat growth and yield by delayed leaf senescence using developmentally regulated expression of a cytokinin biosynthesis gene. Front. Plant Sci 10: 1285.
- Zhang Y-M, Guo P, Xia X, Guo H, Li Z (2021) Multiple Layers of Regulation on Leaf Senescence: New Advances and Perspectives. Front Plant Sci 12: 788996.
- Gregová E, Hlozáková TK, Šliková S, Gálová Z (2014) PROTEIN EXTRACTION OF MAIZE (ZEA MAYS) FOR PROTEOMIC 2 - DE ANALYSIS. Journal of Microbiology Biotechnology and Food Sciences 4(3): 263–265.
- Guan LM, Scandalios JG (2002) Catalase gene expression in response to auxin-mediated developmental signals. Physiologia Plantarum 114(2): 288-295.
- Zavaleta-Mancera HA, López-Delgado H, Loza-Tavera H, Mora-Herrera M, Trevilla-García C, et al. (2007) Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J Plant Physiol 164(12): 1572-82.
- Gupta DK, Palma JM, Corpas FJ (2018) Antioxidants and Antioxidant Enzymes in Higher Plants. Springer Nature: Dordrecht, GX, Netherlands.
- Sharma P, Jha A, Dubey RS, Pessarakli M (2012) Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. Journal of Botany 1: 217037: 26.
- Priya M, Dhanker OP, Siddique KHM, Rao BH, Nair RM, et al. (2019) Drought and heat stress-related proteins: an update about their functional relevance in imparting stress tolerance in agricultural crops. Theor Appl Genet 132(6): 1607-1638.






























