Caffeine Content in Energy Drinks can be Determined using a Validated Liquid-Liquid Extraction Method Coupled to UV-Visible Spectrophotometry
Abena Brobbey, Barbara Adu-Brimpong, John Nii Addotey*, Samuel Asare-Nkansah, Isaac Ayensu, Enoch Appiah, and Alexander Derizie
Department of Pharmaceutical Chemistry, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Ghana
Submission: June 13, 2023; Published: July 03, 2023
*Corresponding author: John Nii Addotey, Department of Pharmaceutical Chemistry, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Ghana. Email: jnaddotey.pharm@knust.edu.gh
How to cite this article: Abena B, Barbara A-B, John Nii A, Samuel A-N, Isaac A, et al. Caffeine Content in Energy Drinks can be Determined using a Validated Liquid-Liquid Extraction Method Coupled to UV-Visible Spectrophotometry. Nutri Food Sci Int J. 2023. 12(2): 555834. DOI: 10.19080/NFSIJ.2023.12.555834.
Abstract
Consumption of energy drinks is a growing public health concern due to their high caffeine concentration and the possible adverse effects of regular consumption of these products. There are reports of increased sales of energy drinks in Ghana which are patronized by all age groups including children, adolescents, and pregnant women. Thus, the need to assess the caffeine content in energy drinks sold on the Ghanaian market. Using a simple liquid-liquid extraction technique coupled with UV-visible spectrophotometry, seven (7) products were analyzed at a wavelength of 273 nm in 0.1 M HCl. Percentage yields obtained from extraction of caffeine from reference caffeine solutions ranged from 96.53-97.14 %. The developed method used was shown to be linear (R2 > 0.99) and have acceptable levels of accuracy (98.84-100.15 %), precision (RSDs < 2) and robustness (RSDs < 2). The caffeine content of the seven energy drinks sampled ranged from 0.134-0.282 mg/ml. The amount of caffeine per serving in the products analyzed ranged from 62.86 to 101.12 mg with product ED05 having the highest amount of caffeine per serving of 101.12 mg.
Keywords: Caffeine, Energy Drinks; Ultra-Violet Spectrophotometry; Liquid-Liquid Extraction
Abbreviations: EFSA: European Food and Safety Authority; LOD: Limit of detection; LOQ: limit of quantitation; RSD: Relative Standard Deviations; ICH: International Conference on Harmonization; HPLC: High Performance Liquid Chromatography; GC: Gas Chromatography; UV: Ultraviolet- visible
Introduction
Consumption of energy drinks is a growing public health concern due to their high caffeine concentration and the possible adverse effects of regular consumption of these products. Energy drinks are one group of highly purchased beverages all over the world and consumed by up to 90 % of world population in varying forms [1].
In Ghana, the rate of sales and purchase of energy drinks have become very alarming owing to taste, affordability, and heightened advertisements on the media. Recently, there have been reports in North America and Germany of the consumption of energy drinks by children, adolescents, and young people [2,3,4] and in developing African countries like Ghana, this is no exception. This is a major challenge to the health economies of these countries. Many developed countries like United States of America, Canada and Austria have put restrictions on the manufacture, labelling, advertisement, and sales of energy drinks, especially in schools and to children less than 18 years [1,5]. However, in developing African countries like Ghana there are no restrictions on the caffeine content of energy drinks and there is incessant advertisement of these products on television, radio, and other social media platforms. Patrons of such products range from children of school going age to the elderly.
Caffeine increases the levels of the neurotransmitter, dopamine, like the action of amphetamines and heroin and this accounts for the addictive nature of caffeine. Caffeine also stimulates medullary and cerebral centers in the brain resulting in increased neuronal firing, which leads to adrenaline release [6]. The adrenaline-like action of caffeine alters moods, increases wakefulness or alertness, and reduces tiredness. These effects are observed at doses of 85-250 mg. At doses beyond 250 mg, side effects such as restlessness, anxiety, nervousness, and insomnia become more pronounced. Consumption of high levels of caffeine has also been found to result in the increase of gastric-acid secretion, kidney malfunction, cardiac arrhythmia, disturbances of the central nervous system such as seizures and delirium [7].
As there are no controls on the consumption of caffeine – containing drinks, they are subject to overuse which can lead to the above-mentioned effects. Thus, it is expedient that the content of caffeine in these energy drinks be investigated to ascertain if they are within pharmacologically safe ranges. This will enable the assessment of the potential effects of cumulative use per time of such caffeine containing energy drinks. Analytical methods which have been described for analysis of caffeine in such products include High Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) [7, 8]. These sophisticated methods are expensive, time consuming and require more skill to carry out. For developing countries such as Ghana, cheaper, faster yet equally sensitive methods are needed to enhance the monitoring of these products as the demand soars. Ultraviolet- visible (UV) spectrophotometry represents an alternative to the above methods. However, the main setback is the possible interference from other ingredients and common colorants like tartrazine on absorbance of caffeine. Therefore, the study aims to develop and validate an extraction method for easy quantification of caffeine in energy drinks. The developed method will not be affected by interference from other ingredients.
Experimental
Materials, Reagents and Equipment
The main reagents used were hydrochloric acid (36.5 %v/v) (Fischer Scientific, USA), ammonia (30 %v/v) (Surechem Ltd., UK), pure caffeine powder (99.8 %w/w) (Aarti Industries Ltd.), chloroform (VWR, UK) and double distilled water (Inhouse). Equipment used was T90+ Double beam UV-Visible spectrophotometer (PG Instrument Ltd, UK) with a 10 mm matched quartz cell.
Method development
Preparation of reference solution
An amount of 10 mg of reference caffeine powder was dissolved in 20 ml of 0.1 M HCl, sonicated for 8 min, and allowed to cool. The solution was then transferred into a 100 ml volumetric flask and made up to the mark with 0.1 M HCl to obtain a final concentration of 100 μg/ml.
Determination of wavelength of maximum absorption for caffeine
A volume of 2 ml of the reference solution was pipetted into a 25 ml volumetric flask and made up to volume using 0.1 M HCl (8 μg/ml). The absorption maximum of caffeine was obtained by scanning this solution of 8 μg/ml from 220 nm to 380 nm using the UV-visible spectrophotometer.
Establishment of the extraction process
The amount of 2 g of caffeine was dissolved in 30 ml of 0.1 M HCl. The solution was transferred into a 100 ml volumetric flask and made up to volume using 0.1 M HCl. Into a separating funnel, 20 ml of dilute ammonia was added to 100 ml of the resulting solution to basify the solution. Red litmus paper was used to confirm basicity when it turned blue. Five (5) successive extractions were performed with 25 ml of chloroform. The chloroformic extracts were pooled and the solvent was allowed to evaporate. The weight of the dry mass determined (D) and percentage yield was calculated. This procedure was repeated using 1 g and 0.1 g of caffeine.
% yield=(D(g)×100)/2
Method validation
This method (which included the extraction and UV-Visible determination) was validated in terms of linearity, limit of quantitation, limit of detection, accuracy, precision (inter-day and intra-day) and robustness in accordance with the International Conference on Harmonization (ICH) guidelines [9].
Linearity
Six (6) solutions were prepared by performing serial dilutions of the reference solution (100 μg/ml). Volumes of 1.4 ml, 1.2 ml, 1.0 ml, 0.8 ml, 0.4 ml, and 0.2 ml were pipetted into six separate 10 ml volumetric flasks. The solutions were made up to the 10 ml mark with 0.1 M HCl and mixed thoroughly. Triplicate absorbance readings were taken for each solution at 273 nm. A calibration plot of the average absorbance readings against concentration was used to establish the linearity of responses obtained.
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The limit of detection (LOD) and limit of quantitation (LOQ) for the developed method were calculated from the linear regression analysis obtained for caffeine. The standard deviations (σ) and the slope (S) of the calibration curve were used in the calculations. The formula used is indicated below.
LOD=3.3σ/S LOQ=10σ/S
Accuracy
The accuracy of the developed method was determined at three concentration levels of 120 %w/v, 100 %w/v and 80 %w/v. Into three (3) separate 10 ml volumetric flasks 1.2 ml, 1.0 ml and 0.8 ml of the reference solution were pipetted and made up to the 10ml mark with 0.1 M HCl to obtain the concentration levels of 120 %w/v, 100 %w/v and 80 %w/v respectively. The absorbances of these solutions were determined in triplicate at 273 nm and percentage recoveries calculated.
Precision
Inter-day and intra-day precision of this method were assessed. For intra-day precision, three different solutions were prepared from the reference solution by pipetting 1.4 ml, 1.0 ml and 0.2 ml into separate 10 ml volumetric flask. These solutions were then made up to the 10 ml mark with 0.1 M HCl and analyzed three times within a day. Triplicate absorbance readings were taken from which relative standard deviations (RSD) were calculated. For inter-day precision, 1.0 ml of the reference solution was pipetted and transferred into a 10 ml volumetric flask and made up to volume with 0.1 M HCl. This solution was analyzed on three consecutive days to obtain triplicate absorbance readings and relative standard deviations (RSD) calculated.
Robustness
The robustness of this method was determined by varying the acid used in the extraction of caffeine while maintaining other parameters. A solution of 0.1 M H2SO4 was used in place of 0.1M HCl. The percentage recoveries were calculated and RSDs.
Determination of Caffeine content in Energy drinks
An accurate volume of 100 ml of the energy drink was measured and transferred into a clean 200 ml separating funnel. Twenty (20) ml of 0.1 M HCl was added and swirled gently for 3 min. The solution was based on adding excess dilute ammonia and subsequently tested with red litmus paper. Five successive extractions were done with 25 ml portions of chloroform. The chloroformed extracts were pooled together and allowed to evaporate to dryness. The residue obtained after evaporation was weighed and analyzed using the developed method.
Results and Discussion
Method development
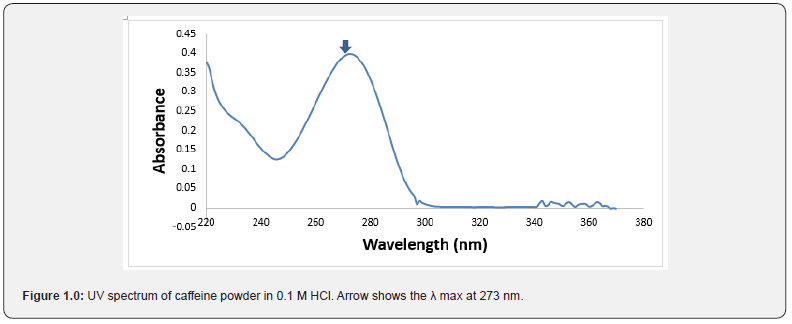
The wavelength of maximum absorption determined for caffeine in acidic medium (0.1M HCl) was 273 nm from the spectrum obtained. Figure 1.0 shows the ultraviolet spectrum of caffeine powder (1.0 μ/ml) obtained after a spectrum scan from 220 nm to 380 nm. The spectrum shows λ value at 273 nm.
Precision of extraction method
The consistency of the extraction procedure was ascertained via replication. Three replicate extractions were performed and percentage yields of 97.02, 96.53 and 97.14 % were obtained after extraction of caffeine from 2.0, 1.0 and 0.1 %v/w of reference caffeine solutions respectively (Table 1.0). This gives a clear indication that the extraction method employed is efficient and effective in obtaining caffeine from solutions.

Analytical method validation
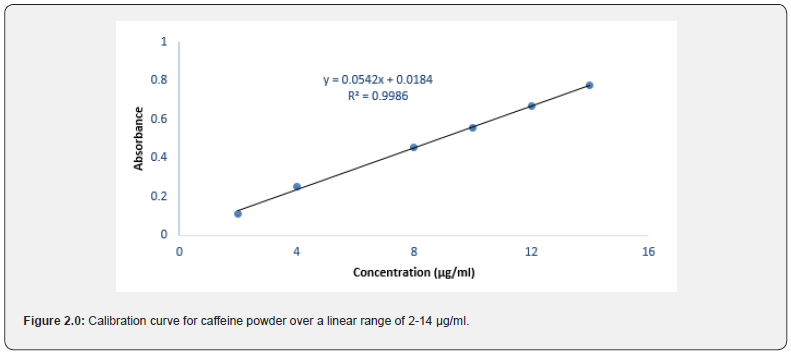
The method used for the determination of caffeine showed a good correlation between the concentrations of caffeine and the absorbances obtained. This was confirmed by a correlation coefficient r of 0.9994 established over a linear range of 2-14 μg/ ml (Figure 2.0).
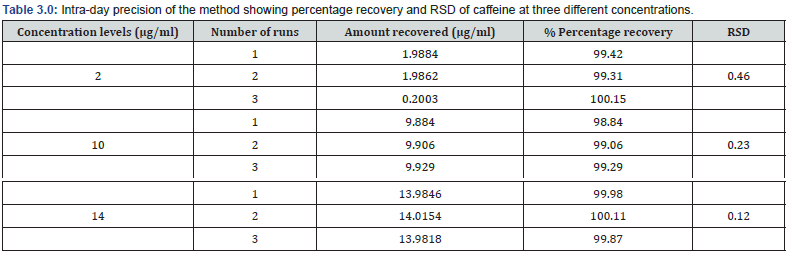
Limit of detection (LOD) and limit of quantitation (LOQ) were calculated to be 0.6 μg/ml and 1.8181 μg/ml indicating high sensitivity of method. This method showed acceptable levels with good recoveries varying from 98.84 to 100.15 % at the three concentration levels of 80 %, 100 % and 120 % (Table 2.0). Additionally, a good level of precision was obtained with the intra-day and inter-day precision having calculated RSDs of < 2 % (Table 3.0 and 4.0).

Monitored over three consecutive days.
Monitored over three consecutive days
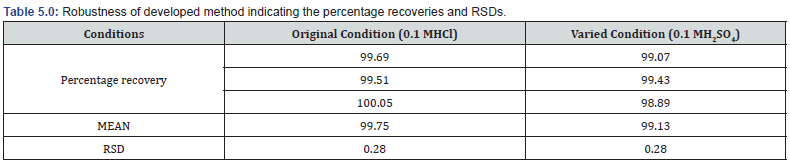
The ability of the method to resist small changes in some parameters was assessed. The main parameter varied was the acid used for extraction of the caffeine while keeping all other factors constant. RSDs calculated for the original and varied condition were all < 2 % indicating robustness of the method (Table 5.0).
Caffeine content in energy drinks
Compounds like caffeine possess chromophores which make it possible to be analyzed using UV-visible spectrophotometry. Seven energy drinks were randomly sampled from the Kumasi market and coded ED01-ED07. Products had different quantity per serving, ED01 and ED02 350 ml, ED03, EDO4 and EDO5 500 ml, EDO6 330 ml and, product ED07 250 ml. The caffeine content of the energy drinks sampled ranged from 0.134 – 0.282 mg/ ml (Table 6.0). The European Food and Safety Authority (EFSA) specifies the daily intake of caffeine from healthy adults to be up to 400 mg, up to 200 mg for pregnant women and for children and adolescents the caffeine intake should not exceed 3 mg/kg body weight per day [10]. It was observed that there was no correlation between the quantity per serving and the amount of caffeine per serving except for EDO5, 500 ml which had the highest quantity per serve of 101.12 mg. Interestingly, product ED03 had 500 ml as quantity per serving and an amount of caffeine per serving of 67 mg whiles product ED01 had 350 ml as quantity per serving and an amount of caffeine per serving of 98.70 mg.

Conclusion
This study sought to provide a simpler, cheap, and easy method which could be used to quantify the content of caffeine in energy drink samples in developing countries as well as help regulatory authorities in these countries to also monitor the caffeine content of energy drinks on the market. The developed UV spectrophotometric method was shown to be linear and had acceptable levels of accuracy, precision, and robustness. The extraction procedure employed was also shown to be efficient with high percentage yields. Seven energy drinks sampled from the market were successfully analyzed using this method and had their caffeine content range from 0.134-0.282 mg/ml. Product ED05 had the highest amount of caffeine per serving of 101.12 mg.
References
- Meltzer HM (2008) Risk assessment of caffeine among children and adolescents in the Nordic countries. Nordic Council of Ministers P: 11-13.
- Visram S, Cheetham M, Riby DM, Crossley SJ, Lake AA (2016) Consumption of energy drinks by children and young people: a rapid review examining evidence of physical effects and consumer attitudes. BMJ open 6(10): e010380.
- Goldman RD (2013) Caffeinated energy drinks in children. Canadian Family Physician 59(9): 947-948.
- Azagba S, Langille D, Asbridge M (2014) An emerging adolescent health risk: caffeinated energy drink consumption patterns among high school students. Preventive Medicine 62: 54-59.
- American Beverage Association (2014) ABA guidance for the responsible labeling and marketing of energy drinks. America Beverage Association.
- Heneman K, Zidenberg Cherr S (2007) Nutrition and Health Info-sheet for Health professionals, Department of nutrition, University of California P: 1-5.
- Nour V, Trandafir I, Ionica ME (2010) Chromatographic determination of caffeine contents in soft and energy drinks available on the Romanian market. Sci Study Res Chem Chem Eng Biotechnol Food Ind 11: 351-358.
- Beckett AH, Stenlake JB (1988) Practical Pharmaceutical Chemistry: Part II, 4th Edition, A&C Black.
- ICH (1996) Q2B Validation of Analytical Procedures-Methodology. Consensus-Guidelines, ICH Harmonized Tripartite Guidelines.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2015) Scientific opinion on the safety of caffeine. EFSA Journal 13(5): 4102.






























