Evolution using of Natural Pigments on Flesh Quality of Monosex Nile Tilapia (Oreochromis Niloticus) Fed with Supplemented Diets
Abdelrahman S Abouzied1, Mohamed M Toutou2* and Ali A Soliman2
1Fish processing and Technology Laboratory, Fisheries Division, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt
2Fish Nutrition Laboratory, Aquaculture Division, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt
Submission: May 22, 2023; Published: June 19, 2023
*Corresponding author: Mohamed M Toutou, Fish Nutrition Laboratory, Aquaculture Division, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt, Email: mtoutou50@yahoo.com
How to cite this article: bdelrahman S Abouzied, Mohamed M Toutou, Ali A Soliman. Evolution using of Natural Pigments on Flesh Quality of Monosex Nile Tilapia (Oreochromis Niloticus) Fed with Supplemented Diets. Nutri Food Sci Int J. 2023. 12(2): 555832. DOI: 10.19080/NFSIJ.2023.12.555832.
Abstract
A study was conducted to investigate the evolution of natural pigments on flesh quality of monosex Nile tilapia (Oreochromis niloticus) fed with supplemented diets to improve whole body composition, pigmentation, and flesh quality. Monosex O. niloticus (marketable size) were tested with three natural pigments of (shrimp by-product meal (SH), red pepper meal (RP), tomato meal (TM) 2%) compared with the basal diet (Ctr). Fish were stocked in concrete tank (5×2×1 m), at a density of 10 fishes per group and fed on a pelleted diet (∼25% crude protein, CP), 3 times daily for 90 days. Fish quality in terms of whole-body composition showed the best of RP and the lowest of Ctr diet. The results of the present work revealed that body weight, weight gain and specific growth rate for the fish fed diets contained different pigments were significantly raised (p > 0.05) compared to the control group. In general results show that red pepper or shrimp by-product meal can be used as source natural pigments in Oreochromis niloticus diets to ensure good pigmentation, better growth, and feed utilization.
Keywords: Nile Tilapia; Flesh Quality; Pigments; Red Pepper Meal; Shrimp By-Product Meal; Tomato Meal
Abbreviations: NIOF: National Institute of Oceanography and Fisheries; QIM: Quality Index Method; QI: Quality Index; GE: Gross Energy; RP: Red Pepper Meal; SP: Shrimp By-Product Meal; TM: Tomato Meal; Ctr: Compare with the Basal Diet
Introduction
Nile tilapia, Oreochromis niloticus has often been considered unique and exceptional for aquaculture construction. This is because of their motivating aquaculture characteristics, such as extraordinary growth rate, an ability to maintain good growth at high stocking densities, a good struggle to disease, noble food conversion rates and a ready acceptance of artificial feed [1]. Pigmentation is one of the important quality attributes of the fish for consumer acceptability. The pigmentation level of an aquatic animal may be an important factor affecting its market value and may also directly indicate its healthiness and quality [2]. It is well known that pigments are synthesized by algae and some microorganisms, as well as higher plants. Animals, including those of aquatic origin, cannot synthesis these pigments, but can convert them into other forms [3]. As a result, all animals must rely on pigments producing organisms for their requirement [4,5]. Natural and synthetic pigments are used in fish diets to enhance skin and fillet coloration. However, synthetic pigments are expensive and comprise 10-15% of the total feed cost while natural pigments could serve as cheaper alternatives [6,7]. Red pepper Capsicum annum, they are a rich source of vitamins A, C, and strong antioxidants, Capsanthin in pepper stimulates the immune system and helps in attacking infectious agents [8]. Capsanthin and capsorubin, which are 80% of the active ingredients, contribute the red color to pepper, red pepper and its products are commercially used as a spice [9]. Tomatoes and their preserves are good sources of healthy ingredients, especially lycopene and other carotenoids, folate, ascorbic acid, vitamin E, fl avonoids, and potassium, which behave like nutrients, and diverse disease preventing molecules. Lycopene and carotene constitute a major source of carotenoids, and the main red pigment lycopene – represents about 80-90% of the total carotenoid content in a tomato fruit [10]. Coloring agent for human food, and as pharmaceutical ingredient, in animal feeds, Oral administration of red pepper meal, shrimp by-product meal, and astaxanthin in Oncorhynchus mykiss [11, 12], red pepper meal in Labidochromis caeruleus [13], astaxanthin, canthaxanthin, and Gammarus spp. in Carassius auratus [14] and beetroot and marigold in Schizothorax richardsonii [15] was successfully used for coloration. In addition, paprika oleoresin [16,17] and capsicum oleoresin [18] also enhance coloration in fish. The main purpose of this study was to evaluate the effect of different dietary of three natural pigments of (red pepper meal (RP), shrimp by-product meal (SH), tomato meal (TM) 2%) on growth performance, body chemical composition and flesh quality of monosex Nile tilapia (Oreochromis niloticus) fed with supplemented diets.
Material and Method
Experimental design
This experiment was conducted at the Fish Nutrition Laboratory, Baltim Research Station, National Institute of Oceanography and Fisheries (NIOF), Egypt. Monosex O. niloticus adults were purchased from a private fish farm, Kafr El-Sheikh Governorate, Egypt. After acclimation in a concrete tank (5×10×1m) for two weeks and fed diet (25% crude protein) before the start of the experiments. Ten fish with an average weight of 100.8±5.5 g was randomly stocked into each of 12 concrete tanks (5×2×1m) representing four treatments with three replicates per each. Experimental tanks were cleaned and supplied with fresh water and aeration. Water quality parameters including temperature, pH (Jenway Ltd., Model 350-pH-meter) and dissolved oxygen (Jenway Ltd., Model 970-dissolved oxygen meter) were measured weekly. Ambient water temperature, dissolved oxygen and pH through the experimental period were 23.0±3.0oC, 6.8±3.0 mg L-1, and 7.8±0.2, respectively. Fish were fed to satiation the experimental diets twice a day every day at 09.00 and 14.00 h, for 12 weeks. The system used here is flowthrough with 20% daily water exchange. Water quality parameters were monitored on a weekly basis throughout the experimental period using standard APHA methodology (1998) [19].
Experimental diets: All the ingredients of the experimental diets were purchased from the local market at reasonable prices. Three natural pigments of (red pepper meal (RP), shrimp byproduct meal (SP), tomato meal (TM) 2%) compare with the basal diet (Ctr). The diet used in the experiment was formulated to cover all nutrients required for tilapia as recommended by the [20]. Dry ingredients were finely ground by using a Lab-Mill, sieved, and then manually mixed to assure their homogeneity. A basal control diet was formulated to fulfill the nutrients requirement of fish contained 25% CP and 448.3 kcal/100g (Table 1). The basal diet was used as the control, and three other experimental diets. Diets were stored in labeled plastic bags and stored in the freezer at -20°C until used. O. niloticus fingerlings were fed test diets of 5 to 3% of the total biomass of the fish per day, fed 3 times daily for 90 days.
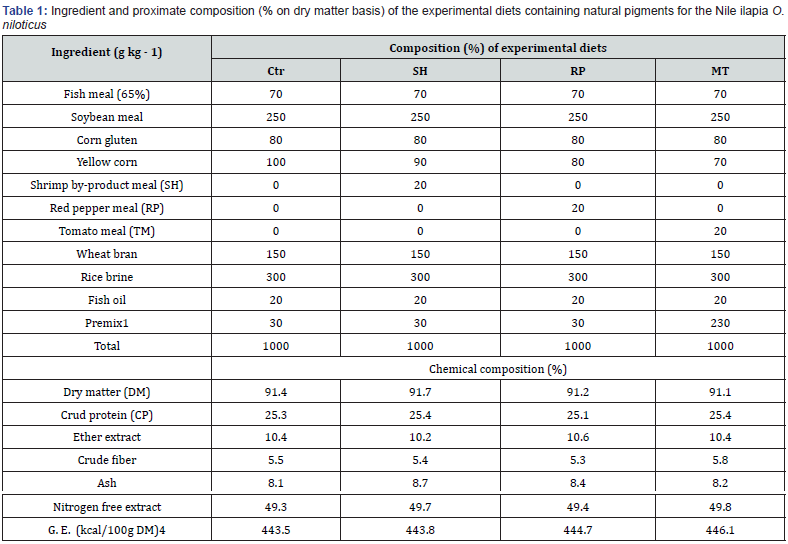
Premix composition
Each 1 kg contains Vit A (400000 i.u.), Vit D3 (100000 i.u.), Vit E (230 mg) Vit K3 (165 mg) Vit B1 (300 mg), Vit B2 ( 80 mg), Vit B6 (200 mg), Vit B12 (1 mg), Vit C (650 mg), Niacin (1000 mg), Methionine (3000 mg), Choline chloride (10000 mg), Folic acid (100 mg), Biotin (2 mg), Pantothenic acid (220 mg), Magnesium sulphate (1000 mg), Copper sulphate (1000 mg), Iron sulphate (330 mg), Zinc sulphate (600 mg), Cobalt sulphate (100 mg), Calcium carbonate up to 1000 g; 4 Gross energy (GE) was calculated as 5.64, 9.44 and 4.11 kcal/100g for protein, lipid and NFE, respectively [20].
Experimental analysis
Feed sample was used for proximate analyses (Table 1) and Fish samples were netted from tank at the period of the feeding trial. They were pooled and homogenized for proximate composition. Moisture, total protein, lipid, and ash contents were all determined by Standard Association of Official Analytical Chemist methodology (AOAC 2007) [21].
Evaluation of growth performance and feed utilization efficiency
Growth performance and feed utilization including weight gain (WG, g), specific growth rate (SGR, % day-1), feed conversion ratio (FCR) and protein efficiency ratio (PER), were determined as follows: WG = (final weight, FW) – (initial weight, IW) (g/fish), SGR = 100 × [(FW)-(IW)] / experimental days, and FCR = feed fed (g) (dry weight) / WG (g). Protein efficiency ratio (PER) = (WGFI), Where Iw and Fw = mean initial and final body weight (g), respectively, Thiobarbituric acid - reactive substances (TBA-RS) value was determined by the method described by [22]. TBA was expressed as mg Malonaldehyde per kg sample.
Sensory evaluation
Sensory evaluation to assess fish quality was performed at the end of experiment. The fish fillets were assessed by a panel of four trained panelists. The samples were evaluated using a 9-point hedonic scale, with 1 being the lowest and 9 the best. Both fresh and cooked samples were assessed. Fresh samples attributes assessed were general appearance and color, while the attributes of cooked samples assessed were texture, aroma, taste, and juiciness.
Statistical analysis
Data were presented as mean±SE. The results were subjected to one-way analysis of variance (ANOVA) to test the effect of treatment inclusion on fish performance. Data were analyzed using SPSS program, Version 16 [23]. Differences between means were compared using Duncan’s (1955) multiple range test at p < 0.05 level.
Results
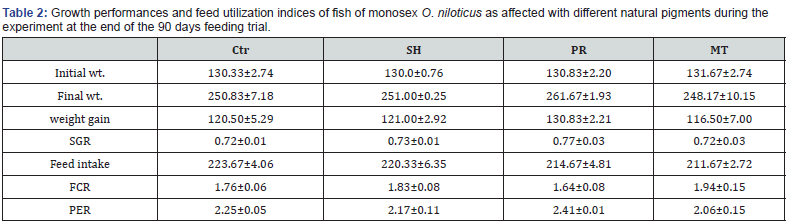
The average initial weight of fish was 130.5±2.3g. Statistical insignificant (p≤0.05) differences in final weight, weight gain and SGR were found among the tested natural pigments compared to the control (Table 2). The obtained data showed that growth performance was insignificantly affected by natural pigments. Fish fed at RP showed the higher final weight (261.67g), weight gain (130.83) and SGR (0.77% day 1). At the end of the feeding assay, feed utilization efficiency indices for all experimental treatments are given in Table 2. It is obvious that the lowest (best) FCR was recorded at RP (1.64) followed by Ctr (1.76), whilst the worst FCR (1.94) was obtained in TM. Also, the best PER (2.41) was recorded in RP dietary group, whereas the lowest value was at TM. The effect of natural pigments on biochemical composition of flesh in the present experiment is illustrated in Table 3. Moisture contents were the best when fish fed RP also ether extract. The highest significant (p≤0.05) ether extract (20.35%) and the lowest significant (p≤0.05) content of crude protein of flesh composition. Ash content showed significant (p≤0.05) differences among the tested natural pigments. The results of TBA-RS values of tilapia fish fillet that was stored for 5 days at 5±0.5°C for the current study are presented in Table 4. Thiobarbituric acid is used as an index to evaluate the degree of lipid oxidation. In this study, the TBA values of SH recorded 0.35±0.01 mg MDA/kg at 0 day of storage. These values increased gradually with prolonged refrigerated storage periods up to 5th day to reach 0.7±0.04 and, it was found that TBA was higher value 2.38±0.01 mg MDA/kg in Ctr.

Means in the same row with different superscript letters are significantly different at (p < 0.05).

The results of sensory evaluation of both raw tilapia samples and fish fillets that were stored for five days at 5 ± 0.5°C for the present study are presented in (Table 5,6). The values are means of riplicate determination ± SD. The sensory evaluation of fish is the using of one or more of the five senses to assess, or form an opinion on, some characteristic of quality. In the present study, the parameters that were used to do the sensory examination are body stiffness, appearance of fish, pupil, odour, gill appearance and gill odour. The mean value of parameters of quality index method (QIM) is the best for RP fish, and it is lowest for Ctr fish indicating that both treatments are at acceptable limits of Quality Index (QI) score. The quality of RP fish is higher than the quality of Ctr fish as the lower values of Quality Index (QI). scores indicate higher quality of fish and the higher values of Quality Index (QI) score indicate lower quality of fish.

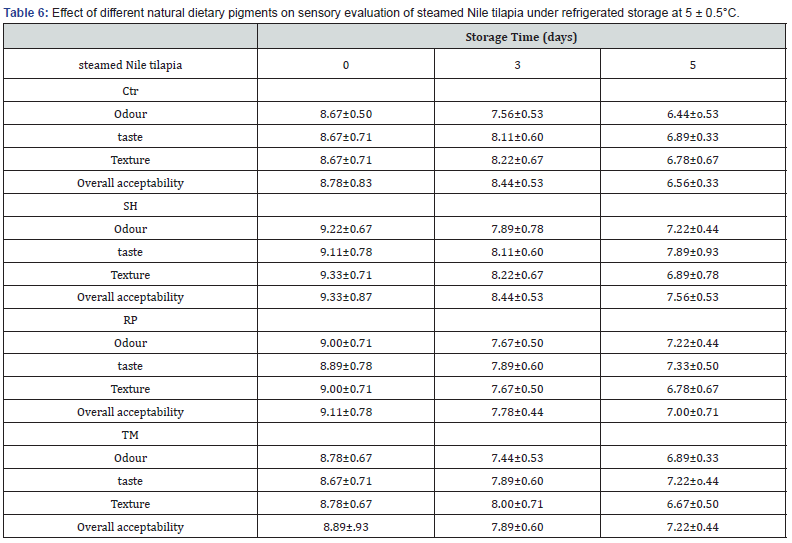
The most important parameters that were used to do the sensory evaluation of fillets of all treatment fish are rancid odour, flesh appearance and flesh consistency. Every parameter has a range of quality grades; SH is the highest grade of quality, Ctr is the sign of poor quality of fillets.
Discussion
Pigmentation is unique of the vital quality qualities of the fish for consumer appropriateness. Natural pigments are responsible for pigmentation of flesh in nutrition fish and skin color in fishes. Red pepper Capsicum annum are inexpensive and abundant natural carotenoid sources. In this study, growth performance was insignificant, although improved in the RP treated. Likewise, 30 to 60 mg/kg paprika improved growth performance of rainbow trout [12]. However, growth was negatively affected by C. annum and its extracts when used in high doses for rainbow trout [24] and Zacco platypus [25]. This was likely due to antinutritional factors such as excessive pungency [25], tannin [26], or saponin [27]. Decreased as the dietary concentration of the carotenoid increased, Similar results were obtained when astaxanthin, C. annum, or its oleoresins were supplemented in diets for Labidochromis caeruleus [13] Zacco platypus [27], and rainbow trout [12,28,17] demonstrating that the accumulation of carotenoids in fish skin lowers lightness and hue. The diets containing carotenoids increased red, yellow, and chroma in the tilapia skin. Likewise, these carotenoids have successfully induced redness and yellowness in skin of koi carp [29], Pagrus auratus [30], Labidochromis caeruleus [13] and goldfish [29]; [17]. Further, chroma was enhanced by the addition of a carotenoid source in red porgy [31]. These studies show how well a much cheaper pigmentation affects skin or fillet color in fish within a short period, enabling the formulation of economically feasible diets. Red or gold tilapia (O. mossambicus) are evaluated by two concepts: a major advantage of red-gold tilapia for aquarium hobbyists is its attractive color while, for consumers, the red color of cultured salmonids, porgy, and snapper raise their market value and consumers often pay a higher price/kg for red tilapia than other tilapias of equivalent weight [32].
Conclusion
Based on the obtained results, the fish fed on natural pigments exhibited the best performance and the best feed utilization indices. This was evidenced by the best PER and the highest energy utilization for those fish. This was supported also by the increase in the flesh quality of monosex O. niloticus fed on RP. It is recommended to add natural pigments to the diets of monosex O. niloticus enhances body composition of fish, feed utilization and fish integrity with increasing economic efficiency.
References
- Ng WK Romano N (2013) A review of the nutrition and feeding management of farmed tilapia throughout the culture cycle. Rev Aquac 5:220-254.
- Chien YH, Jeng SC (1992) Pigmentation of kuruma prawn, Penaeus japonicus Bate, by various pigment sources and levels and feeding regimes. Aquaculture 102: 333-346.
- Goodwin TW (1984) The Biochemistry of Carotenoids. 2nd ed. Chapman and Hall, London 2: 64-96.
- Gupta SK, Jha AK, Pal AK, Venkateshwarlu G (2007) Use of natural carotenoids for pigmentation in fishes. Natural Product Radiance 6(1): 46-49.
- Torrissen OJ (1989) Pigmentation of salmonids: Interactions of astaxanthin and canthaxanthin on pigment deposition in rainbow trout. Aquaculture 79: 363-374.
- Latscha T (1989) The role of astaxanthin in shrimp pigmentation. Advances in Tropical Aquacult. Aquacop Ifremer Actes de Collogue 9: 319-325.
- Buttle LG, Crampton VO, PD Williams (2001) The effect of feed pigment type on flesh pigment deposition and color in farmed Atlantic salmon. Salmo salar L Aquacult Res 32(2):103-111.
- Mobli M, Piraste B (1994) Vegetable Production. Isfahan University of Technology, Iran pp:877.
- Tepiü A, Zekoviü Z, Kraviü S, Mandiü A (2009) Pigment content and fatty acid composition of paprika oleoresins obtained by conventional and supercritical carbon dioxide extraction. CyTA - Journal of Food 7(2): 95-102.
- Shi J, Le Maguer M (2000) Lycopene in tomatoes. Chemical and physical properties are affected by food processing. Crit Rev Food Sci Nutr 40: 1-42.
- Yanar M, Kumlu M, Celik M, Yanar Y, N Tekelioglu (1997) Pigmentation of rainbow trout (Oncorhynchus mykiss) with carotenoids from red pepper. Isr J Aquacult Bamidgeh 49(4): 193-198.
- Diler I, Hossu B, Dilek K, Emre Y, H Sevgili (2005) Effects of natural and synthetic pigments in diets on flesh coloration and growth of rainbow trout (Oncorhynchus mykiss W). Isr J Aquacult Bamidgeh 57(3): 175-184.
- Yılmaz S, S Ergün (2011) Effect of red pepper (Capsicum annuum) on pigmentation of blue streak hap (Labidochromis caeruleus). Isr J Aquacult Bamidgeh 63(5): 1-6.
- Yeşilayer N, Aral O, Karsli Z, Öz M, Karaçuha A, et al. (2011) The effects of different carotenoid sources on skin pigmentation of goldfish (Carassius auratus). Israeli J Aquacult Bamidgeh 63: 1-9
- Jha GN, Sarma D, Qureshi TA, MS Akhtar (2012) Effect of beetroot and marigold flower meal on growth performance, carcass composition and total carotenoids of snow trout (Schizothorax richardsonii). Isr J Aquacult Bamidgeh 64:1-7.
- Scabini V, Fernandez-Palacios H, Robaina L, Kalinowski T, MS Izquierdo (2011) Reproductive performance of gilthead seabream (Sparus aurata L 1758) fed two combined levels of carotenoids from paprika oleoresin and essential fatty acids. Aquacult Nutr 17(3): 304-312.
- Yeşilayer N, M Erdem (2011) Effects of oleoresin paprika (Capsicum annum) and synthetic carotenoids (canthaxantin and astaxanthin) on pigmentation levels and growth in rainbow trout Oncorhynchus mykiss W. J Anim Vet Adv 10(14): 1875-1882.
- Harpaz S, D Padowicz (2007) Color enhancement in the ornamental dwarf cichlid Microgeophagus ramirezi by addition of plant carotenoids to the fish diet. Isr J Aquacult Bamidgeh 59(4):195-200.
- APHA (1998) Standard methods for the examination of water and wastewater, 20th edition. American Public Health Association, Washington DC.
- National Research Council (NRC) (1993) Nutrient Requirements of fish. National Academy Press Washington, DC 1-12.
- Association of Official Analytical Chemists AOAC (2007) Official Methods of Analysis (18th ed) Gaithersburg: Association of Official Analytical chemists.
- Kirk RS, Sawyer R (1991) Pearson’s Composation and Analyses of Foods (9th ed). Jurong: Longman Singabore publishers (Pte) Ltd.
- SPSS (1997) Statistical package for the social sciences, Versions16, SPSS in Ch, Chi-USA.
- Ingle de la Mora G, Arredondo Figueroa JL, Ponce Palafox JT, Barriga-Soca IDA, JE Vernon Carter (2006) Comparison of red chili (Capsicum annum) oleoresin and astaxanthin on rainbow trout (Oncorhynchus mykiss) fillet pigmentation. Aquaculture 258(1-4):487-495.
- Lee CR, Pham MA, SM Lee (2010) Effects of dietary paprika and lipid levels on growth and skin pigmentation of pale chub (Zacco platypus). Asian Aust J Anim Sci 23(6): 724-732.
- Esayas K, Shimelis A, Ashebir F, Negussie R, Tilahun B, et al. (2011) Proximate composition, mineral content and antinutritional factors of some capsicum (Capsicum annum) varieties grown in Ethiopia. Bull Chem Soc Ethiop 25(3): 451-454.
- Rao AV, DM Gurfinkel (2000) Dietary saponins and human health. pp. 255-270. In: W Olezek, A Marston (eds). Saponins in Food, Feedstuffs and Medicinal Plants: Proc. Phytochemical Society of Europe. Kluwer Academic Publ the Netherlands pp: 291.
- Kouakou N’Goran DV, G Choubert (2006) Effect of varying the concentration of dietary astaxanthin on rainbow trout, Oncorhynchus mykiss, muscle pigmentation. J Appl Aquacult 18(2): 61-73.
- Hancz C, Magyary I, Molnar T, Sato S, Horn P, et al. (2003) Evaluation of color intensity enhanced by paprika as feed additive in goldfish and koi carp using computer-assisted image analysis. Fish Sci 69(6): 1158-1161.
- Booth MA, Warner Smith J, Allan GL, BD Glencross (2004) Effects of dietary astaxanthin source and light manipulation on the skin colour of Australian snapper Pagrus auratus (Bloch & Schneider, 1801). Aquacult Res 35(5): 458 464.
- Kalinowski CT, Izquierdo MS, Schuchardt D, LE Robaina (2007) Dietary supplementation time with shrimp shell meal on red porgy (Pagrus pagrus) skin color and carotenoid concentration. Aquaculture 272(1-4): 451-457.
- Lovshin LL (1998) Criteria for Selecting Nile Tilapia and Red Tilapia for Culture. CRSP Research Reports p:13.






























