Comparative Evaluation of Amino Acid Profile of Crayfish Crackers Made from Blend of African Bread Fruit (Treculia Africana) Deine and Varieties of Yellow Root Cassava (Manihot esculenta Cranz)
Oganezi NC*, Uzoaga LN, Okoronkwo CU and Okorie O
Department of Food Science and Technology, Abia State University, Uturu, Nigeria
Submission: May 11, 2023; Published: June 06, 2023
*Corresponding author: Oganezi NC, Department of Food Science and Technology, Abia State University, Uturu, Nigeria. Email: chinonyeremwog@yahoo.com
How to cite this article: Oganezi NC, Uzoaga LN, Okoronkwo CU, Okorie O. Comparative Evaluation of Amino Acid Profile of Crayfish Crackers Made from Blend of African Bread Fruit (Treculia Africana) Deine and Varieties of Yellow Root Cassava (Manihot esculenta Cranz). Nutri Food Sci Int J. 2023. 12(1): 555829. DOI: 10.19080/NFSIJ.2023.12.555829.
Abstract
Crayfish crackers were produced using starch from three varieties of genetically modified high carotenoid cassava roots namely UMUCASS 37, 45 and 46 to which varied concentrations of African breadfruit and 10% crayfish were added. These were subjected to amino acid analysis to evaluate their nutritional profile. commercially sold prawn crackers was used as control. Results indicated that the addition of African breadfruit and 10% crayfish increased the amino acid contents in varied concentrations. The most abundant amino acid was glutamic acid while the most concentrated essential amino acid was leucine. The inclusion of 20 and 30% ABF plus 10% CFE to the respective starch samples from the various yellow root cassava in the production of crayfish crackers yielded snacks with amino acids that can meet with WHO (1991) reference pattern for amino acids. Predicted protein efficiency ratio (P-PER) of the crayfish crackers ranged between 1.34 and 1.67 for samples produced using starch from UMUCASS 37, 1.20 and 1.21 for samples produced using starch from UMUCASS 45 and 1.68 – 2.06 for samples produced using starch from UMUCASS 46. Cracker samples which contained UMUCASS 46 starch had higher P-BV than those which had UMUCASS 37 or UMUCASS 45 starch. The order of nutritional value of starch from the three yellow root cassava starch to produce nutritious snack such as crayfish cracks (i.e., a simulated form of prawn crackers) is UMUCASS 46 > UMUCASS 45 > UMUCASS 37.A higher concentration of >20% African breadfruit improved the amino acids content of the crayfish crackers.
Keywords: Crayfish Crackers; Yellow Root Cassava; African Breadfruit; Prawn Crackers; Predicted Protein Efficiency Ratio; Snack; Predicted Biological Value
Introduction
Snack products are defined as a broad range of light meal products that are eaten between regular meals [1]. Snacks are increasing in popularity due to their flavor, convenience and change of food habits [2]. All over the world, adults consume energy aside from traditional meals such as breakfast, lunch, and dinner [3]. Concerns about diet-health relationships have led many people to include healthier snacks in their diets including those with functional as well as probiotic properties [4]. Snacking has its purported advantages and disadvantages, but this depends on the nutritional needs and physiological state of an individual.
Micronutrient malnutrition is a global health challenge affecting almost half of the global population, causing poor physical and mental development in children, and resulting in a range of illnesses [5]. Snacking may affect micronutrient intake such as vitamin A and minerals such as iron, zinc, iodine, and selenium resulting in a phenomenon known as hidden hunger. Malnutrition is most prevalent in young girls, women and pre-school children who are suffering particularly from low consumption of vitamins and micronutrients [5]. However, the motivation to snack depends on factors such as hunger [6], location [7], social food culture and environment [6], cognitive factors [8] and hedonic eating [9].
Snacks produced by different processes are available in the market (Riaz, 2006). Social norms emphasize healthful eating which will probably elevate the intake of nutrient rich snacks [10]. A combination of staple foods with varied concentration of nutrients is one avenue of developing snacks which supply both macro and micro-nutrients which inadvertently can curb nutritional deficiencies. In the wake of product development using different food sources to generate snack foods, there is need to evaluate nutrients such as protein quality in such formulations. It was on this basis that an analogue of prawn crackers that is crayfish crackers were produced using blends of Treculiar africana (i.e., African breadfruit) and varieties of yellow root cassava to which crayfish stock was added were evaluated for protein quality..
The Nigerian crayfish (Procambarus clarkii) also known as ‘crawfish’, ‘crawdad’ is freshwater crustacean resembling lobster and taxonomically are members of the super families Astacoideae and Parasitacoideae. It is a good source of easily digestible highquality protein, has low fat but contains essential fatty acids namely omega-3 and omega-6 [11]. Crayfish has been reported to have high nutritional value such as having high total unsaturated fatty acids, fatty acids, high quality protein for which 55.703% are essential amino acids & minerals [12]. Treculiar africana (African bread fruit) belongs to the family Moraceae and it grows in every green and deciduous forest in many parts of West and Tropical Africa [13]. Treculiar africana seeds are eaten in various forms such as roasted seeds eaten with either palm kernel or coconut meat; the dehulled seeds can be boiled to a soft consistency and eaten as porridge or mixed with other cereals or tubers such as yam or shredded cassava [14]. Cassava is a carbohydrate rich tuber crop with 64 to 72% starch which has a very different starch structure in terms of branch length, distribution, amylose content and granular structure when compared with those found in cereals [15]. The high rate of vitamin A and other micronutrients deficiency in many parts of African especially sub-Saharan Africa has resulted to research in seeking avenues of improving the quality of commonly consumed foods among different human populations through biofortification using genetic engineering. Genetically engineered pro-vitamin A cassava has been developed to tackle vitamin a deficiency affecting a good number of the subsaharan African population [16]. A good number of yellow cassava roots with varied provitamin A activity has been developed. UMUCASS 36, 37, 38 were the first batch of yellow root cassava developed and have pro-vitamin A content between 6-8ppm while a second batch with an average of 10ppm pro-vitamin A content namely UMUCASS 44, 45 and 46 were second in the series [17]. It is envisaged that the utilization of these improved yellow root cassava in combination with other indigenous foods products will help curb hidden hunger occasioned by Vitamin A deficiency and other nutrient deficiencies.
Materials and Methods
Source of Raw materials: Yellow flesh cassava varieties namely UMUCASS 37 (TMS01/1412), UMUCASS 45 (TMS 01/0539) and UMUCASS 46 (TMS 01/0593) were sourced from the National Root Crops Research Institute, Umudike. African breadfruit (Telferiar africana) seeds, crayfish, salt, and mixed spices were bought from a local market in Umuahia North Local Government area of Abia State, Nigeria.

Production of cassava starch concentrate, cassava starch concentrate was produced by the method described by [18]. This can be shown in the flow chart as Figure 1.
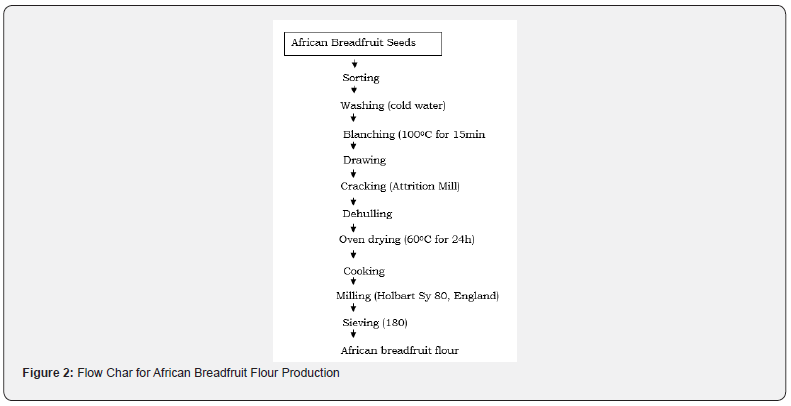
Production of African breadfruit (Treculiar Africana) flour: African bread fruit seed. This can be shown in the flow chart as Figure 2.
Production of crayfish stock
Crayfish stock was prepared by dispensing 200ml water into 500g ground crayfish in a stainless-steel pot. These were put to boil until the water reduced to half of its original volume. The stock solution was seived to get crayfish stock solution.
Blending formula of crayfish, cassava starch concentrates, African bread fruit flour with mixed spices
Table 1 shows the ratio of blends of cassava starch concentrate, crayfish stock African breadfruit and mixed spices for the different crayfish cracker samples. They were mixed in 150m of water to form a malleable dough. After mixing to get a malleable dough, each dough sample was formed into cylindrical rolls of 5mm diameter. After which, they were steam cooked for 1h. The steam cooked rolls were allowed and then wrapped with cling film before refrigerating for 12h to set or firm up for easy slicing. A serrated knife was used to cut the rolls into 1mm thickness and subsequently dried in a hot air oven (Gallenkamp, England) at 70°C for 3h. Each crayfish cracker sample was fried in hot oil for them to puff up and subsequently drained off oil by blotting using kitchen paper before storing in labeled airtight containers.
Evaluation of Amino acid content. Amino acid profile was done by Ion Exchange chromatography using the methods described by (Benitez, 1989) while tryptophan was determined by the method described by (Robel,1967) using Technical Sequential Multi sample (TSM) amino acid analyser (Technicon Instruments cooperation, New York, USA). Two grams of each cracker sample was hydrolyzed using Sulphuric acid. Subsequently, 10 μl of the hydrolyzed sample was loaded into the cartridge of the TSM amino acid analyzer respectively. The net height of each peak produced on the chart record of the TSM amino acid analyzer (each representing an amino acid) was measured and calculated. Tryptophan was hydrolyzed after de-fatting 2 g of the respective cracker sample using chloroform/ methanol mixture (1:1). Ten (10) ml of 4.2M NaOH was added to the defatted sample which degraded all the other amino acids except tryptophan. Oxidation of tryptophan was inhibited by the addition of drops of thioglucollic acid to the mixture in the glass ampoule. Alkaline pH was maintained using 5 ml acetate buffer (pH8.0). 5 μl of each hydrolyzed cracker sample was dispensed into the cartridge of the TSM amino acid analyzer respectively. The net height of the peak produced on the chart record of TSM amino acid analyzer (representing tryptophan) was measured and calculated. Norleucine was used as an internal standard. Amino acid values from the chromatogram peaks were calculated whereby half height of each peak on the chart was found and width of the peak on the half height was accurately measured and recorded. Area of each peak was then obtained by multiplying the height by the width at half height the Norleucine equivalent (NE) for each amino acid in the mixture. Norleucine equivalent (NE) for each amino acid in the standard mixture was calculated using the formula.
NE = (Area of Norleucine Peak)/ (Area of each amino acid)
A constant S was calculated for each amino acid in the standard mixture:
Where Sstd = NEstd x Molecular weight x μMAAstd
Finally, the amount of each amino acid present in each sample was calculated in g/16gN or g/100g protein using the following formula:
Concentration (g/100g protein) = NH x W@NH/2 x Sstd x C
Where C = ((Dilution X 16)/ (Sample Wt (g) X N% X 10 X Vol.loaded)) ÷ NH x W (nleu)
Where: NH = Net height
W = Width @ half height
nleu = Norleucine
Protein quality indices were determined based on the amino acid content of the respective cracker sample.
Determination of protein Quality Indices
Estimation of Amino Acid score and predicted protein Efficiency Ratio (p-PER): Amino acid scores of the respective cracker samples were determined based on whole Hen’s egg (Paul et al., 1976). Essential amino acids were grouped namely methionine+cysteine and phenylalanine+tyrosine was taken as two distinct units. Amino acid scores (AMSS) were estimated by using the formula stated by (FAO/UN/WHO, 1991) as:
AMSS= …………………………….1
Determination of Predicted Protein Efficiency Ratio (P-PER)
The predicted protein efficiency ratio (P-PER) was calculated from the amino acid composition using the equation by (Alsmeyer et al., 1974).
P-PER = - 0.468 + 0.454 (Leu) – 0.105 (Tyr)...................................2
Essential Amino Acid Index (EAAI)
The essential amino acid index was calculated using the equation stated by (Oser, 1959) as:
EAA Index .........................3
Where P = food protein, S = standard protein (whole egg), n= number of amino acids (counting pairs such as methionine and cysteine as one).
Determination of Biological value (BV)
The biological value (BV) of the respective seed spices were calculated using the equation stated by (Oser, 1959).
Biological Value = 1.09 (EAAI) – 11:7................................................ .............4
Results and Discussion
Table1 shows results of the amino acid composition of crayfish cracker samples made from blends of crayfish stock (CFE), African breadfruit flour (ABF) and starch from varieties of yellow root cassava (i.e provitamin A cassava roots) (UMUCASS 37,45, 46). Results indicated the presence of varied concentrations of amino acids in the various samples. The addition of African breadfruit (ABF) and crayfish stock resulted to a gradual increase in amino acid content of the various cracker samples. All the cracker samples were sufficient in leucine and tyrosine based on FAO 1991 reference pattern for amino acids which is 1.10g/100g protein for both amino acids but varied for the other amino acids. The inclusion of African breadfruit (ABF) resulted to a slight loss in the respective amino acids in the ratio of 10 ABF: 80 UMUCASS: 10CFE when compared with OABF: 90UMUCASS: 10 CFE. Blends of 20 ABF: 70 UMUCASS: 10 CFE and 30 ABF: 60 UMUCASS: 10CFE resulted in a gradual increase in amino acid content in the respective crayfish cracker blends. The most abundant amino acid in all the crayfish cracker blends was glutamic acid. Glutamic acid ranged between 6.20g/100g protein for sample I (0ABF:90 UMUCASS 37:10CFE) and 17.33g/100g protein for sample IX (0ABF: 90UMUCASS 46:10 CFE) while the commercially sold prawn cracker used as control had glutamic acid content of 12.87/100g. The least concentrated amino acid was cysteine and it ranged between 0.36 100g protein for samples II, VI and VII (10ABF: 80UMUCASS37:10CFE; 10ABF:80UMUCASS45:10CFE and 20ABF:70UMUCASS45:10CFE) respectively and 0.90g/100g protein for sample XII (30ABF:60UMUCASS46:10CFE). The commercially sold prawn cracker had cysteine content of 0.97g/100g protein. Glutamic acid is reported to be the predomimant dietary amino acid especially in vegetable protein [19]. Dietary glutamic acid may be the main component accounting for what was reported to have an inverse inhibition of blood pressure, hence dietary glutamic acid has a favorable effect on blood pressure [19].
The most abundant essential amino acid in the blends of the protein crackers was leucine. It ranged between 3.99g/100g protein for sample V (0AFB:90UMUCASS45:10CFE) and 6.59g/100g protein for sample XII (30ABF: 60UMUCASS 46:10 CFE). Tryptohan was the least concentrated amino acid in the various blends of the crayfish crackers, and it ranged between 0.63 g/100g protein for sample II (10AFB:80UMUCASS 37:10CFE) and 1.02g/100g protein for sample XII (30AFB:60UMUCASS46:10CFE). Cysteine content of the commercially sold prawn cracker was 0.84g/100g protein. Crayfish crackers of samples X, XI and XII (i.e blends of 10AFB:80UMUCASS46:10CFE; 20AFB:70UMUCASS46:10CFE and 30ABF:60UMUCASS 46:10 CFE) respectively, had higher tryptophan content than the tryptophan content of the commercially sold prawn cracker. The abundance of essential amnio acids in the crayfish cracker blends was in this order. leucine > lysine> valine > isoleucine >phenylalanine> threonine histidine> methionine> tryptophan. Leucine is a branched chain amino acid and has been reported to regulate several cellular processes such as protein synthesis, tissue regeneration and metabolism such as glucose control through improved insulin sensitivity on tissues namely the liver and skeletal muscle; it can change the secretion pattern of adipokines in the adipose tissue to a more favorable profile [20].
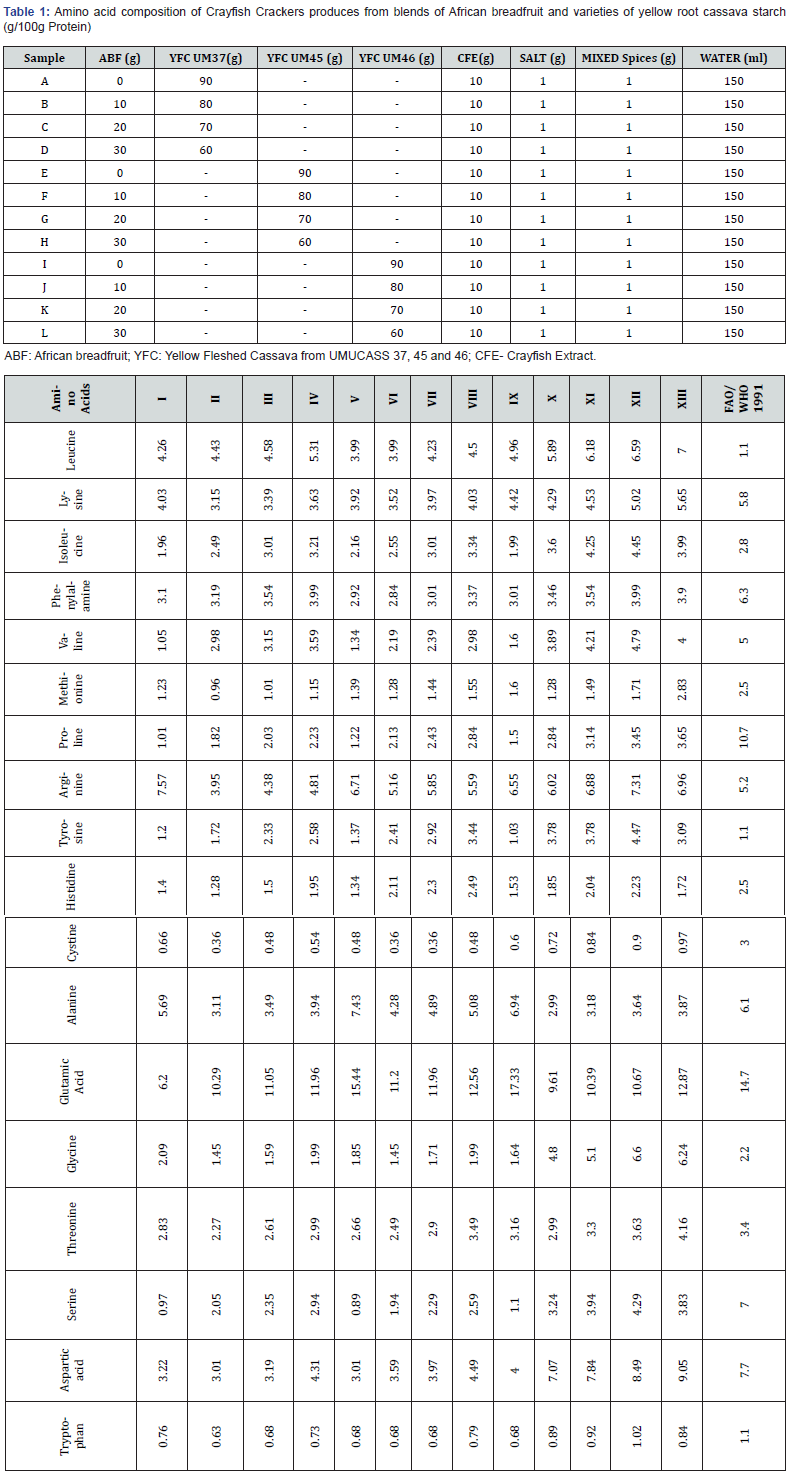
I = 0%ABF, 90%UMUCASS37, 10%CFE; II = 10%ABF, 80%UMUCASS37, 10%CFE; III =20%ABF, 70%UMUCASS37, 10%CFE; IV = 30%ABF, 60%UMUCASS37, 10%CFE; V = 0%ABF, 90%UMUCASS45, 10%CFE; VI = 10%ABF, 80%UMUCASS45, 10%CFE; VII = 20%ABF, 70%UMUCASS45, 10%CFE; VIII = 30%ABF, 60%UMUCASS45, 10%CFE; IX = 0%ABF, 90%UMUCASS46, 10%CFE; X = 10%ABF, 80%UMUCASS46, 10%CFE; XI = 20%ABF, 70%UMUCASS46, 10%CFE; XII= 30%ABF, 60%UMUCASS46, 10%CFE; XIII = Commercially sold prawn crackers (Dietking). ABF = African Breadfruit (Treculiaafricana), UMUCASS 37,45,46 = Genetically modified yellow fleshed high carotenoid cassava starch, CFE= Crayfish Extract
Some amino acids in the crayfish cracker blends decreased with substitution using African bread fruit especially in blends made from starch of UMUCASS 37 and 45 and a few from blends added to starch from UMUCASS 46. The amino acids which gradually increased with the inclusion of ABF at 10, 20 and 30% were leucine, isoleucine, phenylalanine, valine, proline, typrosine and senine. However, the inclusion of 30% ABF plus 10% CFE to cassava starch from the respective high carotenoid cassava varieties resulted in an increase in the amino acid content of the crayfish cracker samples. Some amino acids decreased slightly following the inclusion of 10 and 20% ABF to the cassava starch and 10% CFE when compared with crackers produced from blends without ABF i.e samples I, V and IX but contained 10% CFE. These amino acids include lysine, isoleucine, methionine, arginine, cystrine, alanine, glycine, threonine, aspartic acid, and tryptophan. The inclusion of 20 and 30% ABF plus 10% CFE to the respective starch samples from the various yellow root cassava in the production of crayfish crackers yielded snacks with amino acids that can meet with WHO (1991) reference pattern for amino acids. However, crayfish crackers produced from blends of varied concentrations of ABF (10 to 30%) plus 10% CFE added to starch from UMUCASS 46 had higher amino acids namely leucine, isoleucine, arginine, tyrosine, and glycine that can meet with WHO (1991) reference pattern for amino acids than those of UMUCASS 37 and 45.
Table 2 shows result on variations in concentration of amino acid of crayfish crackers made from blends of African Breadfruit and varieties of yellow root cassava starch compared with commercially sold prawn crackers. Results indicated that glycine had the highest variation with a value of 65.38% while phenylalanine had the least value of 12.17% compared with the amino acids of commercially sold prawn crackers. Considering the coefficient of variation of essential amino acids between the control (i.e commercially sold prawn crackers) and the test samples (i.e., cracker from blends of crayfish/African bread fruit/yellow root cassava starch), results indicated that the coefficient of variation was highest for valine (39.80%) and lowest for phenylalanine (12.17%). The order of variation in essential amino acid content between the crayfish cracker samples and control sample was in this order: valine > methionine > arginine > isoleucine > histadine > leucine > threonine > lysine > tryptophan > phenylalanine. This indicated that there were wide differences in some amino acids of the test’s samples and the control.

I = 0%ABF:90%UMUCASS37:10%CFE; II = 10%ABF, 80%UMUCASS37, 10%CFE; III =20%ABF, 70%UMUCASS37, 10%CFE; IV = 30%ABF, 60%UMUCASS37, 10%CFE; V = 0%ABF, 90%UMUCASS45, 10%CFE; VI = 10%ABF, 80%UMUCASS45, 10%CFE; VII = 20%ABF, 70%UMUCASS45, 10%CFE; VIII = 30%ABF, 60%UMUCASS45, 10%CFE; IX = 0%ABF,90%UMUCASS46, 10%CFE; X = 10%ABF, 80%UMUCASS46, 10%CFE; XI = 20%ABF, 70%UMUCASS46, 10%CFE; XII= 30%ABF, 60%UMUCASS46, 10%CFE.
Amino acid classes predicted protein efficiency ratio, essential amino acid index and biological value of the crayfish cracker samples and commercially sold prawn crackers is shown in Table 3. The total amino acid concentration of the test samples ranged between 49.23g/100g protein and 83.25g/100g protein while the commercially sold prawn cracker had a total amino acid content of 84.62 g/100g protein. It was observed that cracker samples from blends of UMUCASS 37 cassava starch plus varied concentrations of African breadfruit (AFB) and 10% crayfish extract (CFE) had lower total amino acid content than cracker samples from blends of cassava starch from either UMUCASS 45 and UMUCASS 46 plus varied concentrations of African breadfruit and 10% crayfish extract. It was also observed that inclusion of 10%, 20% and 30% African bread fruit resulted to a gradual increase in total amino acids except those of sample II (10AFB:80 UMUCASS 37:10CFE) and sample VI (10 AFB: 80UMUCASS 45: 10CFE) which had values of 49.14 and 54.17g/100g protein respectively. It can be deduced that total amino acid content clearly increased with the inclusion of 20% and 30% plus 10% CFE to starch from UMUCASS 37 and 45. The inclusion of 10% to 30% AFB plus 10% CFE to starch of UMUCASS 46 resulted to higher total amino acids content of crayfish cracker samples than those from UMUCASS 37 and 45. Total amino acids content of the control (i.e commercially sold prawn crackers) was 84.62g/100g protein.
Total non-essential acid content of crayfish crackers ranged between 21.04 and 42.51g/100g protein while the control had a value of 43.57g/100g protein. The total essential amino acid content of crayfish crackers with and without histidine increased gradually with the inclusion of AFB to the respective starch samples from the yellow root cassava starches used in the production of crayfish crackers. Total essential amino acids of crayfish crackers from the blends of 30AFB:60UMUCASS 46:10 CFE (i.e., sample XII) compared well with that of the control. Generally, the addition of varied concentrations of AFB plus 10% CFE to starch of UMUCASS 46 used in the production of crayfish crackers resulted to higher essential amino acid content than essential amino acids of crayfish crackers from UMUCASS 37 and 45 starch blends. Essential aliphatic amino acids (EAAA) ranged between 10.10 and 19.46g/100g protein. With a similar inclusion of varied concentrations of AFB plus 10% CFE to the respective starch from the yellow root cassava, the order of concentration of EAAA in the crayfish cracker was in this order: EAAA of crayfish crackers from UMUCASS 46> EAAA of crayfish crackers from UMUCASS 37 > EAAA of crayfish crackers from UMUCASS 45. Aliphatic amino acids have large hydrophobic side chains and are regarded as branched chain amino acids (BCAAS) [21]. They consist mainly of leucine, isoleucine, and valine. Their molecules are rigid, and their mutual hydrophobic interactions are important for correct folding of proteins as this chain tends to be in the protein molecule [21]. Total acidic amino acids, total basic amino acids and total sulfur amino acids ranged between 9.42 and 19.16g/100g, 8.38 and 14.56g/100g protein and 1.32 and 2.61g/100g protein respectively. Crayfish crackers produced using blends of cassava starch from UMUCASS 46 plus AFB and CFE had higher total acidic, basic and sulfur amino acids than crackers from blends containing starch from UMUCASS 37 and 45. Total acidic basic and sulfur amino acids of the control was 21.92. 14.33 and 3.80g/100g protein respectively. Although the concentration of cysteine in the test samples were lower than that of the control, results indicated that those of samples X (10AFB:80UMUCASS 46:10CFE) and sample XI (20AFB:70 UMUCASS 46:10CFE) had higher cysteine content that can spare methionine. Sulfur amino acids provide sulfur for sulfation reactions in the body while cysteine has sparing effect on methionine. [22]. Nutritionally, methionine alone is capable of supplying sulfur required by the body excluding supplying sulfur for sulfur containing vitamins, Thiamin, and biotin [22].
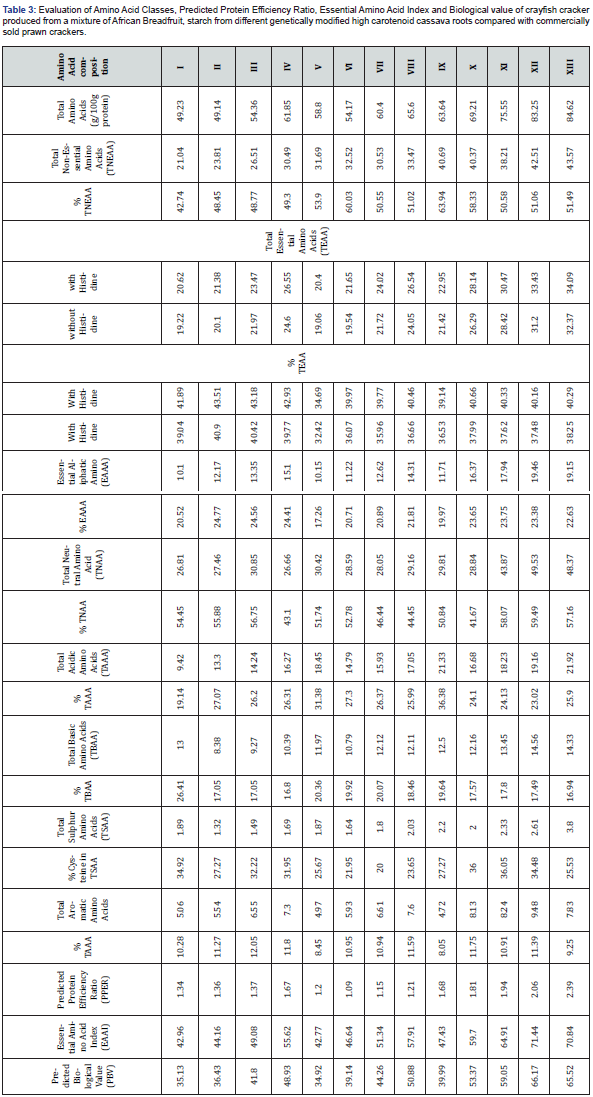
I=0%ABF:90%UMUCASS37:10%CFE; II = 10%ABF, 80%UMUCASS37, 10%CFE; III =20%ABF, 70%UMUCASS37, 10%CFE; IV = 30%ABF, 60%UMUCASS37, 10%CFE; V = 0%ABF, 90%UMUCASS45, 10%CFE; VI = 10%ABF, 80%UMUCASS45, 10%CFE; VII = 20%ABF, 70%UMUCASS45, 10%CFE; VIII = 30%ABF, 60%UMUCASS45, 10%CFE; IX = 0%ABF,90%UMUCASS46, 10%CFE; X = 10%ABF, 80%UMUCASS46, 10%CFE; XI = 20%ABF, 70%UMUCASS46, 10%CFE; XII= 30%ABF, 60%UMUCASS46, 10%CFE.
Predicated protein efficiency ratio (P-PER) is one of the quality parameters used for protein evaluation (FAO/WHO/UN, 1991). Predicted protein efficiency ratio (P-PER) of the crayfish crackers ranged between 1.34 and 1.67 for samples produced using starch from UMUCASS 37, 1.20 and 1.21 for samples produced using starch from UMUCASS 45 and 1.68-2.06 for samples produced using starch from UMUCASS 46. The addition of AFB plus CFE enhanced the P-PER of crackers produced using starch from blends of cassava starch from the respective yellow root cassava varieties. The control had a P-PER of 2.39. [23] reported that P-PER is influenced by leucine and a leucine content above 5.00 will result in an appreciable P-PER value. Much of protein’s benefit synthesis, it helps turn on the body’s switch to build muscle and spare muscle when dieting [24]. A protein efficiency ratio below 1.5 appropriately describes a protein of low or poor quality [25].
Crayfish/Prawn crackers are basically carbohydrate snack foods. The inclusion of proteinous food sources such as the African breadfruit and crayfish improved its nutritional value. It can be deduced that samples IV, IX, X, XI and XII had P-PER above 1.50 and are not very poor in proteins. It is worthy of note that sample XII (30AFB:60UMUCASS 46:10CFE) had a P-PER of 2.06 which compared well with the control i.e commercially sold prawn crackers which had a P-PER of 2.39.
The essential amino acid index (EAAI) of the crayfish crackers ranged between 42.96 and 71.44 while the control had a value of 70.84. Crayfish cracker samples which had a value of 50% and above were samples IV (30AFB:60UMUCASS 37:10CFE), VII (20AFB:70UMUCASS 45:10CFE), VIII (30AFB:60 UMUCASS 45.90CFE), X (10AFB:80UMUCASS 46:10CFE), XI (20AFB:70 UMUCASS 46:10CFE) and XII (30AFB:60 UMUCASS 46:10 CFE) and they had values of 55.62, 51.34, 57.41, 59.70, 64.90 and 71.44 respectively . Sample XII had a comparable EAAI with that of the control. Predicted. Biological value (P-BV) is an index of the amount of nitrogen from a food that is absorbed by the body. The predicted biological value of the crayfish cracker samples ranged between 34.92 and 66.17 while the control had a value of 65.52. Cracker samples which contained UMUCASS 46 starch had higher P-BV than those which had UMUCASS 37 or UMUCASS 45 starch.
The essential amino acid scores (EAAS) based on provisional amino acid scoring pattern for crayfish crackers made from blends containing varieties of yellow root cassava is shown in Table 4. Crayfish crackers produced from blends containing UMUCASS 37 and UMUCSS 45 yellow root cassava starch were all insufficient in all the essential amino acids except blends which contained 30% AFB plus 10% CFE which showed sufficiency in phenylalanine + tyrosine. Results indicated that some crayfish cracker blends containing UMUCASS 46 yellow root cassava starch were sufficient in some of the essential amino acids. The incorporation of 10 to 30% AFB to starch of UMUCASS 46 yellow root cassava resulted in samples sufficient in phenylalanine + tyrosine (i.e samples X, XI and XII) with values of 1.21, 1.22 and 1.41 respectively. Sample XI was sufficient in isoleucine and phenylalanine + tyrosine with values of 1.06 and 1.22 respectively while sample XII was in is sufficient isoleucine, phenylalanine + tyrosine and tryptophan with values of 1.11, 1.41 and 1.02 respectively. The commercially sold prawn crackers i.e., control was sufficient in all the essential amino acids except isoleucine, tryptophan, and valine.

EAA-Essential Amino Acid, PAAESPa-Provisional Amino Acid (egg) scoring pattern, EAACM EAAC-Essential Amino Acid concentration, AASAmino Acid Scores, I = 0%ABF:90%UMUCASS37:10%CFE; II = 10%ABF, 80%UMUCASS37, 10%CFE; III =20%ABF, 70%UMUCASS37, 10%CFE; IV = 30%ABF, 60%UMUCASS37, 10%CFE; V = 0%ABF, 90%UMUCASS45, 10%CFE; VI = 10%ABF, 80%UMUCASS45, 10%CFE; VII = 20%ABF, 70%UMUCASS45, 10%CFE; VIII = 30%ABF, 60%UMUCASS45, 10%CFE; IX = 0%ABF,90%UMUCASS46, 10%CFE; X = 10%ABF, 80%UMUCASS46, 10%CFE; XI = 20%ABF, 70%UMUCASS46, 10%CFE; XII= 30%ABF, 60%UMUCASS46, 10%CFE.
Blends of yellow cassava root which did not contain African breadfruit namely samples I, V and IX were grossly insufficient in valine and isoleucine. [26] reported that essential amino acids acting in limiting capacity are lysine, methiomine + cysteine, threonine, and tryptophan. All the crayfish cracker from blends of AFB, starch from high carotenoid vitamin A yellow root cassava and 10% crayfish extract were all insufficient in these limiting amino acids except sample XII which was only sufficient in tryptophan and its EAAC compared favorably with the commercially sold prawn crackers [27,28].
Conclusion
It can be deduced from this research that the inclusion of African bread fruit and 10% crayfish extract to starch from high carotenoid cassava varieties improved the amino acids content in varied degrees. The order of nutritional value of starch from the three yellow root cassava starch to produce nutritious snack such as crayfish cracks (i.e a simulated form of prawn crackers) is UMUCASS 46 > UMUCASS 45 > UMUCASS 37. A higher concentration of >20% African breadfruit improved the amino acids content of the crayfish crackers.
References
- Ozturt S, Mutlu S (2019) Physiochemical properties, modifications, and applications of resistant starches: chemical, Technological and Health properties. Straches for Food Application 297-332.
- Serna-Saldivar SO (2016) Snacks foods: Types and composition. In laballero, B, Finglas, PM and Toldra F Encydopedia of Food and Health. The new trend is the development of nutrition and health-promoting snacks.
- Hess JM, Jonnalagadda SS, Slavin JL (2016) What is snacks? A review of the definitions of snacking, motivations to snack, contributions to dietary intake and recommendations for improvement. Advances in Nutrition 7 (3): 466-475.
- Niro CM, De Medeiros A, Bresolin JD Dionisio AP (2022) Banana leathers as influenced by polysaccharide matrix and probiotic bacteria. Food Hydrocholoids for Health
- Kiran A, Wakeel A, Mahmood K, Mubaraka R, Hafsa, et al. (2022) Biofortification of staple crops to alleviale human malnutrition: contributions and potential in developing countries. Agronomy 12(2): 452.
- Bellisle F (2014) Meals and snacking, diet quality and energy balance. Physiological behaviour 134: 38-43.
- Marshal D, Bell R (2003) Meal construction exploring the relationship between eating occasion and location. Food Quality references 14(1): 53-64.
- Robinson E, Aveyard P, Daley A, Kolly K, Lewis A, et al. (2013) Eating attentively: a systematic review and meta-analysis of the effect of food intake memory and awareness on eating. American Journal of Clinical Nutrition 97(4):728-742.
- Hennegan JM, Loxton, NJ, Maltar A (2013) Great expectations eating expectances as mediators of reinforcement sensitivity and eating. Appetite 71:81-88.
- Njike VV, Garvi TM, Shuval O, Shuval, Edshteyn, I, et al. (2016) Snack food, satiety, and weight. Advances in Nutrition 7(5): 866-878.
- Simonyan JB (2016) Comparative analysis of crayfish marketing in selected markets of Akwa Ibom and Abia States, Nigeria Pg 35-41.
- Farrag MMS, El-Geddawy M-A MA, Agmed ZSA (2022) more evidence for the nutritional quality and future exploitation of the invasive crayfish Proambarus clarki (Girard, 1852) from the River Nile, Egypt. The Egyptian Journal of Aquatic Research 48(2): 151-156.
- Oscabor VN, Ogar DA, Okafor PC, Egbang GE (2009) profile of the African breadfruit (Treculia Africana). Pakistan Journal of Nutrition 8(9): 1005-1008.
- Ojimelukwe PC, Ugwuona FC (2021) The traditional and medicinal use of African breadfruit (Treculia Africana Deine) an underutilized ethnic food of ibotribe of southeast Nigeria. Journal of Ethnic Foods 8 (21): 1-13.
- Odemelam CS, Perceived B, Ahmad Z, Chang MW, Scholey D, et al. (2020) Characterization of yellow root cassava and food products: Investigation of cyanotic and B carotene concentrations. Binc Research Notes, 13: 1-7
- Ayetigbo O, Latif S, Abass A, Muller J (2018) Comparing characteristics of root flour and starch of biofortified yellow flesh and white flesh cassava variants and sustainability considerations: A review. Sustainability 10:3089.
- International Institute of Tropical Agriculture ITA (2014) Nigeria releases more cassava with higher provitamin A to fight micronutrient deficiency.
- Onaboly A, Abass A, Bokanga Mand Olalunbosun Y (1998) New Food products from cassava. International institute of Tropical Agriculture, Ibadan, Nigeria.
- Stamler J, Brwon IJ, Daviglus ML, Chan Q, Kesteloot H, et al. (2009) Gutainic acid, The main dietary amino acid and blood pressure: The INTERMAP study. Circulation 120(3): 331-228.
- Pedroso JAB, Zamplen TT, Donato J (2015) Reviewing the effects of L-Leucine supplementation in the regulation of food intake, energy balance and glucose homeostasis. Nutrients 7(5): 3914-3937.
- Amachi NL, Oluagha WE (2010) Effect of boiling on amino acid composition of jackfruit (Artocarpus heterophyllus) seed from southeast Nigeria. Journal of Advances in Food science and technology 3(4): 1755-181.
- Fukagawa NK, Yu YM, Young VR (1998) Methionine and cysteine kinetics at different intakes of methionine and cysteine in elderly men and woman. American Journal of Clinical Nutrition 68: 330-338.
- Amaechi NC, Njoku BS, Adiele AM (2015) Evaluation of amino acids profile of cridoscolus acontifolius and ceiba pentandia leaves grown in South-Eastern Nigerian. International Journal of Applied Research and Technology 4 (6): 88-95.
- Volek JS (2006) How do you need much of this branched chain amino acid to get results? Health and wellness News.
- Friedman M (1996) Nutritional value of protein from different food sources: A review. Journal of Agriculture and Food Chemistry 44 (1): 6-29.
- Bingham S (1977) Dictionary of nutrition. Barrie and Jenkins, London 78-28.
- Belschant AA, Lyon CK, Kohler GO (1975) Sunflower, safflower, seasane and castor protein. In: Pine, N.W (Ed). Food Protein Sources. University Press Cambridge UK 79-104.
- Food and Agricultural organization of the United Nations (FAO/UN): World Health Organization (WHO) (1991) Protein quality evaluation. Report of FAO/WHO expert consultation. FAO Food and Nutrition Paper, 51, FAO Rome 19-21:180.






























