Identification And Dosage of Caffeine by Liquid Chromatography UV-Visible in Energy Drinks Marketed in Benin Republic
Yemoa Achille1*, Agbokponto Janvier Engelbert1, Assanhou Assogba Gabin2, Mizehoun-Adissoda Carmelle3, Garba Sabiratou1 and Doffon Parfait1
1Laboratory of Analytical Chemistry and Drug Analysis (LCAM), Bénin
2Laboratory of Galenic Pharmacy and Pharmaceutical Technology, Bénin
3School of Nutrition, Bénin
Submission: September 30, 2022; Published: November 09, 2022
*Corresponding author: Yemoa A, Université d'Abomey Calavi, Faculté des Sciences de Santé de Cotonou, Laboratoire de Chimie Analytique et Analyse des Médicaments (LCAM), Cotonou, Bénin
How to cite this article: Yemoa Achille, Agbokponto Janvier Engelbert, Assanhou Assogba Gabin, Mizehoun-Adissoda Carmelle, Garba S, et al. Identification And Dosage of Caffeine by Liquid Chromatography UV-Visible in Energy Drinks Marketed in Benin Republic. Nutri Food Sci Int J. 2022. 11(4): 555816. DOI: 10.19080/NFSIJ.2022.11.555816.
Abstract
The consumption of energy drinks is experiencing a sharp increase in the world as in Benin with a flourishing market. Many questions remain as to their composition and their potential health effects. Although their composition varies depending on the brand. The substances most encountered in these drinks are caffeine, taurine, group B vitamins, sugars, and derivatives. Several cases of caffeine intoxication have been reported by US poison control centers following the consumption of these energy drinks. These facts, despite their seriousness, however, remain poorly documented. We have undertaken through the present study, the identification, and the assay by liquid chromatography UV-visible of caffeine in 34 samples of energy drinks collected in 15 supermarkets of Cotonou in Benin Republic after some preliminary tests of visual inspection, measurements of the beverage volume and pH. From the physicochemical analysis of the drinks, it appears that all the samples (n = 34) contained caffeine at levels varying from 12.7mg/ 100mL to 51.3mg/ 100mL (NAFDAC standard: 14.5 to 32mg / 100mL). The non-conformities noted were under assay (1/34 samples; 12.7±0.2mg/100mL); overdose (1/34 samples; 51.3±1.1 mg/100mL)); risk of overdose (7/34 samples). All samples passed the tests for volume (251.7 < <506.7mL) and pH (2.7 pH <3.8; Standard: 2.7 pH <4.0) while 20/34 samples did not pass the visual inspection test. It is urgent that the public authorities in charge of food security ensure the control to protect the health of the populations.
Keywords: Quality Control; Energy Drinks; Caffeine; Identification; Dosage
Abbreviation: FSS: Faculty of Health Sciences; LCAM: Laboratory of Analytical Chemistry and Drug Analysis; DCM: dichloromethane; CSHPF: Council of Public Hygiene of France; DCM: dichloromethane; TLC: Thin Layer Chromatography
Introduction
The growing supply of varied products, combined with good availability, gives consumers the possibility of consuming sugar-sweetened beverages anywhere, anytime, and often at a lower cost [1]. Among these sugary drinks are energy drinks. They represent one of the fastest growing soft drink markets in the world [2,3]. The first energy drink appeared in the United States in 1949 and was marketed under the name “Dr. Enuf “ [4]. In Europe, they were first launched in 1987; then the market grew worldwide, becoming very popular after the introduction of Red Bull in 1997.
Since then, the energy drink market has grown dramatically, with various brands being promoted all over the world. Annual consumption of energy drinks in 2013 exceeded 5.8 billion liters in approximately 160 countries [5]. The total estimated value of the US retail market for energy drinks was approximately USD 12.5 billion in 2012 and this market grew by 56% from 2006 to 2012 [6]. In the United States, energy drinks represent the second most used dietary supplement by young people; approximately 30% regularly consume energy drinks [7]. In South Africa, between 2009 and 2014, the annual volume of sports and energy drinks sold increased from 97.7 million liters to 167.7 million liters, from around two to three liters per capita in just five years [8]. In Benin in 2019; 746 151 kg of energy drinks were imported for a total amount of 238 318 127 FCFA according to the General Directorate of Customs and Indirect Rights).
Moreover, Energy drinks most often contain caffeine, taurine, glucuronolactone, group B vitamins, sugar and/or sweetener. Some may contain ginseng or guarana [9]. Caffeine is the almost ubiquitous substance in So-called Energy Drinks. It is the main ingredient of Energy Drinks [4] and is the subject of particular attention. In addition, the pleasure stimulating sensation produced by caffeine, often at low doses, can be replaced by psychological symptoms, such as anxiety and depressive neuroses at high doses [10], and its intoxications occur for very variable quantities ingested; caffeine tolerance does in fact show very marked individual variability [11]. Thus, more than 40 cases of caffeine poisoning have been reported by one of the American poison control centers following the consumption of energy drinks [12].
Moreover, in the current state of knowledge, the adverse effects described linked to Energy Drinks could be attributed to caffeine, in association with predispositions or consumption methods [9]. Faced with cases of caffeine poisoning, the Superior Council of Public Hygiene of France CSHPF) recommends a daily consumption of less than 300 mg of caffeine, which corroborates published scientific data. For children under 12, the maximum recommended daily intake is estimated at 2.5 milligrams/ kilogram mg/kg). Thus, faced with the many adverse effects of energy drinks and cases of caffeine intoxication resulting from the consumption of energy drinks, several countries have imposed regulations concerning the marketing, composition, and advertising of these drinks [13].
However, in Benin Republic, the lack of strong regulations known to all on these energy drinks, as well as the lack of scientific studies carried out on these drinks marketed in the country would favor an underestimation, or even a passing over in silence of multiple abuses with consequences of consumer poisoning. It is to fill this gap that we propose to undertake this work which aims to assess the compliance of energy drinks marketed in Benin Republic from the point of view of the recommended doses of caffeine.
Materials and Methods
This was a descriptive and analytical cross-sectional study that took place over a period of fourteen (14) months, from December 2019 to January 2021. The drinks analyzed were collected from supermarkets in the city of Cotonou. Cotonou is a city located on the coastal strip that stretches between Nokoue Lake and the Atlantic Ocean) in Benin Republic. We collected our samples in supermarkets because they represent the formal circuit. In addition, the drinks are stored in better conditions, thus ensuring good food safety. The various analyzes were carried out at the Laboratory of Analytical Chemistry and Drug Analysis LCAM) of the Faculty of Health Sciences (FSS) of Cotonou. It is a laboratory registered in a quality assurance approach.
Materials: Chemical and Reagents
Methanol HPLC gradient grade, Acetonitrile HPLC gradient grade, Dichloromethane analytical grade and Sodium Carbonate (99.9%). were purchased from Scharlau Chemical Barcelona, Spain). (Caffeine Ph. Eur grade) (98.5%) was purchased from VWR International BVBA Leuven, Belgium), Ultrapure water (H2O) was obtained from a Pure lab Chorus 1 Plus water purification system from ( Veolia Aubervilliers, France).
Samples collection
The study covered all energy drinks available and sold in Cotonou supermarkets. Preliminary work was done, before the sampling, which consisted in listing all the supermarkets in Cotonou as well as the brands of energy drinks available in these supermarkets. The included in our study were energy drinks containing caffeine sold in supermarkets in Cotonou; and available in the supermarkets included in our study. The main criterion for non-inclusion concerned beverages whose expiry date was less than six months at the time of our sampling.
Our sampling was done considering the batches of beverages available and the places of collection. This is non-probability sampling for convenience. We have therefore defined a sample as a set of four (04) units of beverages having the same brand, the same manufacturing batch and coming from the same collection site. This number of units was set according to the laboratory tests to be carried out. All brands of energy drinks marketed in supermarkets in Cotonou during the period of our collection were collected in reusable bags. Each sample received an identification code and was subsequently accompanied by a sampling sheet specifying the date and place of collection, the quantity sampled and the purchase price. All samples were sent to LCAM. They are then classified, in cupboards, by place of collection with precision of the denomination, the name of the manufacturer, the presentation, the batch number, the dates of manufacture and expiry. They were stored at laboratory temperature (25°C) until the end of our analyses.
Study of labeling
This part consists in the verification for each beverage sample, of the following information sales denomination; the weight; name or company name or registered trademark + address of the manufacturer; country of origin and/or provenance; identification of the manufacturing batch; instructions for use and precautions for use; date of manufacture/packaging/date of minimum durability; list of ingredients; mention “high caffeine content” and quantity of caffeine present in the drink in mg/liter and Mention “not recommended for children, pregnant women and breastfeeding women”.
Nominal beverage volume control
The test was carried out on three beverage bottles for each sample and according to the following method. We empty the contents of each container into a 500mL test tube for drinks with a volume of less than 500mL and into a 1000mL test tube for 500mL drinks; and then we read the volume on the test tube after the air bubbles have disappeared. An average of the three measured volumes were then determined. The average volume measured must be substantially equal to the announced volume.
Caffeine extraction
The extraction was done on 100mL of energy drink, taken using a test tube and introduced into a 250mL beaker. We alkalinize the drink with a weak base such as 1 M sodium carbonate and adjust the pH to 9. Then add 20mL of dichloromethane (DCM) and maintain under magnetic stirring for 20 minutes. Figure 1 summarizes the caffeine extraction procedure.

Instrumental Analysis Methods
pH measurement
The pH measurement was made by directly immersing of the electrode of the previously calibrated pH meter (VWR; Belgium) in 100mL of energy drink contained in a 250mL beaker. For the various measurements, the electrode of the pH meter is rinsed with milli-Q water and dried using blotting paper. It was then introduced into the test socket. The different pH values were read after stabilization of the reading display. Three tests were carried out per sample, thus making it possible to determine a mean and a standard deviation. The pH value should be between 2.6 and 4.0 [14].
Thin Layer Chromatography
Each sample was monitored on TLC plates as stationary phase made of silica gel 60F254S (Merck; Germany) while the mobile phase was a mixture of ethanol: water 6: 4; (v/v). The lamp detection-UV was done at 254nm for caffeine. We prepared a 1mg/ mL solution of reference standard of caffeine in ethanol. The test solutions were prepared under the same conditions as previously described after caffeine extraction. (Nineteen 19) different deposits were made on each plate. The deposited volume was 5 μL for each solution. All samples were analyzed in duplicate on the TLC plate. We identified the caffeine present in each sample by comparing the Rf value and spot intensity of the caffeine reference chemical to that of the analyzed sample.
HPLC Analyses
The analyzes were performed on a Hitachi VWR High system (VWR international; Pennsylvanie, USA) equipped with a quaternary pump HITACHI VWR 5160, an autosampler HITACHI VWR 5260, and a UV-DAD detector HITACHI VWR 5430. The system was controlled with Chromaster software version 1.1 (VMR; USA). The chromatographic separations were carried out in isocratic mode, on a Lichrocart ® (C18; 5 μm dp) column 250×4 mm; ID), maintained in a compartment thermostated at (30°C, applying as mobile phase an isocratic mixture of acetonitrile: water ( 20:80; v/v); the pump flow rate was 1mL/min. The sample solutions were maintained in a controlled compartment at 8°C. The injection volume was 10 μL. The UV detection wavelength was fixed at 273nm. However, the UV spectra were recorded online from 200 to 400nm.
Sample Solutions for HPLC Analysis
Solution for calibration
A stock solution of calibration standard of caffeine (400μg/ mL was prepared in a mobile phase consisting of a mixture of acetonitrile: water 20: 80; v/v). Dilutions were carried out to obtain solutions at 04 different concentrations levels: 5μg/mL, 10μg/mL, 15μg/mL, and 20μg/mL. These solutions were injected into the HPLC to draw a calibration curve from which the caffeine contents of the different samples were determined.
Assay preparation:
We dissolved in 20mL of mobile phase, all the quantity of extract obtained from of 100mL of energy drink to obtain a stock solution; after that, we made appropriate dilution (1:100; v/v). To obtain 7.5μg/mL assuming that one bottle or can may contain 15mg/mL of caffeine, filter each solution on a microporous membrane preferably 0.45μm pore size or less) and dilute as done for the standard solutions to have about 7.5μg/mL of caffeine. Three tests were carried out for each sample
Calculation of caffeine content
By using the equation of the calibration curve of the chemical reference substance presented in the form y = ax+b, with y representing the area under the curve, x the concentration of the injected solution, caffeine contents of the samples were calculated by applying the formula below:
m = (AUC-b)/a x Š2000/(0.858)
with 2000 the dilution factor and 0.858 the extraction yield, m: caffeine content mg/100mL), AUC: area under the curve, b: intercept of the calibration line, a: slope coefficient of the calibration curve.
Results and Discussion
Sample Characteristics
A total of 34 samples of energy drinks were collected with four 04) units per sample. The different origins of the manufacturers of these collected energy drinks are grouped together in table 1. The energy drinks collected during our study are presented in table 2 below. For the rest of the process the 34 samples were coded E1 to E34) for confidentiality and were submitted to each test. All our samples have passed the visual inspection test for labelling verification. Fourteen 14) samples out of the 34 presented their label in French and these labels were indelible. The noncompliance rate is 20/34 or 58.8%.
Over the 34 samples analyzed, out of 20 samples the name and address of the manufacturer were mentioned. The noncompliance rate is 14/34 or 41.2%. Four non-conformities were recorded out of the 34 samples analyzed; 12.5% and concerned the absence of indication of the country of origin. On the packaging of one 01) sample out of 34, or 2.9%, the volume was not written. On 21 samples were mentioned the dates of manufacture and expiry. The non-compliance rate is 38.2%. Of the 34 samples analyzed, the presence of the mention “high caffeine content”; 23 samples had marked this mention on their label, given a non-compliance of 32.3%.

Among the 34 samples analyzed, 10 did not bear this mandatory statement “not recommended for pregnant, breastfeeding women and children”, we recorded a non-compliance rate of 29.4%. All our samples were packaged either in a bottle 01 (sample) or in a can. These packaging’s were in good condition, not rusty and the closure device of the vials was also in good condition. No packaging irregularity was therefore noted.
Volume Measurement Test and pH Measurement
The results of the sample volume measurement are shown in table 3 and are 100% consistent.
The results of the pH measurement of the samples are recorded in table 3 opposite and comply 100% with the specifications of the standard taken as reference.
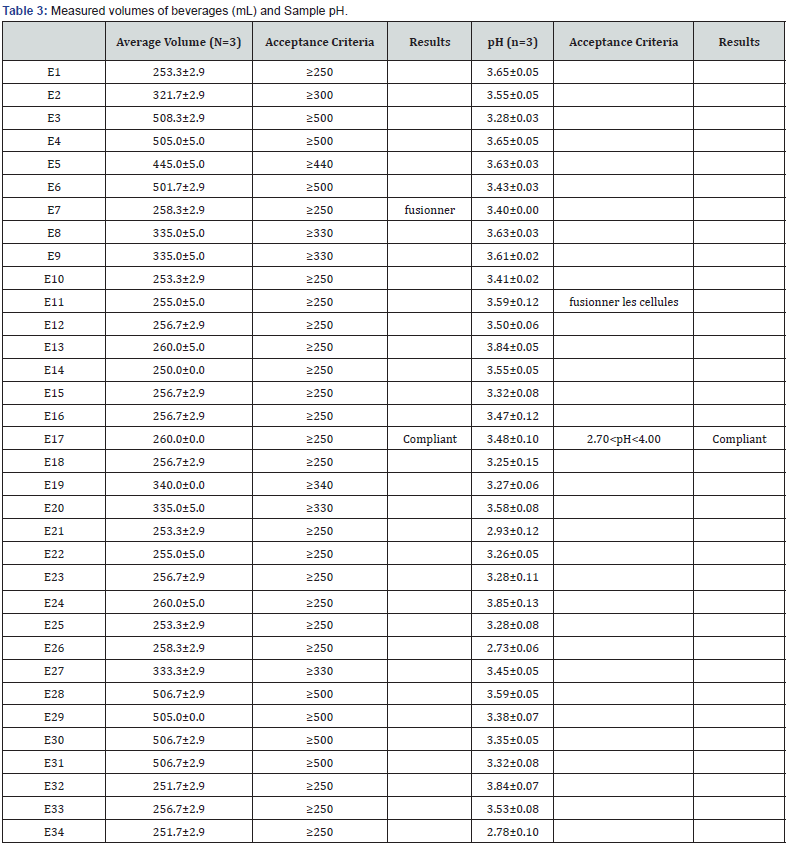
Identification test
The presence of caffeine in the reference solution is indicated by an orange-yellowish spot and a frontal ratio of approximately 0.9. The caffeine spots in the chromatograms obtained with the test solutions correspond in terms of color, size, shape, and distance traveled to those in the chromatograms obtained with the caffeine reference solution (figure 2). All samples, 100% passed the identification test. The caffeine once identified by TLC in beverages, we moved on to HPLC-UV visible assay.
Caffeine determination by HPLC-UV visible
As illustrated in figure 3A, HPLC results confirmed that caffeine was detected (tR= 3.66 min for the chromatogram of the SCR (a), and the chromatogram of the sample (b)). The comparison of the spectra of the reference chemical substance and the sample shows a perfect superposition (figures 3B, 3C). We can therefore confirm the presence of caffeine in this analyzed sample. The same process was observed for the other samples. The caffeine content in these samples was calculated using the calibration line drawn from the areas under the curve of the SCRs and the samples.

Table 4 presents the results of the caffeine assay in the samples by HPLC_UV Visible. The reference standard is that of the National Agency for Food and Drug Administration and Control (NAFDAC). In total, nine (09) samples were non-compliant. One (01) sample (E19) was under-dosed in caffeine and the eight (08) other non-compliances related to overdose: one (01) sample (E26) presented a risk of overdose and seven (07) samples (E12, E14, E28, E29, E30, E31, E33) were overdosed.
Table 5 presents the summary of the various tests carried out on the samples. From the analysis of this table, it appears that our samples passed all the tests, with the exception of the visual inspection test (58.8% non-compliance) and the caffeine assay by HPLC-UV visible (26.5% non-compliance). In addition, 14.7% are non-compliant with both tests at the same time. The total noncompliance rate is 70.6%, or 24/34 samples.


Discussion
This study contributed to the evaluation of the quality of energy drinks containing caffeine and marketed in Benin. It included thirty-four (34) samples of caffeinated energy drinks from supermarkets in Cotonou.
The size of our study population is thirty-four (34) samples. This size is larger than that of Rai et al., who collected ten (10) energy drink samples. Their study focused on the determination by UV-Visible liquid chromatography of caffeine and taurine in energy drinks [15]. It was also greater than the size of the study population of Sapna et al., carried out in 2011 in New Delhi [16].
Our work focused on energy drinks marketed in Cotonou supermarkets and containing caffeine. Each brand of drink found in the supermarket was represented by a sample. This low number of samples is explained by the health situation which has been raging in the world since December 2019, and which is responsible for numerous stock-outs in supermarkets. Moreover, during our study, we were not interested in traditional markets. Our samples were collected in the supermarkets of Cotonou, which represent the formal circuit, and which offer better traceability of the food products purchased there. Also, the products are stored in better conditions.
All our samples have undergone a visual identification test. From the analysis of the results of the visual identification, it appears that 41.2% of our samples passed the preliminary visual inspection test 58.8% non-compliance). Our study was based on primary packaging and packaging. Our analysis shows that beverages sold in supermarkets have a multitude of nonconformities in their packaging and labels. The most marked non-conformities concern the absence of mandatory information such as “not recommended for children, pregnant women and breastfeeding women”, “high caffeine content” and quantity in mg/100 mL, as well as the date of manufacture and storage for other samples. In addition, some samples had their label not written in French and the label was not indelible. The fact that these mentions are absent does not guarantee the absence of risks for the health of consumers.
On the labels of 5 samples, (14.7%), the caffeine content was not mentioned, thus exposing consumers to the risk of overdose and/or caffeine intoxication. This reported rate is lower than the 33.3% of samples with no mention of caffeine content on the label obtained by Sapna et al., [16]. The labels of some samples were not written in French, whereas the official language in our country Benin is French. This non-compliance does not allow all consumers to have access to the information on the label such as the list of ingredients and their content, as well as the precautions for use and contraindications. This could therefore expose consumers to all kinds of risks allergy to given substances, overdose, etc.). However, the information is not indelibly written on some samples increases the risk of counterfeiting/falsification of these drinks.
Similarly, a lack of information relating to the name and address of the manufacturer on certain samples during the visual inspection poses a problem of traceability and the impossibility of alerting and/or recalling the batch. The absence of the date of manufacture on certain samples constitutes departures from good manufacturing practices and increases the risk of consuming spoiled drinks.
The pH of our samples ranged from 2.7 to 3.9, values consistent with the specifications of the standard taken as a reference for the pH of energy drinks [17]. These pH values are close to those found by Rai et al., in 2016 2.96-3.81), during a study based on the determination of caffeine and taurine contained in energy drinks by HPLC-UV [15]. However, Humagain et al., in 2013 obtained during their studies on energy drinks, pH values ranging from 6.5 to 6.7 [18]. These energy drinks have an acidic pH due to the physico-chemical properties of caffeine. Moreover, caffeine is only soluble in a cold aqueous medium when the medium is acidic. However, for better oral health, the pH of foods and beverages should be above 4.5 [19], a value corresponding to the critical pH. The critical pH being the pH below which the demineralization of the dental enamel occurs. The pH of all our samples was therefore below the critical pH, thus exposing consumers to dental erosions.
Regarding the measurement of the average volume of the samples, no non-compliance was noted. The caffeine identification by TLC in all samples was 100% positive. Regarding the assay by HPLC, for the thirty-four samples collected, we have a compliance rate of 73.5%. One 01) sample E19), 2.9%) was underdosed, seven 07) samples E12, E14, E28, E29, E30, E31, E33) were overdosed, 20.6%) and one sample E26) presented a risk of overdose. The caffeine content of our samples ranged from 12.7 to 53.2mg/100mL.
Underdosing may be due to a fault in the manufacturing system, negligence during manufacturing. It can also be a voluntary and deliberate act in order to maximize one’s profit. In 2010, Violeta et al., had caffeine contents when measuring caffeine in energy drinks marketed in Romania that ranged from 16.82 mg/100 mL to 39.48mg/100mL [20]. Also, Pena et al., in 2005 determined caffeine levels in energy drinks marketed in Portugal, varying from 2.1 to 217.5mg/100mL [21]. In addition, during our study, the eight samples whose non-compliance with the standard was related to overdose had caffeine levels significantly higher than indicated by the labels. Sapna et al., had 38% of their samples not within the label limit [16].
The caffeine overdose of these energy drinks could be a deliberate act by the manufacturers to make consumers addicted to their energy drink brand. Indeed, caffeine is a powerful stimulant of the central nervous system. Its action on this system leads to an increase in alertness and improved mental and physical performance. Taking these drinks overdosed in caffeine will therefore make consumers feel alertness and an improvement in mental and physical performance greater than those obtained with other drinks, thus making these drinks look like the best on the market. This situation exposes consumers to the risk of caffeine intoxication with manifestations such as tachycardia, palpitations, behavioral disorders, etc. All these preliminary results call on the authorities in charge of food regulations to be extra vigilant because the health risks for consumers are enormous.
Conclusion
This preliminary study relating to the identification and dosage by UV-Visible liquid chromatography of caffeine in energy drinks, included thirty-four (34) samples collected in the supermarkets of Cotonou in Benin Republic. Various reference analytical methods were used in this study. The different tests carried out are the visual identification; the measurement of pH and average volume; the identification by thin layer chromatography and the dosage by HPLC-UV Visible. It shows a total non-compliance rate of 70.6%, of which 23.5% relate to overdose, exposing consumers to numerous dangers, cardiovascular and neuropsychiatric manifestations. This study confirms the reality of the circulation of low-quality energy drinks in Benin. Other subsequent larger studies should be conducted in Benin following the same rules of good laboratory practice while including the other substances of interest contained in these drinks.
Acknowledgement
We would like to thank Pierre Fabre Foundation for its financial support. We also thank Mr. Ahmed Amoussa for his contribution to this work.
References
- Quebec Coalition on Weight Issues (2012) The Inside of Sugary Drink Marketing: The Product: A varied offer to meet a segmented market. Quebec: CQPP 1: 71.
- Euromonitor International Global sports and energy drinks; where consumer lifestyle and « lifestyle branding » meet.
- Superior Council of Health (2009) OPINION OF THE SUPERIOR COUNCIL OF HEALTH N° 8622 "Energy drinks". Brussels: CSS p. 11.
- Reissig CJ, Strain EC, Griffiths RR (2009) Caffeinated energy drinks-a growing problem. Drug and alcohol dependence 99(1-3): 1-10.
- Bailey RL, Saldanha LG, Gahche JJ, Dwyer JT (2014) Estimating caffeine intake from energy drinks and dietary supplements in the United States. Nutrition reviews 72 : 9-13.
- Zucconi S, Volpato C, Adinolfi F, Gandini E, Gentile E, et al. (2013) Gathering consumption data on specific consumer groups of energy drinks. External Scientific Report for European Food Safety Authority 10(3): 1-190.
- Simon M, Mosher J (2007) Alcohol, energy drinks, and youth: a dangerous mix. San Rafael CA: Marin Institute p. 21.
- Nicholas S, Corné VW, Mashekwa M, Aviva T, Karen H (2017) Energy drink consumption and marketing in South Africa. Préventive Medicine 105: 32-36.
- ANSES (2013) Evaluation des risques liés à la consommation de boissons dites « énergisantes ». Maison-Alfort: ANSES p. 195
- Khan K, Asif M, Arshad MJ, Naeem M (2006) Extraction and chromatographic determination of caffeine contents in commercial beverages. Journal of Applied Sciences 6: 831-834.
- Bigard AX (2010) Dangers des boissons énergisantes chez les jeunes Risks of energy drinks in youths. Archive de pédiatrie 17(11): 1625-1631.
- McCarthy D, Mycyck M, DesLauriers C (2008) Hospitalization for caffeine abuse is associated with concomitant abuse of other pharmaceutical products. Am J Emerg Med 26(7): 799-802.
- Higdon JV, Frei B (2006) Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr 46(2): 101-23.
- Kenya standard (2019) Energy drinks- specification p. 10.
- Rai PR, Rai HB, Dahal S, Chaudhary S, Shrestha S (2016) Determination of caffeine and taurine contents in energy drinks by HPLC-UV. J. Food Sci. Techol 9: 66-73.
- Sapna J, Ramakant S, Nimisha J (2011) Caffeine content of energy drinks pp. 1-15.
- Karau MG, Kihunyu NJ, Kathenya MN, Wangai NL, Kariuki D, etal. (2010) Determination of caffeine content in non-alcoholic beverages and energy drinks using HPLC-UV method. African journal of drugs & alcohol studies 9(1): 15-21.
- Humagain B, Baniya J, Lamsal KP (2013) Initiation for formulation of policy to regulate the unnecessary use of energy drinks, technical report, WHO, country office for Nepal. Katmandu: forum for protection of consumer rights-Nepal.
- Forsythe SJ, Hayes PR (1998) Food Hygiene Microbiology and HACCP. 3ème édition p.
- Violeta N, Ion T, Mira EI (2010) Chromatographic determination of caffeine contents in soft and energy drinks available on the romanian market. St. Cerc. St. CICBIA 11(3): 351-358.
- Pena A, Lino C, Silveira MIN (2005) Survey of caffeine levels in retail beverages in Portugal. Food Additives and Contaminants 22(2): 91-96.






























