Comparative Effect of Arbuscular Mycorrhizal Fungi and Biostimulants on the Antioxidant and Nutritional Potential of Moringa oleifera
Fowe Michelle Carole Djouhou1,2*, Dieudonné Nwaga2 and Elie Fokou1
1Department of Biochemistry, Laboratory for Food Science and Metabolism, University of Yaoundé I, Cameroon
2Department of Microbiology, Laboratory of Soil Microbiology, Biotechnology Centre, University of Yaoundé I, Cameroon
Submission: April 24, 2019; Published: August 13, 2019
*Corresponding author: Fowe Michelle Carole Djouhou, Department of Biochemistry, Laboratory for Food Science and Metabolism, University of Yaoundé I, Cameroon
How to cite this article: Djouhou Fowe Michelle Carole, Nwaga Dieudonné, Fokou Elie. Comparative Effect of Arbuscular Mycorrhizal Fungi and Biostimulants on the Antioxidant and Nutritional Potential of Moringa oleifera. Nutri Food Sci Int J. 2019. 9(2): 555758. DOI:10.19080/NFSIJ.2019.09.555758.
Abstract
The influence of arbuscular mycorrhizal fungi (AMF) and biostimulants was evaluated on the antioxidant and nutritional potential of Moringa oleifera grown in the botanical garden of the University of Yaoundé I. Four treatments (Control or without fertilization (N0), 20g of AMF (N1), 10 g biostimulants (N2) and 20g AMF + 10g biostimulants (N3)) were made up and the experiment was designed in randomized block design with twenty-two replications. Moringa seeds were sown and four months after planting, leaves and roots were collected for the determination of their antioxidant and nutritional potential. Obtained data were analyzed using ANOVA at p < 0.05. It appeared that the arbuscular mycorrhizal fungi and biostimulants modulated the antioxidant and nutritional potential of Moringa oleifera with a remarkable influence of arbuscular mycorrhizal fungi on protein synthesis. Total phenolic content varied from 220mgEAG/100g (AMF + biostimulants) to 253mgEAG/100g (biostimulants) in leaves and from 164mgEAG/100g (biostimulants) to 245mgEAG/100g (control) in roots. Biofertilization increased the activities of both catalase and peroxidase in leaves and roots. Compared to control, AMF and biostimulants combination increased the nutritional content of about 90%, 2%, 26% in leaves and 6%, 3% and 14% respectively for lipids, sugars and proteins. AMF + biostimulants treatment was proved to be the best because of the greatest radical scavenging activities and larger quantities of chemical compounds in their plants.
Keywords: Arbuscular mycorrhizal fungi; Bio-stimulants, Nutritional and antioxidant potential; Moringa oleifera
Introduction
The world has become aware of environmental issue in recent years affecting crop production, human nutrition and wellbeing. In tropics, the major limiting factor of crops is soil nutrient deficiency resulting from land degradation; and affecting the growth, nutrient content and uptake of the plant [1]. To restore the productive potential of soils, some techniques can be used; fertilization is one of them [2].
Fertilizers are any organic or inorganic material of natural or synthetic origin that are added to soil to supply one or more plant nutrients essential for plant growth. Since synthetic compounds are highly polluting, hazardous and costliest, researchers are extensively working to develop natural products as they are less hazardous, cheaper and easily available.
In recent decades, agricultural growing practices have been evolving towards organic, sustainable or environmental friendly systems. Modern agriculture implicates reduction of chemical inputs without reducing the crop yield and quality.
Actually, research activity in the matter of agricultural systems has for years been oriented to increase yield without considering the quality of the products and the rational use of resources. In contrast, attention now is mainly focused on product quality and the sustainability of the cultivation systems. Moreover, cultivation management pays more attention to the reduction of production costs by lowering inputs [3].
This leads to the development of organic-based fertilizers and microorganisms which enhance the bioavailability and mobility of plant nutrients and, thus, improve the uptake of nutrients by plant roots. Good fertility is fundamental to successful plant growth, and the application of fertilizers and manures is an essential growing activity. Arbuscular mycorrhizal fungi (AMF) and biostimulants are products that can achieve the balance between contributions and needs of plants [4].
AMF constitute a group of roots obligate biotrophs that exchange mutual benefits with about 80% of plants and allow them to explore larger volumes of soil to absorb more water and nutrients, enhance their transport, and increase absorption of immobile mineral elements. Their action leads to the stimulation of plant growth [5].
Relative to biostimulants, they are biological derived fertilizer additives and similar products that are used in crop production to enhance plant growth, health and productivity. They help plants to tolerate abiotic stresses, improve nutrient-use efficiency and improve quality attributes [4]. As environmental changes can have an impact on the chemical and biochemical composition of plant’s organs, the objective of this study was to evaluate the effect of AMF and biostimulants as substrate amendment on the antioxidant and nutritional potential of M. oleifera.
Materials and Methods
Experimental site and substrate
The present work was conducted at the Faculty of Science, University of Yaoundé I from January to June 2013. The area lies between latitude North 3 degrees 52 minutes 0 seconds (3°52’0’’) and longitude East 11 degrees 31 minutes 0 seconds (11°31’0’’).
Soils that provided support for the experiment were basically a forest topsoil collected in Yaoundé (Centre region), Cameroon. It consisted of a mixture of an Oxisol soil and sand (proportion 3-1) that was sieved using a mason sieve (0.5cm) and sterilized in an autoclave at 121 °C for 30 min. Once the mixture cooled, it was introduced into perforated plastic bags (20 × 20cm).
Arbuscular mycorrhizal fungi and bio-stimulants
Inoculum, provided by the Laboratory for Soil Microbiology, University of Yaoundé I consisted of two local selected strains of AMF: Scutellospora gregaria and Gigaspora margarita (20spores/g of sterilized soil). Bio-stimulants were a mixture of volcanic material (podzolan), fish waste and animal bones finely ground.
Treatments
The 3 litres containing plastic bags according to treatments were ranged on shelves. Four treatments (Control: without fertilization (N0), 20g of AMF (N1), 10g biostimulants (N2) and 20g AMF + 10g biostimulants (N3)) were made up and the experiment was designed in a factorial design with twentytwo replications. AMF and biostimulants were applied in pots and Moringa oleifera seeds sown. Biostimulants were applied in two steps. The first dose was 5g at sowing stage and the second dose, 5g five weeks after sowing. Watering was done twice a day with tap water in the absence of rain.
Sample collection
Four months after planting, M. oleifera leaves and roots were collected, cleaned and air-dried at room temperature. They were kept away from high temperatures and direct sun light to avoid destroying active compounds. They were then pounded with an electric mill.
Preparation of plant extracts
The process followed the established extraction procedure of plant samples, using hydro-methanol (30:70 v/v) as solvents. Serial extractions were done using 5g of plant powder, in 50ml of solvent in Erlenmeyer flask. The mixture was stirred at two hourly intervals for 24 hours. Rotavapor was used to recover methanol.
Determination of Total Phenolic Compounds
The amounts of phenolic compounds in leaves and roots were estimated by using Folin-Ciocalteu reagent [6]. Using the standard (Gallic acid) curve, the total phenolic compound content was calculated and expressed as Gallic acid equivalent in mg/g of sample.
Antiradical activity: DPPH (2.2-Diphenyl-1- picrylhydrazyl) test [7]
The free radical scavenging activity of the extract was measured in terms of hydrogen donating or radical scavenging ability using the stable free radical DPPH. EC50 value (the concentration required to scavenge 50% DPPH free radicals) was calculated. The ability to scavenge the DPPH radical was calculated using the following equation:
DPPH scavenging effect (%) = [(A0 - A1)/A0 x 100].
Where A0 is the absorbance of the control reaction and A1 the absorbance in the presence of the sample.
Catalase and peroxidase Activities
Catalase activity was measured according to the method described by Anderson et al. [8] where one unit of catalase was defined as the amount of enzyme required to decompose 1μmol of H2O2 in 1min. The reaction was initiated by the addition of 1.0ml of freshly prepared 20mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically at 240nm for 1min. The enzyme activity was expressed as units/g. One unit is the amount of enzyme activity required to decrease the absorbance at 240nm by 50% units.
The peroxidase activity was measured using the method described by [9]. This method is based on the measurement of the enzymatic activity with an electron donor (guaiacol), which is oxidized to tetraguaiacol in the presence of hydrogen peroxide. A peroxidase unit is defined as the amount of enzyme that produces 1μmol/min of oxidized guaiacol.
Water, protein, fat and sugar content
The water content was determined according to the method described by Tee et al. [10]. It is based on the mass loss of the samples at 105 °C.
The total protein content was measured by the Kjeldahl method based on the conversion of organic nitrogen to ammonium sulphate. The nitrogen content (N) is converted into protein using 6.25 as the converting factor.
The total lipid content was determined based on their differential solubility in certain organic solvents such as hexane. They are extracted in hot hexane by continuously refluxing for 12 hours [11].
The sugar content was determined by the method described by Dubois et al. [12] based on dehydration followed by cyclization of C6 sugars (hexoses) and C5 (pentoses) in hot acidic medium to give respectively hydroxymethylfurfural and furfural. The latter react with phenol to give a complex with a maximum absorption at 490nm.
Statistical analysis
The data were subjected to statistical analysis to verify and evaluate the difference between the antioxidant and nutritional potential of Moringa oleifera leaves and roots four months after planting. Data were statistically analysed with SPSS software, followed by post hoc analysis using Fischer’s LSD. The difference was considered significant if P<0.05. Results were expressed as mean ± S.D.
Results and Discussion
Hydro-methanol was chosen as extraction solvent for both phenolic content and scavenging activity because of it wide solubility properties for low molecular and moderately polar substances, including the antioxidant-active phenolic compounds.
Phenolic content and scavenging activity
The influence of the different treatments on the leaf and root phenolic content and effective concentration 50 (EC50) is presented by Figure 1.

In leaves, values went from 219.83 ± 0.26 mgEAG/100g (AMF + biostimulants) to 253.55 ± 0.17 mgEAG/100g (biostimulants) while in roots, they ranged from 164.23 ± 0.02 mgEAG/100g (biostimulants) to 245.35 ± 0.69 mgEAG/100g (control).
The sample concentration required to decrease the initial concentration of DPPH to 50% (EC50) is a commonly used parameter for determining the antioxidant activity. These values are compared to a positive control (Gallic acid), which has an EC50 of 26.90mg/g of DPPH.
The application of biostimulants decreased the total phenolic compounds of both M. oleifera leaves and roots, but AMF increased this parameter compared to the control treatment.
The leaves of M. oleifera have a better scavenging activity than roots; this could be explained by their high secondary metabolite content. The antioxidants react with DPPH, a stable purple coloured free radical and convert it into a colourless 2.2-diphenyl-1-picryl hydrazine. Antioxidants, on interaction with DPPH, either transfer an electron or hydrogen atom to DPPH, thus neutralizing its free radical character [13]. Moringa oleifera l eaf a nd r oot e xtracts s ignificantly r educed D PPH radicals. The degree of discoloration indicates the scavenging potential of the antioxidant extract, which is due to the radical scavenging ability.
Phenolic compounds because of their structure have the ability to give a proton to free radicals which become more stable. The DPPH scavenging ability of the extract may be attributed to its hydrogen donating ability.
These results indicate that there is no correlation between antioxidant activity and total phenolic content (P>0.05). However, different results were reported on this aspect; some authors found correlation between phenolic content and scavenging activity; whereas the others found no such relationship, since other compounds are responsible for the antioxidant activity [14,15]. The low antiradical activity of roots compared to leaves could be explained by the existence of chemical compounds that mask the groups of molecules responsible for the scavenging activity.
Enzymatic activity of catalase and peroxidase
Plants are continuously exposed to stress. It is therefore important that they synthesize protective molecules or compounds. The leaves and roots of Moringa oleifera were analyzed for the enzymatic antioxidant levels/activities such as catalase and peroxidase. The results (Tables 1) showed that the biostimulants and AMF + biostimulants treatments present the highest enzymatic activities.
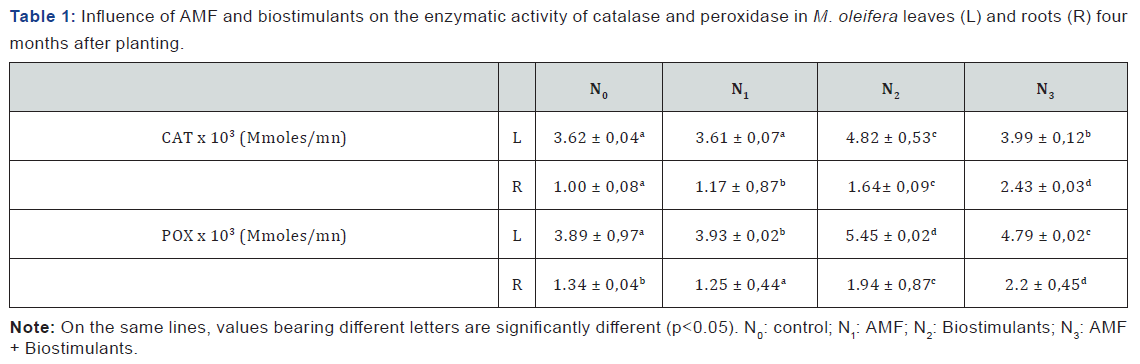
Fertilization increased the activity of both enzymes. In leaves, catalase activities reached from 3.61 x 103 ± 0.07 Mmoles/min (AMF) to 4.82 ± 0.53 x 103 Mmoles/min (biostimulants) and peroxidase from 3.89 x 103 ± 0.37 Mmoles/ min (control) to 5.45 x 103 ± 0.02 Mmoles/min (biostimulants). In the roots, these activities ranged from 1.00 ± 0.87 x 103 Mmoles/min to 2.43 ± 0.08 x 103 M moles/min (catalase) a nd from 1.25 x 103 ± 0.44 Mmoles/min to 0.45 ± 2.2 x 103 Mmoles/ min (peroxidase).
In Moringa oleifera l eaves, c atalase a nd p eroxidase activities were important than in root and the activity is more important in treated plants compare to control. On one hand, this could be explained by the fact that the leaves exposed to environmental conditions, face oxidative stress and need to protect their selves. On the other hand, antioxidant enzymes form the first line of defence against free radical-induced damage, offer protection against free radicals. Peroxidase is an enzyme that catalyses the reduction of hydroperoxide, including hydrogen-peroxide and function to protect the cell from peroxidative damage [16]. It has been found that in crops, pest and bio-aggressor attack level can be an indicator of the nutritional composition. Actually, plant rich in some minerals and nutrients are suitable for some bio-aggressors and pests (article to be submitted) and they need to synthetize protective compounds to fight again such situations.
Water, protein, fat and sugar content
The influence of the AMF and biostimulants on water, protein, lipid and sugar contents of Moringa leaves and roots is presented in the Table 2. Nutrient concentrations were significantly (P < 0.05) influenced by soil amendment.
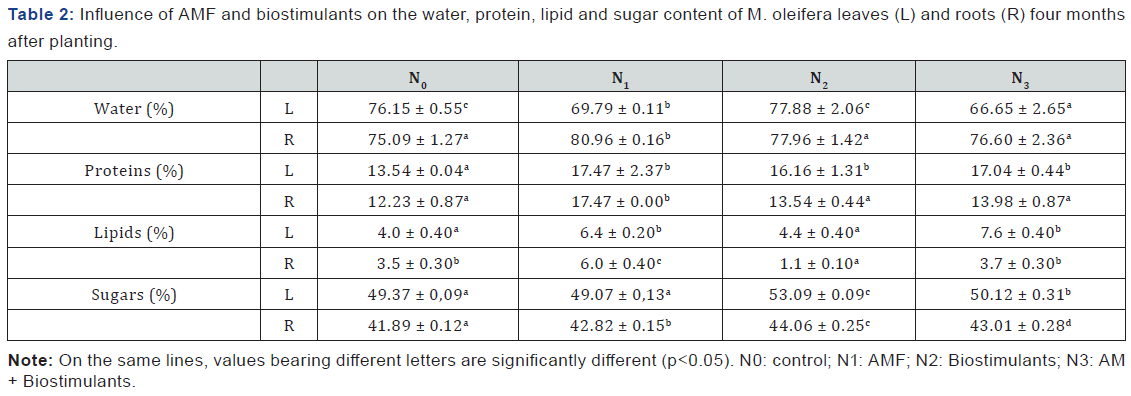
The water content of plant ranged from 66.65 ± 2.65 % to 77.88 ± 2.06 % and from 75.09 ± 1.27 to 80.96 ± 0.16 % respectively for leaves and roots.
The lipid values of the leaves varied from 4.0 ± 0.40 % (Control) to 7.6 ± 0.40 % (AMF + Biostimulants). Roots values ranged from 1.1 ± 0.10 % (Biostimulants) to 6 ± 0.40 % (AMF).
For the sugar content values ranged from 49.07 ± 0.13 % (AMF) to 53.09 ± 0.09 % (Biostimulants) for leaves and from 41.89 ± 0.12 % (Control) to 44.06 ± 0.25 % (Biostimulants) for roots.
The use of AMF and biostimulants increased the levels of macronutrients (total sugars, lipids and proteins) of the plant. Microorganisms, because of the mycorrhizal symbiosis reduced the soluble carbohydrate content of the leaves and roots compared to the control plants. This is understandable because during mycorrhizal symbiosis, when providing the host plant with nutrients, increasing the abiotic (drought, salinity, heavy metals) and biotic (root pathogens) stress resistance, AMF receive between 4 and 20 % of photosynthetically fixed carbon from host plant [17].
From this study, we notice that AMF have a remarkable influence in protein synthesis. These protein contents indicate the best metabolism functioning of the plant; AMF highly stimulate plant metabolism. The level of crude protein content is of particular nutritional significance as it may meet animal’s protein and energy requirements and boost the immune system against diseases [18-20].
Crop nutrient deficiencies are now recognized as an important contributor to the global burden of disease. Macro and micronutrients are very important for a healthy diet and prevent nutritional problems. The positive effect of AMF and biostimulants has boosted Moringa oleifera biomass for leaves and roots, and secondary metabolites quality, thus enhancing its antioxidant capacity, making it more applicable for use in health food.
Conclusion
Soil bio-fertilization positively influences the nutritional and antioxidant potential of Moringa oleifera . Indeed, the application of AMF and biostimulants significantly modify the EC50 of leaves and roots. This latter is an indicator of antioxidant potential of an extract. Due to the fact that tropical soils gradually lose their fertility after a formal continuous culture, it is important to find biological alternatives to this problem in order to reduce the risk of environmental destruction. Biostimulants enrich soil with nutrients and substances when AMF promote their absorption. However, mycorrhizal symbiosis reduces the sugar content. The increase of enzymatic activity of catalase and peroxidase in Moringa leaves compared to roots may be a plant response to oxidative stress probably caused by environmental conditions such as abiotic (drought, salinity, heavy metals) and biotic (root pathogens) stresses.
Acknowledgemnet
The authors thank all the members of the Laboratory for Food Science and Metabolism (LabSAM), Department of Biochemistry, University of Yaoundé I and members of the Laboratory for Soil Microbiology, Department of Microbiology, University of Yaoundé I.
References
- Dania SO, Akpansubi P, Eghagara OO (2014) Comparative Effects of Different Fertilizer Sources on the Growth and Nutrient Content of Moringa (Moringa oleifera) Seedling in a Greenhouse Trial, Advances in Agriculture, Article ID 726313, p. 6.
- Klein HD, Cesar J (1999) Plantes fourragères et maintien de la fertilité du sol. Cultures fourragères tropicales [Forage plants and maintenance of soil fertility. Tropical forage crops]. In: Roberge G, Toutain B Cirad-emvt, pp. 321-358.
- Bulgari R, Cocetta G, Trivellini A, Vernieri P, Ferrante A (2015) Biostimulants and crop responses: a review. Biological Agriculture & Horticulture 31(1): 1-17.
- Childs K, Beeson RC (2000) The effect of biostimulant. Drenches on root growth of woody ornamentals. Ornamental Outlook, Août, pp. 26-28.
- Djouhou FMC, Fokou E, Nwaga D (2018) Potential of Moringa oleifera leaf powder and beneficial microorganisms (Mycorrhizal fungi and rhizobia) in modulating plant symbiosis and yield. Sch J Agric Vet Sci 5(4): 236-243.
- Vinson JA, Yong H, Xuehui S, Ligia Z (1998) Phenol antioxidant quantity and quality in foods: vegetables. Journal of Agricultural and Food Chemistry, 46(9): 3630-3634.
- Molyneux P (2004) The use of the stable free Radical Diphenyl Picryl Hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin Journal of Science and Technology, 26(2): 211-219.
- Anderson MD, Prasad TK, Stewart CR (1995) Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109(4): 1247-1257.
- Lin CC, Kao CH (1999) NaCl induced changes in ionically bounds peroxidase activity in roots of rice seedlings. Plant Soil 216: 147-153.
- Tee ES, Rajam K, Young SI, Khor SC, Zakiyah HO (1996) Laboratory procedures in nutrient analysis of foods. Kuala Lumpur, Malaysia: Division of Human Nutrition, Institute for Medical Research.
- Bourely J (1982) Observation sur le dosage de l’huile des graines de cotonnier [Comments on cotton seed oil determination]. Coton et Fibres Tropicales 27(2): 183 -196.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith E (1956) Colorimetric method for determination of sugars and related substances. Agricultural Research Service 28: 350-356.
- Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohani DP, et al. (2003) Comparative antioxidant activity of individual herbal components used in ayurvedic medicine. Phytochemistry 63(1): 97-104.
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, et al. (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 47(10): 3954-3962.
- Shih MC, Kuo CC, Chiang W (2009) Effects of drying and extrusion on colour, chemical composition, antioxidant activities and mitogenic response of spleen lymphocytes of sweet potatoes. Food Chemistry 117(1): 114-121.
- Sharma R, Vaghela JS (2010) In vivo antioxidant activity of Moringa oleifera leaf and pod extracts against carbon tetra chloride induced liver damage in albino mice. Journal of Chemical and Pharmaceutical Research 2(6): 275-283.
- Müller V, Spanheimer R, & Santos H (2005) Stress response by solute accumulation in archaea. Curr Opin Microbiol 8(6): 729-736.
- Kyriazakis I, Houdijk JG (2006) Nutritional control of parasites. Small Ruminant Resources 62(1-2): 79-82.
- Brisibe EA, Umoren UE, Brisibe F, Magalhaes PM, Ferreira JFS, et al. (2009) Nutritional Characterization and Antioxidant Capacity of Different Tissues of Artemisia annua Food Chemistry 115(4): 1240-1246.
- Adewole MB, Ilesanmi AO (2011) Effects of soil amendments on the nutritional quality of okra (Abelmoschus esculentus [L.] Moench). Journal of Soil Science and Plant Nutrition 11(3): 45-55.






























