Antisickling and Antioxidant Properties of Omega-3 Fatty Acids EPA/DHA
Kotue TC1*, Djote WNB1, Marlyne M1, Pieme AC2, Kansci G1 and Fokou E1
1Laboratory for Food Science and Metabolism, Department of Biochemistry, Faculty of Science University of Yaounde 1, Cameroon
2Laboratory of Biochemistry, Physiology and Pharmacology, Faculty of Medicine and Biomedical Science / UHC-University of Yaounde 1, Cameroon
Submission: May 01, 2019; Published: June 27, 2019
*Corresponding author: Kotue TC, Laboratory for Food Science and Metabolism, Department of Biochemistry, Faculty of Science University of Yaounde 1, Cameroon
How to cite this article: Kotue TC, Djote WNB, Marlyne M, Pieme AC, Kansci G, Fokou E. Antisickling and Antioxidant Properties of Omega-3 Fatty Acids EPA/DHA. Nutri Food Sci Int J. 2019. 9(1): 555752. DOI:10.19080/NFSIJ.2019.09.555752.
Abstract
Background: Therapeutic treatment of Sickle cell disease (SCD) is complex and very expensive. However, natural products have been used to manage sickle cell crises. Omega-3 fatty acids EPA/DHA reduce the number of crisis in SCD.
Objectives: This study aimed to assess the antisickling and antioxidant properties of omega-3 fatty acids EPA/DHA.
Methods: The evaluation of the rates of inhibition of induced sickling by sodium metabisulfite 2% and the potential reversal of sickle cells into normal spherical erythrocytes was performed using microscopic enumeration of red blood corpuscles of the sickling. The evaluation of membrane stability effect, FRAP, DPPH°, and OH° assays was determined using colorimetric method.
Results: Sodium metabisulfite increased the sickling of RBCs from 26.3±1.6 to 79.42±5.2% during 3 hours. Omega-3 fatty acids EPA/DHA 0.2% showed the best antisickling (79.05±1.2%) and reversibility (64.82± 2.7%) rate; the best membrane stability compared to other concentrations. Omega-3 fatty acids EPA/DHA revealed an appreciable reducing power at 26.08±0.3mg FeII/100g. It also showed an inhibitory activity on free radicals of DPPH and hydroxyl radical at IC50 3.7±0.0 and 11.28±0.3mg/mL respectively.
Conclusion: Omega-3 fatty acids EPA/DHA have antisickling, anti-haemolytic and antioxidant properties. The results obtained are in addition to those of the authors who showed that Omega-3 fatty acids EPA/DHA reduce the number of crisis in SCD.
Keywords: Sickle cell disease; Omega-3 fatty acids EPA/DHA; Antisickling; Antioxidant
Introduction
Omega 3 fatty acids are fats commonly found in marine and plant oils. They are polyunsaturated fatty acids (PUFA) with a double bond (C=C) starting after the third carbon atom from the end of the carbon chain. The fatty acids have two ends: - the acid (COOH) end and the methyl (CH3) end. The location of the first double bond is counted from the methyl end, which is also known as the omega (ω) end or the n end [1]. Omega-3 long-chain polyunsaturated fat acid, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are dietary fats with an array of health benefits [2]. They are incorporated in many parts of the body including cell membranes [3] and play a role in anti-inflammatory processes and in the viscosity of cell membranes [1,4]. EPA and DHA are essential for proper fetal development and healthy aging [5]. EPA and DHA are also the precursors of several metabolites that are potent lipid mediators, considered by many investigators to be beneficial in the prevention or treatment of several diseases [6]. Omega-3 fatty acids have been found to play a role in atherosclerosis and peripheral arterial disease. It is thought that both EPA and DHA improve plaque stability, decrease endothelial activation, and improve vascular permeability, thereby decreasing the chance of experiencing a cardiovascular event [7]. DHA is present in large amounts in neuron membrane phospholipids, where it is involved in proper function of the nervous system, which is why it is thought to play a role in Alzheimer’s disease [8].
The findings from a previous retrospective and cross-sectional study suggest that omega-3 fatty acids might be beneficial in Sickle Cell Disease (SCD) [9,10]. In fact, SCD is a group of autosomal recessive genetic blood disorders characterized by a single point mutation in the sixth codon of the β-globin gene. Under low oxygen tension, the resultant abnormal hemoglobin S polymerizes and causes rigid and sickle-shaped red blood cells. Sickle cell pain is the commonest manifestations of the disease in which episodic micro vessel occlusion in one or more sites induces tissue damage accompanied by severe pain and inflammation [11,12]. The pain may be acute or chronic, somatic or visceral, unilateral or bilateral, localized or diffuse [13]. Painful crisis affects nearly all patients often beginning in late infancy and recurring throughout life [14] and it is the major cause of hospital admissions [15]. Moreover, adults who experience painful crises more than three times per year tend to have shorter life expectancies [16]. Clinically, omega-3 fatty acid treatment reduced the median rate of clinical vasoocclusive events, blood transfusion. Subsequent to treatment there was a remarkable reduction in the frequency of pain episodes requiring hospital presentation [17]. Similarly, [18] have demonstrated a significant decrease in the number of crisis and steadystate hemolysis in 16 Nigerians sickle cell patients treated with Cod liver oil containing EPA and DHA. Several studies have also demonstrated that dietary supplementation with omega-3 fatty acids fish results in increased incorporation of these fatty acids into the RBC membrane, which can influence RBC deformability [19,20]. However, the antisickling and antioxidant properties of omega-3 fatty acids EPA/DHA have not been reported. Thus, this in vitro study was undertaken to investigate the effect of omega-3 fatty acids EPA/DHA on blood obtained from patients with sickle cell disease.
Methods
Omega-3 fatty acids EPA/DHA
Omega-3 fatty acids EPA/DHA pure fish oil capsules were bought in a pharmacy. Each capsule contains 750μL in proportion EPA/DHA (3v/2v). The following concentrations of 0.2% v/v, 0.4v/v and 0.6 v/v of the oil were obtained by diluting EPA/DHA in normal saline diluted ethanol (1:4 in normal saline).
Collection of sickle cell blood
Sickle cell blood (HbS) samples from 8 males and 8 females, aged 16 and above were obtained from Central Hospital of Yaounde-Cameroon. The permission of Regional Bioethics Committee of Centre with authorization CEN° 00504/ CRERSHC/2018 was obtained for all the research procedures. A total of Two milliliters (2ml) of venous blood samples were collected from each patient in the sodium EDTA tubes and stored for the experiment.
Antisickling activity In vitro induction of sickling
About 100μL of HbS blood cell suspensions were mixed with 100μL of 2% sodium metabisulfite solution (Na2S2O5) and incubated at 37 °C. The time course of the sickling of HbS erythrocytes was analyzed microscopically according to the method described by [21]. The number of cells was counted every one hour after take 10μL of the mixture diluted 200 times using Marcano liquid. The number of cell was counted every one hour and the percentage of sickling cells was calculated using the formula:
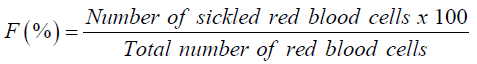
A curve of percentage of sickling in function of time was realized. This permitted the deduction of maximum time necessary to obtain maximum sickling.
In vitro anti-sickling activity of omega-3 fatty acids EPA/DHA
For the assay, in 100μL of sickled cell blood (HbS) preincubated with 2% Na2S2O5 w as a dded t o 1 00μL o f s olution of different concentrations of omega-3 fatty acids EPA/DHA (0.2% v/v; 0.4v/v and 0.6 v/v) previously prepared.
Each mixture was incubated at 37 °C for 3 h (time necessary to obtain maximum sickling). After incubation, 10μL of the mixture was diluted 200 times using Marcano liquid. 10μL of each sample was examined under the light microscope and both sickle cells and total cells were counted from five different fields of view across the slide. For the negative control, the solution containing the extract was replaced by the saline solution. The percentage of sickling inhibition was calculated using the formula:
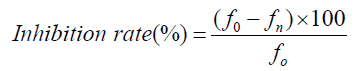
 is the % of sickling of the mixture SS blood and 2 % Na2S2O5.
is the % of sickling of the mixture SS blood and 2 % Na2S2O5.
 is the % of sickling of the mixture SS blood 2% Na2S2O5 and omega-3 fatty acids EPA/DHA with each concentration.
is the % of sickling of the mixture SS blood 2% Na2S2O5 and omega-3 fatty acids EPA/DHA with each concentration.
Reversibility assay of omega-3 fatty acids EPA/DHA
For the reversibility assay, in 100μL of sickle cell blood (HbS) was added to 100μL of solution of different concentrations of omega-3 fatty acids EPA/DHA.
The experiment was followed as mentioned above. The percentage of sickling cells was calculated after every 1 hour till the maximum reversibility of sickling was attained.
The percentage of sickling was determined the same way. Calculation was done after every 1 hour until maximum reversibility of sickling was attained. These percentages permitted to calculate the rate of reversibility of sickling according to the following formula.
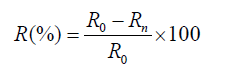
R is the reversibility rate (%)
R0 is the initial percentage of sickling and Rn is the maximum percentage of sickling obtained with different concentrations of omega-3 fatty acids EPA/DHA.
Evaluation of the erythrocyte membrane stability effect of omega-3 fatty acids EPA/DHA
The evaluation of the erythrocyte membrane stability effect of omega-3 fatty acids EPA/DHA was done using a method proposed by [22]. The osmotic f ragility of t he erythrocytes is based on the measurement of the stabilizing effect of their membrane after 24 h of incubation using omega-3 fatty acids EPA/DHA at different concentrations. Varying concentrations of normal saline were prepared (0 - 0.85% NaCl). To 5.05mL reaction vessel containing 4.5mL of each NaCl concentration and 0.5ml of each EPA/DHA concentration, 0.05ml SS blood was added. The mixture was incubated at 37 °C for 24 h and then centrifuged at 3000 rpm for 15min. The optical density of the supernatant was read at 540nm against blank made of 0.85% buffered saline concentration. The percentage of hemolysis was calculated using the formula below:
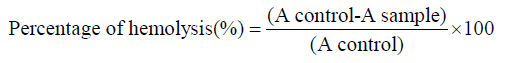
Results were presented graphically as percent hemolysis plotted against the concentration of NaCl.
Total antioxidant activity by ferric reducing antioxidant power assay (FRAP)
Evaluation of the total antioxidant activity by ferric reducing antioxidant power assay (FRAP) was done according to [23]. For this evaluation, 0.1mL of omega-3 fatty acids EPA/ DHA (2mg/mL) was mixed with 3mL of freshly prepared FRAP reagent. After incubation (up to 5minutes) in darkness at room temperature (~25 °C), absorbance was read against a suitable blank at 593nm. The test was carried out in triplets. The concentration of omega-3 fatty acids EPA/DHA was calculated using the standard equation obtained by using standard FeSO4. The final results were expressed in mg of Fe (II)/100g of omega-3 fatty acids EPA/DHA. Gallic acid was used as control.
Evaluation of the Scavenging activity for DPPH
The scavenging effect of omega-3 fatty acids EPA/DHA on DPPH radical was estimated by method described by [24]. A solution of 0.1mM DPPH in methanol was prepared, and 1.0mL of this solution was mixed with 3.0mL of omega-3 fatty acids EPA/DHA solution of varying concentrations. The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 517nm. Gallic acid was used as standard. The ability to scavenge DPPH radical was calculated by the following equation:
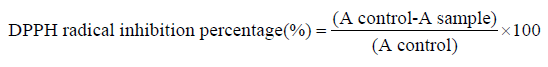
The inhibition percentages calculated permitted the realization of curves, percentage inhibition in function of extract concentration. The test was carried out in triplets. A curve of % DPPH bleaching activity versus concentration was plotted using OriginPro 8 Software to determine IC50 concentration that account for 50% inhibition.
Evaluation of the hydroxyl scavenging activity
Hydroxyl radical assayed as described by [25]. To 1.5mL of each dilution of the extra omega-3 fatty acids EPA/DHA solution of varying concentrations, we added successively 60μdL of FeCl3 1Mm; 90μL of 1.1 o-Phenanthroline 1Mm; 2.4mL of phosphate 0.2M, pH 7.8 and 150μL of H2 02 0.17M. The mixture is then homogenized and incubated at normal temperatures for 5minutes. After 5 minutes absorbance was read at 560nm against the blank. Gallic acid was used as the standard. The antiradical activity of omega-3 fatty acids EPA/ DHA expressed in percentage of inhibition of the hydroxyl radical was determined following the formula:
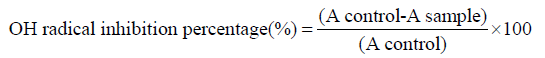
The inhibition percentages calculated permitted the realization of curves percentage inhibition in function of omega-3 fatty acids EPA/DHA concentrations using OriginPro 8 Software. These curves were used to determine IC50 concentration that account for 50% inhibition. The test was carried out in triplets.
Statistical analysis
The results were expressed as mean ± standard deviation. Data was analyzed using Analysis of Variance (ANOVA) of Kruskall-Wallis with the software Sigma Start version 3.01A analysis software. Statistical data were considered significantly different at 95% confidence interval (p <0.05).
Results and Discussion
In vitro induction of sickling with 2% metabisulfite
Sodium metabisulfite 2%, added to sickled red blood cells induced sickling of red blood cells (Figure 1).
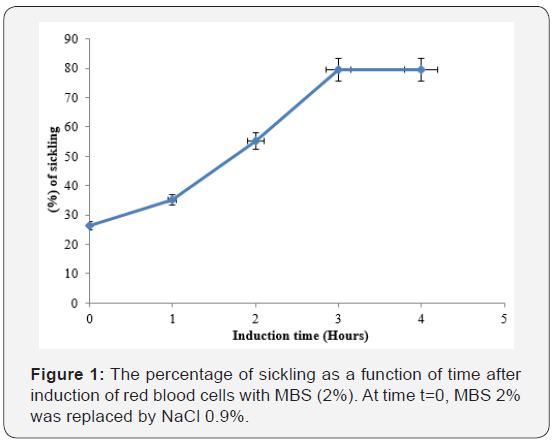
At the beginning, sickling percentage was 26.3±1.6 and after 3 hours of incubation sickling increased to 79.42±5.2% and remained constant with time. The maximum number of sickled cells was obtained after 3 hours, suggesting that this is the time necessary to obtain maximum sickling. In this study, the percentage of sickling RBCs obtained after 3 hours of incubation is lower than 96.5% and 80% values obtained respectively by [21] at the same period and [26] after 1 hour. MBS 2% create hypoxic conditions for RBCs leadind to loss of the morphology and sickled erythrocytes. In vitro deoxygenating of RBCs by MBS (2%) caused progressive aggregation and polymerization of the individual hemoglobin molecules [27,28]. The process of gelation (polymerization) of hemoglobin molecules increases the formation of sickling cells.
Inhibitory and reversibility of sickling activities of omega-3 fatty acids EPA/DHA
After calculating the rate of inhibition of sickling, it was realized omega-3 fatty acids EPA/DHA (0.2%) significantly (p<0.05) inhibited sickling giving inhibition rates of 79.05±1.2% more than 70.541±2.6% and 69.24±2.24% for omega-3 fatty acids EPA/DHA concentrations of 0.4 and .06% respectively. According to reversibility rate, we noticed a significant (p<0.05) reversal effects with omega-3 fatty acids EPA/DHA (0.2%) giving rate of 64.82±2.7 more than 59.77±2.5 and 59.69±2.3 for omega-3 fatty acids EPA/DHA concentrations of 0.4 and 0.6% respectively (Table 1).

Indeed, these results show that omega-3 fatty acids EPA/ DHA have antisickling properties at a certain percentage. Furthermore, it has been reported that Tiger nut oil (Cyperus esculentus) and black seed oil (Nigella sativa) containing omege-3 in their profile also possess antisickling properties [29]. Their effect may also be due to the presence of omega-3. However, none mode of action of this effect has been demonstrated.
Membrane stability effect of the extract
Figure 2 represents the effect of omega-3 fatty acids EPA/ DHA on membrane stability. Hemolysis decreased for all concentrations in the presence of sodium chloride (0.9%) until 13.54% for omega-3 fatty acids EPA/DHA (0.2%) significantly (p<0.05) upper than that exhibited by omega-3 fatty acids EPA/DHA 0.4 and 0.6% and the control.
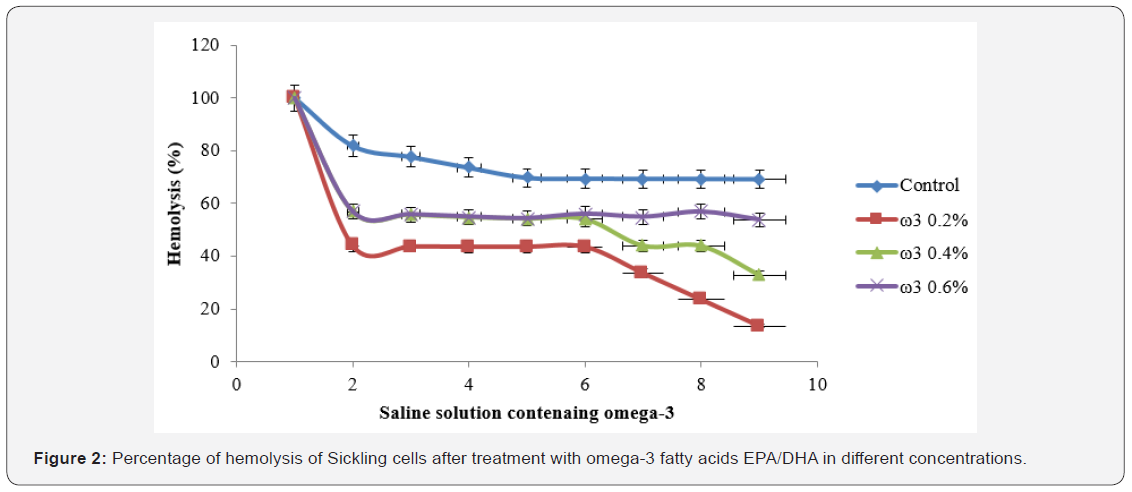
The effect of omega-3 fatty acids EPA/DHA on the membrane stability of RBCs can be evaluated by comparing the haemolysis rates of untreated and treated sickle RBCs with omega-3 fatty acids EPA/DHA at different concentrations. A decrease in the percentage of haemolysis as a function of omega-3 at different concentrations was generally noted. This decrease is related to the appreciable protective effect of omega-3 fatty acids EPA/ DHA on the erythrocyte membrane, hence, their resistance against haemolysis. Furthermore, membrane stability of RBCs was dose-independent with increasing concentration of omega-3. The membrane of erythrocytes is predominantly made up of lipids and the proportions of the different types of lipids affect membrane integrity, structure and function [30]. For example, the erythrocytes of rabbits fed on diet rich in omega-3 fatty acids EPA/DHA show greater resistance to lysis in hypotonic saline relative to red blood cells of control animals on normal diet; suggesting that omega-3 fatty acids confer protection against haemolysis [31].
Antioxidant properties of omega-3 fatty acids EPA/DHA
Table 1 show the evaluation of the antioxidant properties of omega-3 fatty acids EPA/DHA using gallic acid as references. Omega-3 fatty acids EPA/DHA exhibited antioxidant potentials and an average reducing power at 26.08±0.3mg FeII/100g of Omega-3 fatty acids EPA/DHA after carrying out FRAP but significantly (p<0.05) lower compared to gallic acid (mg FeII/100g). It also showed a significant (p<0.05) inhibitory activity on free radicals of 2,2-Diphenyl-1picrylhydrazyl (DPPHo) and hydroxyl radical (HOo) at IC50 3.7±0.0mg/mL and 11.28±0.3mg/mL respectively but remains lower than gallic acid. (Table 2)
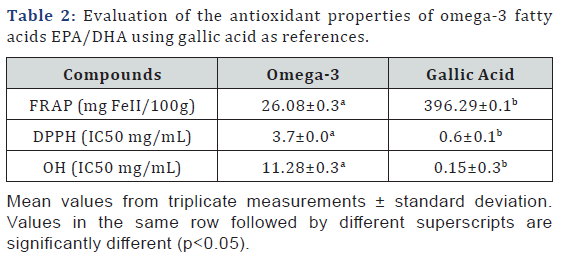
It was also found that the Tiger nut oil (Cyperus esculentus) and black seed oil (Nigella sativa) treatments resulted in an increase in the antioxidant presence of sickle cell samples when tested in vitro [29]. I n f act, oxidative phenomena play a significant role in the physiopathology of sickle cell disease. Sickle RBCs produce greater quantities of superoxide radical, hydrogen peroxide (H2O2) and hydroxyl radical than do normal RBCs [32]. Despite the evident beneficial effects of n-3 fatty supplementation for patients with SCD, there was a lingering concern that the fatty acids, because of their high double bond index and susceptibility to peroxidation [33], might exacerbate the inherent oxidative stress associated with the disease. We discover with this study that omega-3 fatty acids EPA/DHA has a global antioxidant capacity and is active on 2,2-Diphenyl- 1picrylhydrazyl and hydroxyl radicals.
Conclusion
Omega-3 fatty acids EPA/DHA have antisickling, antihemolytic and antioxidant properties. The results obtained are in addition to those of the authors who showed that Omega-3 fatty acids EPA/DHA was effective at reducing the frequency and severity of haemolysis, vaso-occlusive episodes, severe anemia, and blood transfusion. The mode of action of these properties studied will be the next point of focus within this research.
References
- Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH (2000) Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 35(12): 1305-1312.
- Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, et al. (2008) Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 69(4): 644-651.
- Lazzarin N, Vaquero E, Exacoustos C, Bertonotti E, Romanini ME, et al. (2009) Low-dose aspirin and omega-3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertil Steril 92(1): 296-300.
- Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, et al. (2011) Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 93(2): 402-412.
- Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, et al. (2007) The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatr Res 62(6): 689-694.
- Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8(5): 349-361.
- Dawczynski C, Martin L, Wagner A, Jahreis G (2010) n-3 LC-PUFA-enriched dairy products are able to reduce cardiovascular risk factors: a doubleblind, cross-over study. Clin Nutr 29(5): 592-599.
- Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, et al. (2003) Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: a case-control study. Br J Nutr 89(4): 483-489.
- Ren H, Ibegbulam GO, Okpala IE, Ghebremeskel K, Ugochukwu CC, et al. (2005) Steadystate haemoglobin level in sickle cell anaemia increases with the proportion of erythrocyte membrane n-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 72(6): 415-421.
- Ren H, Ghebremeskel K, Okpala I, Ugochukwu C, Crawford M, et al. (2006) Abnormality of erythrocyte membrane n-3 long-chain fatty acids in sickle cell haemoglobin C (HbSC) disease is not as remarkable as in sickle cell anaemia (HbSS) Prostaglandins. Leukot Essent Fatty Acids 74(1): 1-6.
- Ballas SK (2005) Pain Management of Sickle Cell Disease. Hematol Oncol Clin North Am 19(5): 785-802.
- Stuart MJ, Nagel RL (2004) Sickle Cell Disease. Lancet 364(9442): 1343-1360
- Ballas SK (1998) Sickle cell pain Progress in pain research and management. Seattle, WA: IASP Press Vol. 11, p. 109.
- Almeida A, Roberts I (2005) Bone Involvement in Sickle Cell Disease. Br J Haematol 129(4): 482-490.
- Brozovic M, Davies SC, Brownell AI (1987) Acute Admissions of Patients with Sickle Cell Disease Who Live in Britain. Br Med J (Clin Res Ed) 294(6581): 1206-1208.
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, et al. (1991) Pain in Sickle Cell Disease. Rates and Risk factors. N Eng J Med 325(1): 11-16.
- Tomer A, Kasey S, Connor WE, Clark S, Harker LA, et al. (2001) Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n23 fatty acids. Thromb Haemost 85(6): 966-974.
- Okpala I, Ibegbulam O, Duru A, Ocheni S, Emodi I, et al. (2011) Pilot study of omega-3 fatty acid supplements in sickle cell disease. APMIS 119(7): 442-448.
- Kestin M, Clifton P, Belling GB, Nestel PJ (1990) n-3 fatty acids of marine origin lower systolic blood pressure and triglycerides but raise LDL cholesterol compared with n-3 and n-6 fatty acids from plants. Am J Clin Nutr 51(6): 1028-1034.
- Kinsella JE, Lokesh B, Stone RA (1990) Dietary n-3 polyunsaturated fatty acids and amelioration of cardiovascular disease: possible mechanisms. Am J Clin Nutr 52(1): 1-28.
- Joppa KM, Vovora A, Eklu K, Agbonon A, Aklikokou K, et al. (2008) Effet de Morinda Lucida Benth (Rubiaceae) et de Newbouldia Leavis p. Beau v. (Bignoniaceae) sur la falciformation. Med Trop 68(3): 251-256.
- Jaja SI, Kehinde MO, Gbenebitse S, Mojiminyi FBO, Ogungbemi AI (2000) Effect of vitamin C on arterial blood pressure, irreversible sickled cells and osmotic fragility in sickle cell anemia subjects. Nig J Physio Sci 16(1-2): 14-18
- Benzie I, Strain J (1996) The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Anal Biochem 239(1): 70-76.
- Jain A, Soni M, Deb L, Jain A, Rout S, et al. (2008) Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J Ethnopharmacol 115(1): 61-66.






























