Protective Effect of Apple Polyphenols on Acute Ethanol-Induced Neurobehavioral Damage in Mice
Fang Wang1,2, Haifeng Zhang3, Xianchu Han3, Yanxia Fan3, Mingming Liu3, Zhenzhen Song3, Lu Li1, Jingyu Yang2,3 and Chunfu Wu2,3*
1School of Functional Food and Wine, Shenyang Pharmaceutical University, China
2Engineering Research Center for Research and Development of Plant Polyphenols of Liaoning Province, China
3Department of Pharmacology, Shenyang Pharmaceutical University, China
Submission: October 26, 2018; Published: January 16, 2019
*Corresponding author: Chunfu Wu, PhD, Department of Pharmacology, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenhe district, Shenyang, 110016, China
How to cite this article: Fang Wang, Haifeng Zhang, Xianchu Han, Chunfu Wu, et al. Protective Effect of Apple Polyphenols on Acute Ethanol-Induced Neurobehavioral Damage in Mice. Nutri Food Sci Int J. 2019. 8(2): 555733. DOI:10.19080/NFSIJ.2018.08.555733.
Abstract
The present study was to determine the protective effect of apple polyphenols on acute ethanol-induced neurobehavioral damages in mice as well as its possible mechanism of action. A loss of righting reflex test in mice was used to study the protective effect of apple polyphenols on the hypnotic effect caused by acute ethanol intake (4.0g/kg, i.p). Rotarod performance test in mice was used to study the protective effect of apple polyphenols on motor ataxia caused by acute ethanol intake (2.4g/kg, i.p). Blood ethanol concentration and alcohol dehydrogenase activity were measured using assay kits. Pretreatment with apple polyphenols (400 and 800mg/kg) for 1, 3, and 7 days significantly shortened acute ethanol-induced duration of righting reflex loss in Loss of Righting Reflex Test. Pretreatment with apple polyphenols (200mg/kg) for 3 and 7 days and apple polyphenols (200, 400 and 800mg/kg) for 1, 3, and 7 days significantly prolonged the latency to fall off the rotarod after acute ethanol injection in rotarod performance test. All doses of apple polyphenols decreased blood ethanol concentration while apple polyphenols (800mg/kg) increased hepatic alcohol dehydrogenase activity after acute ethanol injection. These results indicate that apple polyphenols have significant protective effects against the hypnotic effect and motor ataxia induced by acute ethanol. These effects may partially occur via a mechanism linked to promoting ethanol metabolism.
Keywords: Apple polyphenols; Ethanol; Neurobehavioral damage; Blood ethanol concentration; Alcohol dehydrogenase
Abbreviations: AP: Apple Polyphenols; AA: Ascorbic Acid; LORR: The Loss of Righting Reflex; ADH: Alcohol Dehydrogenase
Introduction
Alcohol excessive use can increase the risk of a number of health problems, such as intoxication and dependence. Alcohol can affect most organs and systems, especially the central nervous system [1]. Cell membranes are highly permeable to alcohol and once alcohol enters the bloodstream, it can diffuse into nearly everybody tissue and show differential effects according to the blood alcohol concentration. In animal experiments, acute alcohol exposure has been shown to produce differential neurobehavioral abnormalities, such as motor impairment [2], reduction of the probability of longer reaction time responses [3] and sedation/hypnosis [4].
Apples have been traditionally regarded as a healthy fruit in many countries. Large quantities of polyphenols can be extracted and purified from apple peel, especially from the unripe apple. Apple polyphenols (AP) have received much attention over the past decades for their diverse roles in human health. The main classes of AP are flavonoids, including procyanidins, quercetin, (-)-epicatechin and (+)-catechin and anthocyanidins, dihydrochalcones such as phloretin and phloridzin and other polyphenolic compounds such as chlorogenic acid [5]. AP are shown to have a wide range of biological activities with very little adverse effect [6], including strong antioxidant activity [7], hepatoprotective effect [8,9], antiallergic activity [10], antiproliferative activity in cancer cells [11], and hypotriglyceridemic effects [12]. Studies have shown that polyphenols extracted from grapes have neuroprotective activities on ethanol-induced morphological damage [13] and oxidative DNA damage of neurons [14].
Taken together, we hypothesized that AP have protective effects on acute ethanol-induced differential neurobehavioral abnormalities and that these effects may occur through a mechanism linked to promoting ethanol metabolism. The aims of this research were first to assess the neuroprotective effects of AP in two acute ethanol-induced neurobehavioral damage models and then to explore the underlying mechanisms of those effects.
Materials and Methods
Materials and chemicals
Apple polyphenols (AP, Appjfnol) was provided by Tianjin Jianfeng Natural Product R&D Co., Ltd (Tianjin, China). Ascorbic acid (AA) was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). AP and AA were dissolved in distilled water before use. Ethanol assay kit was purchased from SEO Co., Ltd (USA) and ADH assay kit was purchased from Nanjing JianCheng Bioengineering Institute (Nanjing, China). Ethanol was purchased from Tianjin Hengxing Chemical Reagent Co., (Tianjin, China) and diluted by saline to 20 % (v/v) before use.
AP Sample preparation and analysis
AP preparation and the polyphenol profiles were analyzed by reversed phase HPLC according to our previous study [8]. The HPLC chromatogram and specifications of AP were summarized in our previously published paper [8].
Animals
Male Swiss-Kunming mice, weighing between 18-22g, were obtained from the Experimental Animal Center of Shenyang Pharmaceutical University. Mice were housed under a 12h lightdark cycle with ad libitum water and food. All mice used in this study were in accordance with the guideline for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and approved by the Animal Ethics Committee of Shenyang Pharmaceutical University.
Loss of righting reflex
Mice were divided into five groups of 20 mice each: (1) ethanol model group: ethanol+distilled water; (2) positive control group: ethanol+ascorbic acid (200mg/kg); (3) AP low-dose group: ethanol+AP (200mg/kg); (4) AP middle-dose group: ethanol+AP (400mg/kg); (5) AP high-dose group: ethanol+AP (800mg/kg). Mice in the ethanol model group, positive control group and AP treatment groups were administered distilled water, ascorbic acid and AP, respectively, for 1, 3 or 7 days via the intragastric route. 60min after the last drug administration, all mice were injected with ethanol (4g/kg) intraperitoneally. Immediately after ethanol administration, the loss of righting reflex (LORR) performance was observed. The duration of LORR was measured as the time interval between the loss and recovery of righting reflex [15].
Fixed-speed rotarod
Mice were divided into six groups of 20 mice each: (1) control group; (2) ethanol model group: ethanol+distilled water; (3) positive control group: ethanol+ascorbic acid (200mg/kg); (4) AP low-dose group: ethanol+AP (200mg/kg); (5) AP middledose group: ethanol+AP (400mg/kg); (6) AP high-dose group: ethanol+AP (800mg/kg). Mice in the control and ethanol model groups were administered distilled water intragastrically, mice in the positive control and AP treatment groups were administered ascorbic acid and AP, respectively, for 1, 3 or 7 days via the intragastric route. The test procedure was performed according to a previously described method with minor modifications [16]. All mice were trained on a fixed-speed rotarod (speed of rod, 16.0 rpm) 24 hours before the test day. Training was considered complete when mice were able to remain on the rotarod for 180 seconds. Animals that did not successfully stay on the rotarod for 180 seconds were excluded from further testing. On the test day, mice in the control group were injected intraperitoneally with distilled water while other mice were injected intraperitoneally with ethanol (2.4g/kg) 60min after the last drug administration. Each mouse was placed on the rotarod at 0-, 10-, 20-, 30-, 40-, 50-, 60-, 70-, 80- min after ethanol injection and the latency to fall from the rotarod was recorded. During each trial, the latency to fall from the rotarod was recorded with a maximum duration of 180 seconds per trial.
Biochemical analysis
Mice were divided into six groups of 20 mice each: (1) control group; (2) ethanol model group: ethanol+distilled water; (3) positive control group: ethanol+ascorbic acid (200mg/kg); (4) AP low-dose group: ethanol+AP (200mg/kg); (5) AP middledose group: ethanol+AP (400mg/kg); (6) AP high-dose group: ethanol+AP (800 mg/kg). Mice in the control and ethanol model groups were administered distilled water intragastrically; Mice in the AP groups were administered AP and mice in the positive control group were administered ascorbic acid for one time via the intragastric route. 60min after administration, mice in the control group were administered distilled water intragastrically while other mice were injected with ethanol (4g/kg) intraperitoneally. Venous blood was collected from the ophthalmic venous plexus 40min after ethanol injection and the blood ethanol concentration was measured using the ethanol assay kit [17]. Liver samples were homogenized and hepatic ADH activity was measured using the ADH assay kit.
Statistical analysis
Values are given as mean ± SEM. In the experimemt Fixedspeed rotarod test, repeated measures ANOVA analysis was used with groups as between-subjects’ factors and times as within-subjects’ factors. Group difference at one time point was analyzed by one-way ANOVA followed LSD test. The data in other experiments were analyzed by one-way ANOVA followed LSD test. SPSS 13.0 (Chicago, IL, USA) was used. P<0.05 was regarded as statistically significant.
Result
Protective effect of AP on ethanol-induced loss of righting reflex in mice
The effect of AP on ethanol-induced LORR is shown in Figure 1. Acute ethanol (4.0g/kg) induced significant LORR in mice and the duration of LORR lasted about 60 min. Pretreatment with AP (200mg/kg) for 1, 3, and 7 days did not cause any change for ethanol-induced LORR. Pretreatment with AP at 400 and 800mg/ kg administered once before ethanol injection shortened the ethanol-induced duration of LORR. Pretreatment with AP for 3 or 7 days also significantly shortened the duration of LORR. Pretreatment with positive control drug ascorbic acid (200mg/ kg) shortened the duration of LORR. (Figure 1)
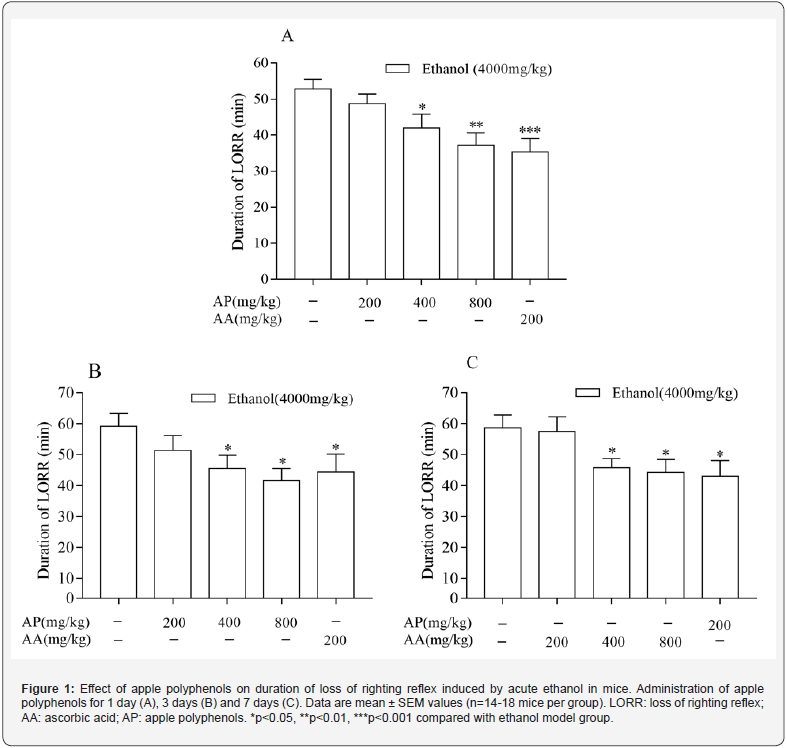
Protective effect of AP on ethanol-induced motor ataxia in mice
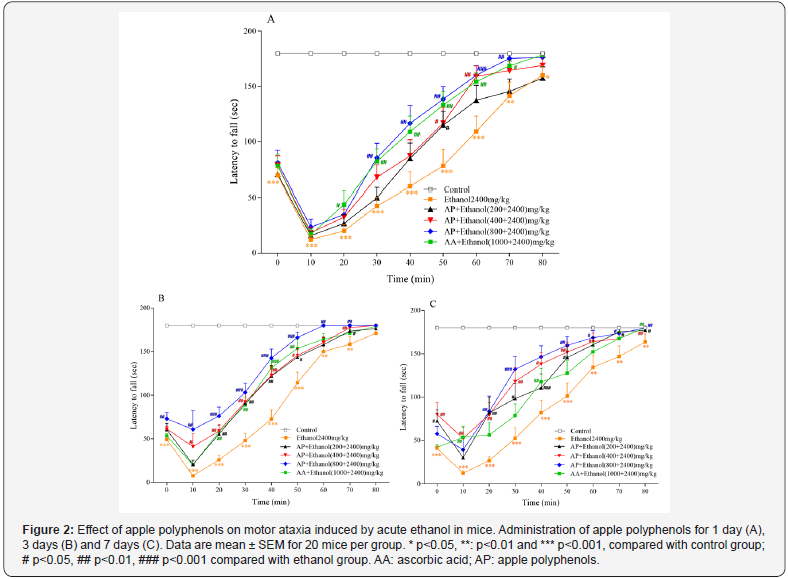
In the motor ataxia experiment, all mice in the control group were able to remain on the rotarod for 180 second at every test time point for the whole experiment period. The latency to fall from the rotarod was shortened when the mice received ethanol (2.4g/kg) and this effect lasted for almost 1 hour (Figure 2). The impairment happened immediately, and the most serious impairment occurred at 10min after ethanol injection. AP at 400 and 800mg/kg administered once before ethanol injection significantly prolonged the latency to fall from the rotarod after ethanol injection. Pretreatment of AP at all three doses for 3 or 7 days prolonged the latency to fall from the rotarod more quickly and significantly after ethanol injection. Pretreatment of AP produced improvement of motor coordination (Figure 2).
Effect of AP on blood ethanol concentration and hepatic ADH activity in mice exposed to ethanol
In order to explore the potential mechanism of protective effect of AP on acute ethanol-induced neurobehavioral damage, the blood ethanol concentration and hepatic ADH activity were detected. The blood ethanol concentration significantly increased in mice exposed to ethanol. Pretreatment with AP (200, 400 and 800mg/kg) significantly decreased the blood ethanol concentration in a dose-dependent manner (Figure 3A). The activity of hepatic ADH was increased in mice exposed to ethanol. Pretreatment with AP (800mg/kg) significantly increased hepatic ADH activity (Figure 3B).

Discussion
Although alcohol consumption is thought to be an integral part of daily life, the World Health Organization has ranked alcohol as one of the primary causes of the global burden of disease in industrialized countries [18]. It is well known that the central nervous system is a particularly susceptible target organ to acute ethanol toxicity. With the dose increase, ethanol causes gradual increase in anxiety, loss of exploration, muscle relaxation and ataxia, sedation and hypnosis [19]. LORR test is a simple and reliable method to assess sensitivity to acute ethanolinduced hypnotic effect [2,20] and fixed-speed rotarod test is a suitable motor performance test for evaluation of acute ethanolinduced cerebellar impairment in animal experiments [21]. These two kinds of model systems are not only commonly used to investigate the mechanism of ethanol-induced neurobehavioral impairment but also for the screening of candidate agents that have protective effect on neurobehavioral damage [22,23]. In the present study, 4.0g/kg of ethanol significantly prolonged the duration of LORR in mice as described in previous studies [24,25]. Pretreatment of AP at the doses of 400 and 800mg/kg for 1, 3 or 7 days significantly improved the acute central inhibition induced by ethanol. Moreover, we also found that pretreatment with AP at 200mg/kg for 3 or 7 days and AP at 400 and 800mg/kg for 1, 3 or 7 days significantly improved acute motor coordination induced by ethanol (2.4g/kg). From the above results, we conclude that AP can especially improve acute neurobehavioral damage induced by excessive doses of ethanol. It has been reported that AP had no significant hematological, clinical, chemical, histopathological or urinary effects at a dose of 2000 mg/kg in a 90-day subchronictoxicity test [6]. Furthermore, both chromosomal aberration and micronucleus tests on AP have found no significant mutagenicity [6]. Furthermore, both chromosomal aberration and micronucleus tests on AP have found no significant mutagenicity6. Based on our present results and the previous reports on the safety of AP, it is suggested that AP has a potential application value with high safety.
Behavioral impairment induced by acute ethanol is associated with blood ethanol concentration, the rate of ethanol metabolism by ADH and the microsomal alcohol-oxidizing system [26]. Blood ethanol concentration is a more accurate indicator of the potential for ethanol-induced damage than ethanol dose administration because of the metabolic differences of ethanol among individuals [27]. Route of administration for ethanol can affect the peak and profile of blood ethanol concentration. In the present study, we chose intraperitoneal injection because this kind of route of administration resulted in a more rapid increase and higher blood ethanol concentration than intragastric gavage which saw about 10% of ethanol metabolized by gastric ADH [27,28]. Our results showed that the increase of blood ethanol concentration in the ethanol model group was similar to a previous report [27]. Pretreatment of AP decreased the blood ethanol concentration in a dose-dependent manner, suggesting that decrease of the blood ethanol concentration is one of the reasons of protective effect of AP on acute ethanol-induced neurobehavioral damage. In animals, intraperitoneal ethanol is absorbed directly from the peritoneal cavity into the portal bloodstream, where it travels to the liver and is metabolized into acetaldehyde by hepatic ADH [27]. ADH is thought to be involved in the major pathway for ethanol metabolism and is an important enzyme that oxidizes alcohol at a fast rate to decrease the alcohol concentration. Our results showed that AP increased hepatic ADH activity, indicating that AP protected the acute ethanol-induced neurobehavioral damage partly via a mechanism linked to promoting ethanol metabolism. We also noticed that though AP decreased blood ethanol concentration 40 min after ethanol injection, the blood ethanol concentration in the AP group was still high. At this time point, mice in AP groups had shown improvement in acute ethanolinduced central inhibition via the LOSS test. This phenomenon indicates that there may be other mechanisms of AP involved in apart from promoting ethanol metabolism and it is worthy to be studied further in the future.
Conclusion
In conclusion, the present study is the first to demonstrate that AP significantly protects against acute ethanol-induced neurobehavioral damage in mice. Moreover, the effects of AP may be partly attributed to the decrease of the blood ethanol concentration and increase in hepatic ADH activity. Our study could serve as a useful reference to allow for future exploitation of the potential of AP to prevent ethanol-induced neurobehavioral damage.
References
- Mukherjee S (2013) Alcoholism and its effects on the central nervous system. Curr Neurovasc Res 10(3): 256-262.
- Ornelas LC, Novier A, Van Skike CE, Diaz-Granados JL, Matthews DB (2015) The effects of acute alcohol on motor impairments in adolescent, adult, and aged rats. Alcohol 49(2): 121-126.
- Wright MJ, Vandewater SA, Taffe MA (2013) The influence of acute and chronic alcohol consumption on response time distribution in adolescent rhesus macaques. Neuropharmacology 70: 12-18.
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, et al (2012) Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol 165(5): 1319-1329.
- Lotito SB, Frei B (2004) Relevance of apple polyphenols as antioxidants in human plasma: contrasting in vitro and in vivo effects. Free Radical Biology and Medicine 36(2): 201-211.
- Shoji T, Akazome Y, Kanda T, Ikeda M (2004) The toxicology and safety of apple polyphenol extract. Food and Chemical Toxicology 42(6): 959- 967.
- Gogia N, Bukia Z, Atamashvili T, Esaiashvili M, Chkhikvishvili I (2015) The amount of polyphenols and antioxidant activity of fruits of different varieties of apple tree-Malus domectica L. Georgian Med News (242): 84-88.
- Yang J, Li Y, Wang F, Wu C (2010) Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J Agric Food Chem 58(10): 6525-6531.
- Wang F, Xue Y, Yang J, Lin F, Sun Y, et al (2016) Hepatoprotective effect of apple polyphenols against concanavalin A-induced immunological liver injury in mice. Chemico-Biological Interactions 258: 159-165.
- Akiyama H, Sakushima J, Taniuchi S, Kanda T, Yanagida A, et al (2000) Antiallergic effect of apple polyphenols on the allergic model mouse. Biol Pharm Bull 23(11):1370-1373.
- Kern M, Pahlke G, Balavenkatraman KK, Bohmer FD, Marko D (2007) Apple polyphenols affect protein kinase C activity and the onset of apoptosis in human colon carcinoma cells. J Agric Food Chem 55(13): 4999-5006.
- Yao N, He RR, Zeng XH, Huang XJ, Du TL, et al (2007) Hypotriglyceridemic effects of apple polyphenols extract via up-regulation of lipoprotein lipase in triton WR-1339-induced mice. Chin J Integr Med 20(1): 31-35.
- Carneiro A, Assuncao M, De Freitas V, Paula-Barbosa MM, Andrade JP (2008) Red Wine, but not port wine, protects rat hippocampal dentate gyrus against ethanol-induced neuronal damage-relevance of the sugar content. Alcohol Alcohol 43(4): 408-415.
- Guo L, Wang LH, Sun B, Yang JY, Zhao YQ, et al (2007) Direct in vivo evidence of protective effects of grape seed procyanidin fractions and other antioxidants against ethanol-induced oxidative DNA damage in mouse brain cells. J Agric Food Chem 55(14): 5881-5891.
- Wu CF, Zhang HL, Liu W (2000) Potentiation of ethanol-induced loss of the righting reflex by ascorbic acid in mice: interaction with dopamine antagonists. Pharmacol Biochem Behav 66(2): 413-418.
- Kamens HM, Andersen J, Picciotto MR (2010) The nicotinic acetylcholine receptor partial agonist varenicline increases the ataxic and sedative-hypnotic effects of acute ethanol administration in C57BL/6J mice. Alcohol Clin Exp Res 34(12): 2053-2060.
- Ieraci A, Herrera DG (2006) Nicotinamide protects against ethanolinduced apoptotic neurodegeneration in the developing mouse brain. Plos Medicine 3(4): e101.
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, et al (2002) Selected major risk factors and global and regional burden of disease. Lancet 360(9343): 1347-1360.
- Linden AM, Schmitt U, Leppa E, Wulff P, Wisden W, et al (2011) Ro 15-4513 Antagonizes Alcohol-Induced Sedation in Mice Through alphabetagamma 2-type GABA(A) Receptors. Frontiers in Neuroscience 5: 3.
- Ponomarev I, Crabbe JC (2002) A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther 302(1): 257-263.
- Shiotsuki H, Yoshimi K, Shimo Y, Funayama M, Takamatsu Y, et al (2010) A rotarod test for evaluation of motor skill learning. J Neurosci Methods 189(2): 180-185.
- Galdino PM, Nascimento MV, Florentino IF, Lino RC, Fajemiroye JO, et al. (2012) The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, beta-caryophyllene, in male mice. Prog Neuropsychopharmacol Biol Psychiatry 38(2): 276-284.
- Yang JY, Wu CF, Song HR (1999) Studies on the sedative and hypnotic effects of oleamide in mice. Arzneimittelforschung 49(8): 663-667.
- Didone V, Quoilin C, Nyssen L, Closon C, Tirelli E, et al. (2013) Effects of L-histidine and histamine H3 receptor modulators on ethanol-induced sedation in mice. Behavioural Brain Research. 238: 113-118.
- Naassila M, Ledent C, Daoust M (2002) Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci 22(23): 10487-10493.
- Naranjo CA, Bremner KE (1993) Behavioural correlates of alcohol intoxication. Addiction 88(1): 25-35.
- Livy DJ, Parnell SE, West JR (2003) Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol 29(3): 165-171.






























