The Plant Polyphenol Metabolism as Functional Architecture and its Nutritional Exploitation
Aurelia Scarano* and Angelo Santino*
ISPA-CNR, Institute of Science of Food Production, CNR Unit of Lecce, Italy
Submission: October 25, 2018; Published: January 04, 2019
*Corresponding author: Aurelia Scarano & Angelo Santino, ISPA-CNR, Institute of Science of Food Production, CNR Unit of Lecce, Italy
How to cite this article: Aurelia Scarano, Angelo Santino. The Plant Polyphenol Metabolism as Functional Architecture and its Nutritional Exploitation. Nutri Food Sci Int J. 2019; 8(1): 555731. DOI: 10.19080/NFSIJ.2019.08.555731.
Abstract
Background: Plant secondary metabolites are characterized by a great diversity of chemical structures. The interaction with surrounding environment has the effect to continuously re-shape secondary metabolites and polyphenols in particular. Due to their interesting healthy properties, polyphenols showed a growing interest and popularity, even though their amounts in most natural sources are often not sufficiently assumed for an optimal dietary intake.
Methods: In this article, we highlight the importance of polyphenol improvement by biofortification, with a closer look to the metabolic engineering approach in vegetables, and how it can be nutritionally exploitable to increase the nutritional value of crops with an important impact on human health.
Results: Some examples of metabolic engineering show how polyphenol-enrichment is achievable by different strategies and increase the levels of nutritionally important secondary metabolites. Furthermore, they are also a useful tool to study the effects of their administration within the context of the original and homogeneous food matrices.
Conclusion:v Crop biofortified by different polyphenol classes can allow the creation of a library of specific foods useful for a better dietary intake in both normal and pathological conditions. Furthermore, it will be useful to improve our knowledge on the biological functions of polyphenols in the context of “structure-function” relationship.
Keywords: Secondary metabolism; Structure-function; Polyphenols; Biofortification
Introduction
Plant secondary metabolism has been studied over years, identifying three major classes of phytochemicals: terpenoids, alkaloids and polyphenols [1]. In the past, the secondary metabolism was thought as “non-essential” and subordinated to the primary metabolism, which is instead committed to the basal energy production and biosynthesis of all the essential components of a living organism. Over the time, the utility of the secondary metabolism has been more widely recognized, as it participates to several processes, which cannot be considered as “secondary”, as in the case of reproduction or defense to biotic or abiotic stresses [1,2]. The initial hypothesis was that different compounds have different roles in the cell [3] and that plants have been evolving to adapt to the environment taking also advantage of the genetic diversity of secondary metabolism pathways [1,4]. In fact, a very interesting theme is related to the inter- or intra-specific specialization and the process of adaptation that occurs contextually with secondary metabolism evolution. To date, more information is available regarding the function of some groups of secondary metabolites. For example, polyphenols are among the most studied secondary metabolites within the plant kingdom and many of their functions have been widely described [5-7]. Despite more studies are still needed to clarify the function related to specific compounds, the actual degree of knowledge allow to consider more specific functions for polyphenols and contextualize them within the plant. Some main polyphenols classes such as flavonoids and anthocyanins are significantly produced in response to UV radiation (also described as sunscreens) or when plants have to counteract reactive oxygen species [8,9]. Others as stilbenes are recognized as phytoalexins, compounds particularly functioning in direct defense towards the attack of phytopathogens [5]. Therefore, research is moving fast to verify the physiological significance of the main polyphenol classes. In this context, it is particularly important to investigate the relationship between the chemical structure and function.
Discussion
The relationship “structure - function - external interaction” and the analogy with the functional and organic architectures
In 1923, the theoretical architect Le Corbusier created a new language and a new philosophy in the modern architecture, which was called functional architecture. The function is shaped according the human needs, following an extreme organization of the home living spaces and elaborating precise modules based on human body size (Modulor) (Vers Une Architecture, Le Corbusier, 1923). In this way, the buildings exactly reflect their own functions, bending to the essential and maximizing the function. Another interesting philosophy of those years is the organic architecture, signed by F.L. Wright, who highlighted the reciprocal exchange between the natural environment and the house living space.
Considering these two different architecture concepts, we can image an analogy with plants and their secondary metabolisms. The specialization in planta has the shape of metabolic modules that are not fixed but quite fluid and dynamic. The fluidity of these metabolic fluxes is strongly affected by environmental factors, which continuously reshape the metabolic systems in the process of adaptation. Therefore, following the genetic background of the metabolic pathways, we can observe a perfect functional architecture inside the plant, but de facto it becomes similar to the organic architecture when it interfaces with the environment and thus adaptation.
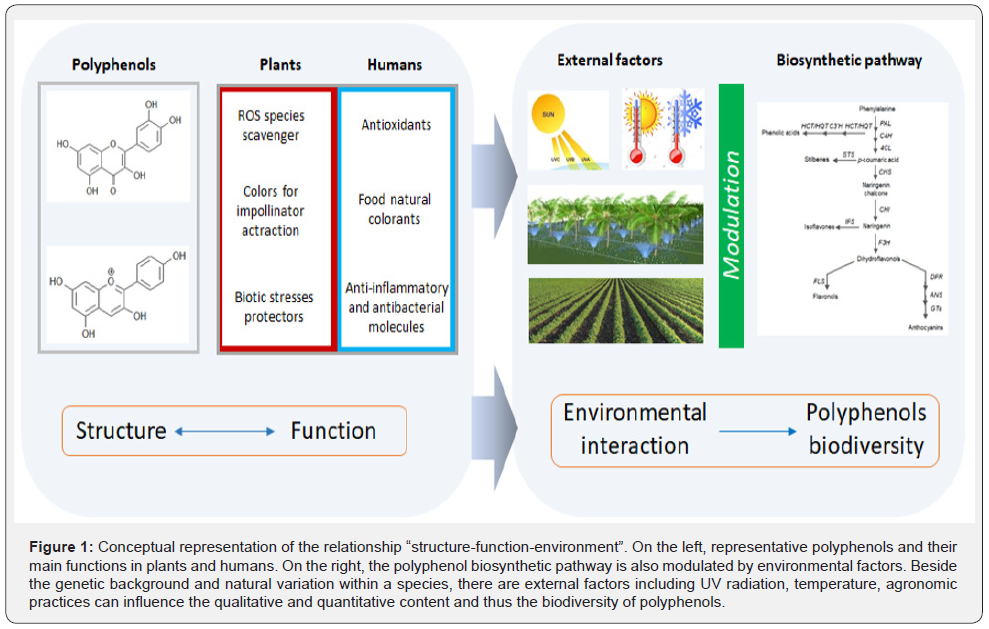
The polyphenols metabolism can be considered a good model reflecting functional and organic architecture at the same time (Figure 1). The polyphenols backbone is based on aromatic rings and structural elements bound to these rings. These elements are responsible of polyphenol biodiversity and allow their classification in different classes such as phenolic acids, flavonoids, stilbenes and lignans (Figure 2) [5,10]. The great natural variability and the wide diversity of polyphenols can be considered an indication of their specialization in planta. Polyphenol aglycones undergo to several reactions, consisting in glycosylation, methylation or acylation which finally lead to the addition of various residues. Furthermore, qualitative and quantitative changes in polyphenol composition can be affected by environmental conditions, as in the case of UV radiation and temperature [11]. Hence, from this point of view, the relationship “structure–function” of polyphenols is also orchestrated and modulated in response to environmental interactions (Figure 1). However, the concept of polyphenols structure-function can be important also for humans. For example, the same antioxidant property of some polyphenols, which is an important feature for plant cell metabolism, is also exploitable for humans as healthy benefit.
The biological feature of the antioxidant activity can be exerted in a direct or indirect way. In the direct way, the molecule has mainly the function of scavenger of reactive oxygen species (ROS), and this is the dominant explanation of the “biochemical scavenger theory” [12]. In this context, Sroka et al. (2003) have observed that the antiradical ability of phenolic compounds is positively related to the number of hydroxyl groups on the aromatic rings and their position: for example, the ortho- or para-positions of hydroxyl groups are related to a higher antiradical activity than the metaposition. Furthermore, the presence of specific substituents, as acetyl or carboxyl groups, and their position in respect to the hydroxyl groups can also influence the antioxidant and anti-radical activities of phenolic compounds [13]. In addition to this direct way to counteract the ROS species, polyphenols can also function in an indirect manner by reducing enzymatic activities like glutathione reductase or superoxide dismutase [14]. Beside their antioxidant properties, polyphenols can exert other important functions in human body, particularly the antiinflammatory activity. Indeed, polyphenols can contribute to the reduction of pro-inflammatory mediators and to the interfering with the induction of NFκB and MAPKs signaling pathways [15]. Other in vitro studies have also suggested that polyphenols are potent inhibitors of vascular endothelial growth factor (VEGF), which is an important pro-angiogenic growth factor implicated in pathological inflammatory processes such as atherosclerosis [16]. This inhibitory activity is related to the molecular structure of polyphenols and is related to several features as: the total number of hydroxyl groups on the B-ring; the hydroxylation of the position 3 on the C-ring; the presence of hydroxyl groups on the B-ring; the presence of the catechol group on the B-ring; the presence of a C2=C3 double bound in the C-ring (as in the case of quercetin); the presence of a galloyl group on the position 3 of flavanols (as in the case of catechins).
Conceptual representation of the relationship “structurefunction- environment”. On the left, representative polyphenols and their main functions in plants and humans. On the right, the polyphenol biosynthetic pathway is also modulated by environmental factors. Beside the genetic background and natural variation within a species, there are external factors including UV radiation, temperature, agronomic practices can influence the qualitative and quantitative content and thus the biodiversity of polyphenols.
How the plant polyphenols can be functional for humans: the problem of the polyphenol dietary intake
Natural sources of polyphenols are fruits such as berries (strawberries, blueberries, blackberries, raspberries, blackcurrants), grape and cherries, common beverages like coffee, wine and black or green tea, and horticultural species such as red or yellow onions, cruciferous species or Solanaceuos species [17]. Nevertheless, an important issue regarding polyphenols bioactivity is related to their bioavailability, since they are ingested within complex food matrices [15,18]. Even though many studies regarding the bioactivity and the mechanism of action of polyphenols, are based on pure or standard compounds [19,20] and do not take into account the real food.
Despite fruits and vegetables are important sources of our daily diet, they often accumulate polyphenols in suboptimal amounts, as in the case of some common horticultural crops [21,22]. Furthermore, changes in lifestyle caused a reduction in the assumption of plant-derived food in daily diet, especially in western countries [23,24]. Consequently, the dietary intake of healthy phytonutrients like polyphenols is at risk to be suboptimal. From this point of view, biofortification of plant foods can provide:
I. A useful tool to enrich polyphenol biodiversity and their content in plant-derived foods;
II. The basis to study in better detail the bioactivity of specific classes within a whole unique food matrix. Furthermore, the metabolic engineering strategy can be also useful to induce the production of new classes of phytochemicals [25,26] normally not synthetized in a crop species, or to modify the metabolic fluxes in a given pathway [23].
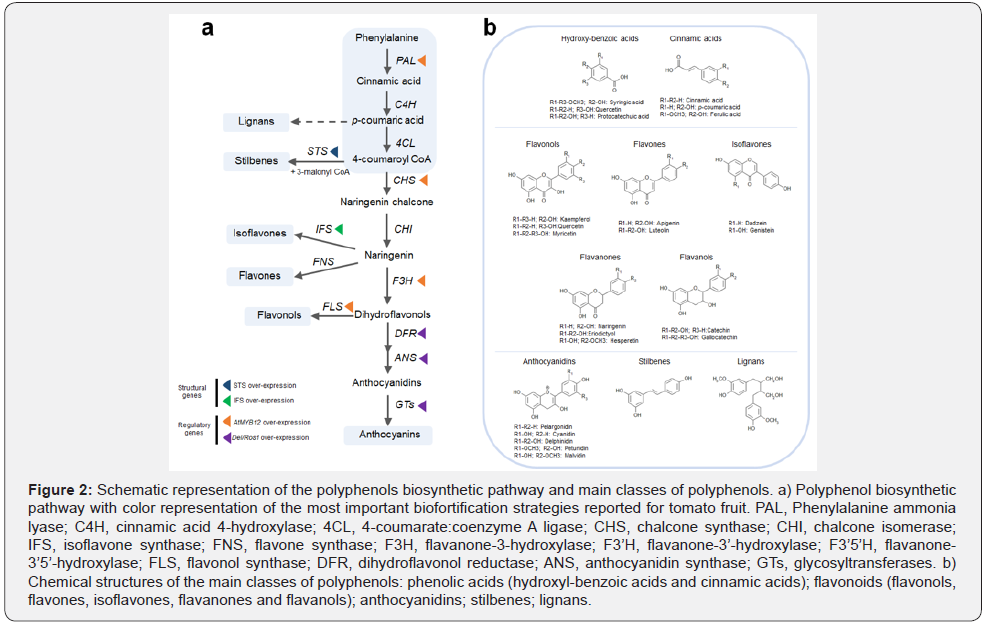
The most widely used strategy of genetic engineering relies on the over-expression of both structural genes (genes directly involved in the biosynthetic pathway) and regulatory genes (genes encoding transcription factors regulating the expression of structural genes) to increase polyphenol levels (Figure 2). Tomato has been widely used as model species for horticultural crops to carry out metabolic engineering interventions due to reliable protocols of plant transformation and regeneration [27-31]. As a result, new tomato lines, able to produce increased set of polyphenols normally absent in tomato fruit or accumulated in few amounts, were obtained. The purple tomato, over-expressing two transcription factors (Delila and Rosea1), is a good example of anthocyanins enrichment. These compounds are normally not synthetized in commonly consumed tomato cultivars. A purple tomato-based dietary administration in a mouse cancer model was able to prolong the life-span of treated mice [21]. In another study, a tomato line called Bronze, enriched in multiple classes of polyphenols was daily administered to an IBD (inflammatory bowel disease) mouse model, inducing beneficial changes in the gut microbiota and alleviating the symptoms of the intestinal inflammation [31]. These studies also provide useful information about the amounts of polyphenols sufficient to induce the beneficial effects in comparison with the natural sources [30,31].
Schematic representation of the polyphenol biosynthetic pathway and main classes of polyphenols. a) Polyphenol biosynthetic pathway with color representation of the most important biofortification strategies reported for tomato fruit. PAL, Phenylalanine ammonia lyase; C4H, cinnamic acid 4-hydroxylase; 4CL, 4-coumarate:coenzyme A ligase; CHS, chalcone synthase; CHI, chalcone isomerase; IFS, isoflavone synthase; FNS, flavone synthase; F3H, flavanone- 3-hydroxylase; F3’H, flavanone-3’-hydroxylase; F3’5’H, flavanone-3’5’-hydroxylase; FLS, flavonol synthase; DFR, dihydroflavonol reductase; ANS, anthocyanidin synthase; GTs, glycosyltransferases. b) Chemical structures of the main classes of polyphenols: phenolic acids (hydroxyl-benzoic acids and cinnamic acids); flavonoids (flavonols, flavones, isoflavones, flavanones and flavanols); anthocyanidins; stilbenes; lignans.
Conclusion
Polyphenols are a good example of secondary metabolites, to explain the importance of metabolic specialization in planta and their possible uses to improve human nutrition. Obtaining polyphenol-enriched food by metabolic engineering can allow to reach sufficient amounts to exert an optimal bioactivity. In such a way, it will be possible in the future to create a library of functional foods useful to define more specific nutritional interventions, for example in the prevention or as adjuvants in pathologies related to a chronic inflammation condition. The enrichment of diet with antioxidant and antiinflammatory compounds could therefore contribute to alleviate inflammatory conditions towards a healthier status.
Acknowledgment
This work was supported by the Apulian Region project “NATURE”.
References
- Kabera JN, Semana E, Mussa AR, He X (2014) Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol 2: 377-392.
- Fraenkel GS (1959) The Raison d’Etre of Secondary Plant Substances These Odd Chemicals Arose as a Means of Protecting Plants from Insects and Now Guide Insects to Food. Science 129 (3361): 1466- 1470.
- Seigler D (2000) Plant secondary metabolism. New Phytol 147: 483- 485.
- Waterman PG (1992) Roles for secondary metabolites in plants. In Proceedings of the 171st Ciba Foundation Symposium on Secondary Metabolites: Their Function and Evolution, pp. 255-75.
- Chong J, Poutaraud A, Hugueney P (2009) Metabolism and roles of stilbenes in plants. Plant Sci 177: 143-55.
- Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5): 270-278.
- Di Ferdinando M, Brunetti C, Agati G, Tattini M (2014) Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Envir Exp Bot 103: 107-116.
- Zhang Y, De Stefano R, Robine M, Butelli E, Bulling K, et al. (2015) Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant Physiol 169(3): 1568-1583.
- Tohge T, Fernie AR (2017) Leveraging natural variance towards enhanced undestranding of phytochemical sunscreens. Trends Plant Sci 2(4): 308-315.
- Li A, Li S, Zhang YJ, Xu XR, Chen YM, et al. (2014) Resources and biological activities of natural polyphenols. Nutrients 6(12): 6020- 6047.
- Zoratti L, Karppinen K, Luengo Escobar L, Häggman H, Jaakola L (2014) Light-controlled flavonoid biosynthesis in fruits. Front Plant Sci 5: 534.
- Cory H, Passarelli S, Szeto J, Tamez M, Mattei J (2018) The role of polyphenols in human health and food systems: a mini-review. Front Nutr 5: 87.
- Sroka Z, Cisowski W (2003) Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Cheml Toxicol 41: 753-758.
- Han X, Shen T, Lou H (2007) Dietary polyphenols and their biological significance. Int J Mol Sci 8(9): 950-988.
- Santino A, Scarano A, De Santis S, De Benedictis M, Giovinazzo G, et al. (2017) Gut microbiota modulation and anti-inflammatory properties of dietary polypheols in IBD: new and consolidated perspectives. Curr Pharm Design 23(16): 2344-2351.
- Cerezo AB, Winterbone MS, Moyle CW, Needs PW, Kroon PA (2015) Molecular structure-function relationship of dietary polyphenols for inhibiting VEGF-induced VEGFR-2 activity. Mol Nutr Food Res 59(11): 2119-2131.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79: 727- 747.
- Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, et al. (2016) The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 8(2): 78.
- Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 134(12 Suppl): 3479S-34785S.
- Romier B, Schneider YJ, Larondelle Y, During A (2009) Dietary polyphenols can modulate the intestinal inflammatory response. Nutr Rev 67(7): 363-378.
- Butelli E, Titta L, Giorgio M, Mock HP, Peterek S, et al. (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of selected transcription factors. Nat Biotechnol 26: 1301-1308.
- USDA Database for the flavonoid content of selected Foods (2015).
- Martin C, Lie J (2017) Medicine is not health care, food is health care: plant metabolic engineering, diet and human health. New Phytol 216(3): 669-719.
- Martin C (2018) A role for plant science in underpinning the objective of global nutritional security? Ann Bot 122(4): 541-553.
- Hirschi K (2009) Nutrient biofortification of food crops. Annu Rev Nutr 29: 401-421.
- Zhu C, Sanahuja G, Yuan D, Farré G, Arjó G, et al. (2013) Biofortification of plants with altered antioxidant content and composition: genetic engineering strategies. Plant Biotech J 11(2): 129-141.
- Bovy A, de Vos R, Kemper M, Schijlen E, Almenar Pertejo M, et al. (2002) High-flavonol tomatoes resulting from heterologous expression of the maize transcription factor gene LC and C1. Plant Cell 14(10): 2509- 2526.
- Giovinazzo G, D’Amico L, Paradiso A, Bollini R, Sparvoli F, et al. (2005) Antioxidant metabolite profiles in tomato fruit constitutively expressing the grapevine stilbene synthase gene. Plant Biotech J 3(1): 57-69.
- Luo J, Butelli E, Hill L, Parr A, Niggeweg R, et al. (2008) AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. The Plant J 56(2): 316-326.
- Zhang Y, Butelli E, Alseekh S, Tohge T, Rallapalli G, et al. (2015) Multilevel engineering facilitates the production of phenylpropanoid compounds in tomato. Nat Commun 6: 8635.
- Scarano A, Butelli E, De Santis S, Cavalcanti E, Hill L, et al. (2018) Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front Nutr 4: 75.






























