- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Ready to Use Therapeutic Food [RUTF] formulation and packaging for malnutrition: an Overview
Vijay D Wagh*
Professor in Pharmaceutics, Gokhale Education Society, Sir Dr. MS Gosavi College of Pharmaceutical Education and Research, India
Submission: December 01, 2017; Published: January 26, 2018
*Corresponding author: Vijay D Wagh, Professor in Pharmaceutics, Department of Pharmaceutics, Sir Dr. M.S. Gosavi College of Pharmaceutical Education and Research, Nashik - 422005, Maharashtra, India, Tel: +919923072990; Email: drvijaydwagh@gmail.com
How to cite this article: Vijay D Wagh. Ready to Use Therapeutic Food [RUTF] formulation and packaging for malnutrition: an Overview. Nutri Food SciInt J. 2018; 4(5): 555648. DOI: 007 10.19080/NFSIJ.2018.04.555648
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Abstract
Therapeutic foods are foods designed for specific, usually nutritional, therapeutic purposes as a form of dietary supplement. The primary examples of therapeutic foods are used for emergency feeding of malnourished children or to supplement the diets of persons with special nutrition requirements, such as the elderly. Ready-to-Use Therapeutic Food (RUTF) is a mixture of nutrients designed and primarily addressed to the therapy of the severe acute malnutrition without complications. The recent success of home-based therapy has been seen in conjunction with the availability of a novel food, a spread form of ready-to-use therapeutic food (RUTF). The main ingredients of the formulation are powdered milk, peanuts butter, vegetal oil, sugar, and a mix of vitamins, salts, and minerals. The effectiveness of ready to use therapeutic food within the person’s own home for the treatment of severe acute malnutrition in children under five years of age has been found not to be different than standard care. In this review article we have enlighten on complete formulation and development aspects till its packaging and labeling. This formulation is a need of low income countries and developing countries to combat malnutrition of children's.
Keywords: RUTF; Processing; Manufacturing; Malnutrition; India
Abbreviations: RUTF: Ready-to-Use Therapeutic Food; FAO: Food and Agriculture Organization of the United Nations; SAM: Severe Acute Malnutrition; MDG: Millennium Development Goal; ICDS: Integrated Child Development Scheme
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Introduction
Ready-to-use therapeutic foods
Ready-to-use therapeutic foods (RUTF) are high energy, fortified, ready-to-eat foods suitable for the treatment of children with severe acute malnutrition. These foods should be soft or crushable and should be easy for young children to eat without any preparation. At least half of the proteins contained in the foods should come from milk products [1]. Data published by the Food and Agriculture Organization of the United Nations (FAO) relative to the trend of the hunger in the world from 2006 to 2009 are worrying: the number of individuals that suffer hunger increased of about two hundred millions, increasing from the about 850 millions in the year 2006 to almost one billion and fifty million in the year 2009. These data were partially modified in the year 2010, when the number of people suffering hunger in the world decreased to about one billion [2]. It is also relevant to mention that children aged between zero and five, have a greater food need, due to both a greater energy and nutritional requirements, and to an immune system not completely developed yet. According to the [World Health Organization [3] and the United Nations Children's Fund [4] reports, about one hundred and fifty millions of children from zero to five years are deeply underweight. Among these, about sixty millions are emaciated and/or are affected by various stages of malnutrition [5,6].About twenty millions suffer from severe acute malnutrition (SAM). The nutrients lack influence negatively all the body functions, dragging the individual to serious pathological conditions, e.g. edema, and death [7]. The fourth Millennium Development Goal (MDG), proposed to beat down of two third the mortality of the children under five years of age, in the time period from 1990 to 2015 and, although the good results achieved lately, the full achievement of the objective seems yet to be very far [2]. Flour based foodstuff enriched of cereals and legumes, so far elective and preferred by the Governmental Organizations, are inappropriate with respect to some main problems that affect the most depressed areas of world, like the sub-saharian Africa and the southeastern regions of Asia. In these areas it should be taken into account, for example, the high environmental temperatures that favour the microbial proliferations in the food to be prepared or already cooked. Moreover, the water available to cook any food is often contaminated. The onset in the last years of a new type of product called Ready-to-Use Therapeutic Food (RUTF) seemed to be a breakthrough for these problematic situations. This novel food mainly consists of peanuts, and is enriched with sugar, powdered milk, vegetal oil, vitamins, and mineral salts. Its peculiarity is: a high energy density [about 540kcal/100g], a complete nutritional contribution with mineral salts, vitamins, amino acids, and essential fatty acids, and a prolonged shelf-life with respect to other products (up to 24 months due to the low percentage of water). Another major advantage of the RUTF composition is the possibility to use it on site within therapeutic programs, e.g. directly at home without the need to go to hospitals or to nutritional therapy centers for a proper use of this food. The RUTF, however, has also some problems. In the first place, the peanut butter can be contaminated by aflatoxins, carcinogenic molecules produced from Aspergillus flavusand Aspergillus parasitic us species toxigenic fungi, especially favored.
When the cereals carioxides are not properly handled or stored [8]. Moreover, the presence of the powdered milk influences heavily [almost for one third] the final cost of the RUTF and, considering the price growing tendency of the raw material, in a few years the economical sustainability of this food would be compromised [4]. For the above mentioned reasons, the scientific community should pledge itself in the development of new formulated RUTF, to reach higher safety standard and a proportional economic sustainability for the depressed areas where such product is destined. This paper is addressed to review and assess the available information, and to give some operating clues to try to give a strong stimulus for the search of alternative RUTF formulations that can include also neutraceuticals compounds [9] from vegetal origin [10], specifically addressed to health condition support and therapy. Nutraceuticals from natural sources have been investigated for their putative chemo preventive and cancer therapeutic properties for the last few decades. The interest in these compounds is in part due to their pleiotropic effects and relatively non-toxic nature and has recently become a hot topic for the commercial world and the biomedical community [11]. It is postulated that neutraceuticals are relatively non-toxic food supplements with many health benefits including prevention of cancer [12]. These aspects make them particularly appealing for the inclusion in RUTF formulations addressed to specific therapeutic use when needed.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Ideal Characteristic Properties of RUTF
o Calorie dense, high in proteins, vitamins & minerals.
o Simple to deliver and administer.
o Easy to use and fast acting.
o Affordable and acceptable cost.
o Should not require train stuff to administer [parent can deliver it to child].
o Culturally acceptable.
o Texture: smooth, uniform paste with small particle size [<200microns].
o No grittiness, no lumps, no oil separation. o Easy to squeeze out of the sachet [13]
o Appearance: light brown to cream
o Packed in single serve packets. [Each packet may contain fixed amount of calories 400- 500calories].
o Requires little preparation before use.
o Adequate shelf life and stability.
o Can be stored in varied climatic condition and temp.
o Resistance to bacterial contamination.
o Does not cause addiction to child.
o Good nutritional quality [i.e. protein, energy and micro nutrient content].
o Highly palatable with a good taste.
o A consistency and texture suitable for feeding to children.
o Require no additional processing prior to feeding.
o Amino acid complementation for maximum protein quality.
o Ingredients should be easily available in developing countries.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Ingredients
The formulation of RUTF was derived from F-100 and uses the same ingredients with the addition of peanut butter [14]. Peanut butter changes the physical properties of the food to a viscous liquid product instead of a powder.
A nutritionally suitable multi-mix for RUTF has four basic ingredients
o A staple as the main ingredient -preferably a cereal.
o A protein supplement from a plant or animal food -beans, groundnuts, milks, meats, chicken, fish, eggs, etc. To be practical such foods must be low-cost, and this requirement has pushed development towards legumes and oilseed as these are cheaper than products containing milk or other animal products.
o A vitamin and mineral supplement -a vegetable and/ or fruit.
o An energy supplement -fat, oil or sugar to increase the energy concentration of the mix. A typical recipe for RUTF is given in Table 1.
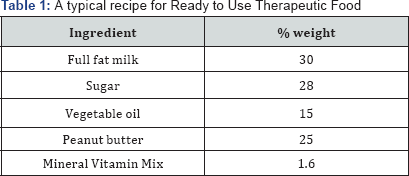
Milk powder
Local supplies of milk powder exist throughout the world; however the milk itself is often imported. Standard commercial techniques to produce milk powder yield a product that is suitable for RUTF production. >50% proteins from milk/dairy products acceptable sources of dairy protein: Skimmed milk powder/Full cream milk powder, Whey protein powder [13].
Vegetable oil
Several types of oil made by standard commercial methods may be used to RUTF, including soy oil, cottonseed oil, rapeseed oil and corn oil. Rapeseed oil and soybean oil have the advantage of providing a good balance of essential fatty acids.
Sugar
Commercial sources of granulated brown or white sugar can be used to make RUTF. The sugar must be ground into a fine powder, a product used in bakeries known as icing sugar or powdered sugar, to reduce the particle size to less than 200 microns.
Peanut butter
This is simply peanuts that have been roasted and ground, without added oil, salt or preservatives. In most areas of the world where peanuts are grown, a commercial food processing company makes peanut butter.
Powdered vitamins and minerals
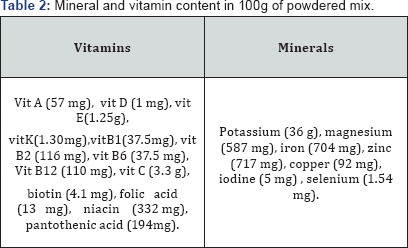
This is a mixture of vitamins and minerals formulated to provide the same amount of micronutrients to the malnourished child as F-100, the standard therapeutic food. Soluble & easily absorbed by patients with SAM. Added minerals water-soluble & shall not form insoluble components when mixed together. Mineral composition that does not alter the acid-base metabolism of patients with SAM: moderate positive non-metabolisable base sufficient to eliminate the risk of metabolic acidosis [13] Table 2.
The World Food Programme and UNICEF have donated ingredients for the production of RUTF in Malawi. The World Food Programme has donated milk, sugar and oil, and UNICEF has donated powdered vitamins and minerals. The RUTF is then used by projects supported and approved by these organizations.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Antioxidants
Antioxidant substances e.g. tocoferols and L-ascorbilpalmitate, are generally added to the RUTF [15] to better stabilize the final product. Particle dimension of the oil emulsion are crucial for the stability of the emulsion [16]. Proteins can act as antioxidant agents with respect to the free radicals action control, and to the chelating metals action, but also have a key role as emulsion forming agents and this way stabilizing the product structure [15].
Emulsifying agents
The lipids added to the foodstuff need to be carefully chosen since the melting temperature depends mainly on the lipids composition. High melting point lipids are preferred to prevent the separation of the components of the RUTF during the storage at room temperature [15]. e.g. Lecithin max 0.5g/100grams, Mono and diglycerides max 2g/100g Level between 1.5 and 2.0g/100g ,can be accepted because there is no adverse effect. All triglyceride oil is decomposed to monoglycerides in the digestion system prior to absorption [13].
Flavoring
Flavoring agent are use in the various food formulation it also use in RUTF formulation for test masking , but artificial flavorings not allowed, only natural flavors are use [13].
Production of RUTF

RUTF is a homogenous mixture of lipid rich and water- soluble foods. The lipids exist as a viscous liquid, and small particles of protein, carbohydrate, vitamins and minerals are mixed throughout this liquid. In order to achieve a homogenous mixture, a specific mixing procedure must be followed. The lipid elements of RUTF are first stirred and often heated; the powdered ingredients are then slowly added to the lipids during vigorous stirring. Once all the powdered ingredients are added, the entire mixture is stirred at higher speeds for several minutes. As long as the powdered ingredients do not have a particle size that is larger than 200 microns, the mixture does not readily separate. When mixtures are made with larger particles, RUTF must be stirred briefly by hand just prior to consumption, to temporarily suspend the large particles in the mixture. The use of oils that are liquids at ambient temperature facilitates the mixing process. Packaging of RUTF can be done from factory bowls or funnels, by hand [simply pouringit] or using a mechanical device [17]Figure 1.
Scale of production
A mechanical mixer is required for all RUTF production. While hand mixing of the ingredients is possible for very small quantities, the quality of the product that is made by hand mixing is so inconsistent it cannot be reliably used. The procedure and equipment used to mix RUTF is contingent upon the quantities of RUTF needed. If a few hundred kg of RUTF is needed each week, small scale production is possible. Small scale production requires a small room dedicated to food production that is free of rodents and other pests. A40 L planetary bakery mixer, such as the MacAdams SM 401, can be used to prepare the RUTF. Such mixers will mix a 25kg batch of RUTF. Ingredients are added by volume to the batch. The containers used to measure the ingredients need to be carefully chosen and calibrated by accurately weighing of the ingredients. Oil and peanut butter should be added directly into the mixing bowl and combined using a mixing speed of 105rpm until homogenous. The Z-shaped kneader blade, rather than a wire whisk device, should be used to minimize the amount of air impregnated into the mixture. Sugar, milk powder, and mineral and vitamin mixture are first hand mixed as dry powders in a dedicated plastic drum, and then emptied into the electric mixing bowl. The RUTF is then mixed at 105rpm for 6 minutes, 210 rpm for6 minutes, and 323 for 6 minutes. These mixing times are necessary to ensure homogeneity of the RUTF and to prevent separation during storage. RUTF can be poured or hand-packed into 250g plastic bottles, a typical daily dose for a malnourished child.
If 500-1500kg of RUTF is needed each week, production is best achieved by partnering with commercial food processing company that has machinery which can efficiently mix, grind and package RUTF. This equipment is commonly found in industrial bakeripes or pastry factories. Several planetary mixers can be used or a larger capacity customized barrel mixer. Whole peanuts can be mixed in with the other ingredients, and this mixture is then run through the same grinder used to make peanut butter. A mechanical or pneumatic semiautomatic device that will fill a container with a prescribed amount can package the RUTF [17].
If more than 3000kg of RUTF are needed every week, an industrial production facility dedicated to RUTF is needed. This can be part of a larger food processing company, which has technical expertise in food production or an NGO formed specifically for the purpose of making RUTF. The machinery required for large scale production is custom designed to mix batches of 200-500kg and automatically package the product. Rather than typical 'batch' production, the product can move continuously from the mixer, to agrinder and then to a packaging device, by using a series of mixing chambers. An operator is needed to add the ingredients to the initial mixing chamber and remove the final filled containers from the packaging device.
The scale of production will determine the methods of quality control and the cost. Quality control is independent on the overall quantity of RUTF produced, and will be more easily implemented at a lower cost with centralized, large scale production. Economy of scale will come up for other aspects of RUTF production, and, if feasible, large scale production should be considered as a long term objective in countries with a high level of severe malnutrition and where a sustained demand is likely.
There are two resources that potential local producers can use to get technical assistance in establishing a production facility, Nutriset [Malaunay, France] and Valid International [Oxford, UK].Standard Planetary Bakery Mixer Automatic Packaging Device for RUTF Single mixing/ packaging unit for large scale of RUTF
Quality control choice if ingredients
Whichever the scale of production is used, quality control is achieved by safe storage of the ingredients, adequate training and supervision of the production personal, and product testing for composition and contaminants. Throughout the world, authorities set standards for food production companies; those organizations involved in RUTF production should adhere to these standards [18]. Key issues in quality control are listed as follows.
Aflatoxin contamination
This toxin is produced by an aspergillus species of fungus, which contaminates the peanuts after they have been harvested, but before they have been ground into peanut butter. The fungus is ubiquitous, fungal growth can be curtailed by storing the peanuts in a cool, dry environment, and can also be controlled using chemical fungicides. Methods to prevent aflatoxin contamination have been described in detail elsewhere [19]. Peanuts should be purchased from a supplier that can ensure that steps to prevent contamination have been implemented during harvest and storage. Aflatoxin contamination is more likely to be seen in peanuts with black discoloration, and among nuts that have a shriveled, irregular appearance. Consumption of aflatoxin can result in hepatic oxidative stress, and predispose the individual to hepatic cancers. RUTF should conform to international standards for maximum aflatoxin content, 10-20ppb [20]. Very high doses of aflatoxin can produce acute intoxications [21]. Moderate doses may depress child growth [22].
Bacterial contamination
The inherent microbiological safety of RUTF allows it to be packaged under clean and dry, but not sterile, conditions. Care must be taken to prevent the introduction of water into RUTF during production. Increasing the water content of RUTF allows bacteria and mold to grow with in the food, promoting product degradation and exposing the malnourished child to potential pathogens. Water is most likely to be introduced from residue left on the mixing bowls and containers after they have been washed. Therefore, it is better to limit the number of times the implements of production are cleaned with soap and water, and to simply dry wipe they clean instead. Typically implements need to be cleaned with soap and water only once a week. If the containers in which the RUTF is to be dispensed are first washed, care should be taken to see that they are completely dry.
Enteric bacterial contamination is most likely to occur from fecal contamination of stored ingredients or during the mixing process. Care should be taken to store in ingredients in areas which are free of rodents. Workers should wash and thoroughly dry their hands before manipulating the RUTF, wear clean plastic gloves, hair coverings and protective coats during RUTF production. Milk and RUTF should be periodically checked for salmonella contamination by standard microbiological methods in reliable laboratories.
Pesticides and heavy metals
Pesticides may cause acute and delayed health effects in human who are administer. Pesticide administer can cause a variety of adverse health effects, ranging from simple irritation of the skin and eyes to more severe effects such as affecting the nervous system, mimicking hormones causing reproductive problems, and also causing cancer. To checked out in the food formulation Table 3.

- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Prevention of oxidation
Oxidation of the fatty acids contained in the RUTF and of some vitamins, mainly vitamin A and C, is the main factor limiting the storage life of RUTF. During the production, some preventive measures should be taken to avoid initiating the oxidation process [23]. While it is helpful to heat the oils during the mixing process to achieve a homogenous mixture, heating to temperatures over45 °C accelerates the oxidation of the lipids, which reduces the period of time that the product is stable after production (shelf life). To prevent oxidation, it is also better to use airtight containers and containers filled as much as possible so that the quantity of oxygen within the container is minimized. The shelf life of locally produced RUTF without airtight packaging is 3-4 months. When RUTF is packaged in airtight foil envelopes under a nitrogen atmosphere (devoid of oxygen), the shelf life can be extended to 24 months.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Composition of RUTF
Errors may be made during the mixing process, which result in RUTF that has a substantially different nutrient content. These are best avoided by careful training of workers mixing the food, use of convenient measures of ingredients for batches of RUTF and periodic compositional testing of RUTF. Measuring a single mineral, such as potassium, by atomic absorption [24] is an inexpensive, reliable way to monitor the vitamin/ mineral content, since the minerals are added as a premix product. If an atomic absorption spectrophotometer is not available, a colorimetric assay for Vitamin C can be substituted [25]. Measuring fat and protein content assure that the other ingredients are being added in appropriate amounts.
Quality control is achieved by adopting operating procedures that are internationally accepted as standards for food production, the Codex Alimentarius [26] and the Hazard Analysis and Critical Control Point Program [HACCP, 17]. These procedures prescribe raw material procurement, storage of ingredients, mixing of ingredients and storage of finished product. In addition to international standards, every nation of the world has a Bureau of Standards which regulates the production of food. These Bureaus also prescribe operating standards, conduct inspections of factories and issue licenses to produce food. Product testing is used to verify the quality of the production process, and should be done with every large batch of finished product, certainly every week. In Malawi, finished product is tested weekly for contaminating microbes [salmonella, staphylococcus, total flora of aerobic mesophilic bacteria, coliforms, E. Coli, yeast, mold], aflatoxin and product composition (fat, protein and potassium). Testing is best done locally so that it can be used to identify lapses in production quality in a timely manner.
Batches of RUTF should not be sent to consumers without verification of product quality. Almost every nation of the world has a laboratory associated with their Bureau of Standards that can conduct the independent testing.
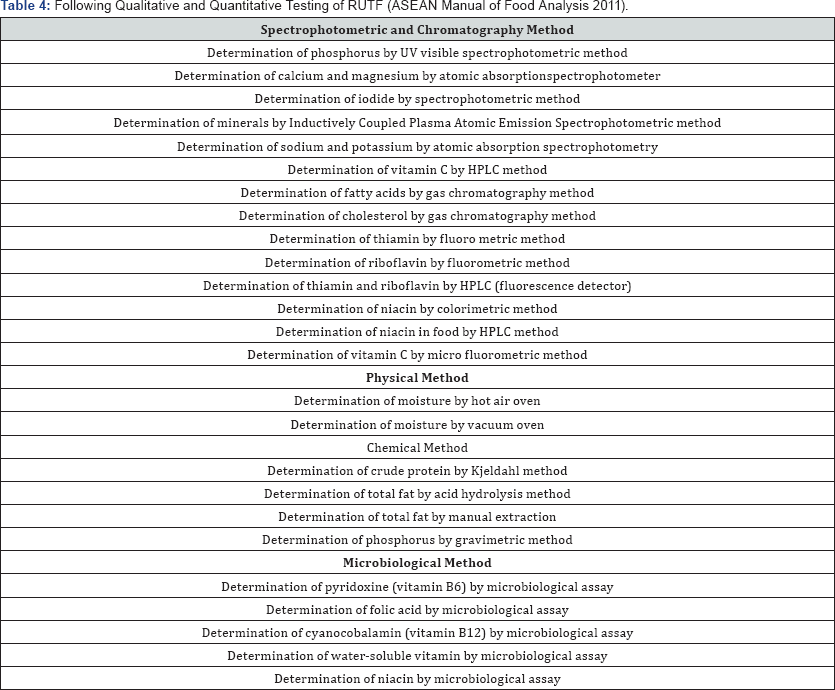
Following Qualitative and Quantitative Testing of RUTF (ASEAN Manual of Food Analysis 2011) (Table 4).
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Safety
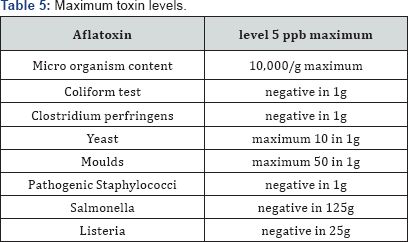
The food should be free from objectionable matter. It must not contain any substance originating from microorganisms or any other poisonous or deleterious substances, including anti nutritional factors, heavy metals or pesticides in amounts that may represent a hazard to health [28] Table 5.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Product Shelf Life
Stability studies need to be conducted on the final product to confirm the product shelf life (2 years).Stability studies should be conducted as following:
I. Long term [0, 3, 6, 9, 12, 18, 24 ]
II. Accelerated data at 40±2 °C for 6 months may support extrapolation of shelf life [0, 3, 6 months] Long term stability studies at 40 °C also expected Stability study
Stability studies must verify
o Organoleptic stability: taste, odour, product consistency and behavior [absence of oil separation, absence of oxidation]
o Integrity of the packing materials.
o Nutritional value and nutrient stability [maintenance of a level of vitamin and minerals over or within specified levels for at least one water soluble and one fat soluble micronutrient].
o Demonstrate absence of microbial growth.
If recipe changes, new stability studies must be provided
Product Efficacy and Acceptability [29]
o Efficacy studies should be conducted for products not using the Joint Statement formulation.
o Acceptability studies should be done on malnourished children in typical beneficiary countries, using products of various ages: 3 months, 6 months and 12 months life.
Packaging and labeling Primary packaging [sachet]
92g only, No detachable parts that present a choking hazard Ex: Aluminium polymer film, polyethylene terephthalate [PET]. Packaging materials [aluminum foil, cups, etc], inks and glue food-contact approved, Requirement for fat - O2 - moisture barrier. Packaging under nitrogen protects from oxidation, Air and water tightness control implemented during the filling process.
Seal: pouch free of damage, hermetically sealed, minimum 2mm [0.010 in], free of impression or design on the seal surface that would conceal or impair visual detection of seald effects.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
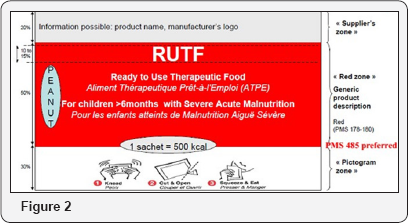
Secondary Packaging (Carton)
Sturdy quality
ECT (Edge Crush test*) >11kN/m with minimum 60% remaining with 90% humidity at temperature of 40 °C able to be stacked to a height of 2.4m, and resistant to puncturing, Plastic bag.
Information printed
I. Red zone: Same information as red zone of sachets, Name and address of the manufacturer, packer, distributor, importer, exporter or vendor including the country of origin
II. Storage conditions: Product to be stored below X degrees Celsius, Net weight, Numbers of units in a carton, Batch number and best before date Figure 2
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Labelling [27]
The following information should be clearly printed out on the label [in English and French]
1) Product generic name: Ready to Use Therapeutic Food [RUTF] Statement "RUTF for Children with Severe Acute Malnutrition"
2) Raw materials should be listed in order of descending quantities
3) Clear pictorial instructions
4) Manufacturing date
5) Best before date
6) Batch/lot number
7) Storage conditions
8) A leaflet should be included in each carton with detail nutritional composition of the product, including composition of the mineral and vitamin premix.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Cost and Sustainability
In general, the cost of the RUTF is high. Usually this food stuff are purchased by Government Organizations or NonGovernment Organizations, and distributed by the local governments. The cost is mainly influenced in the case of a local production by the availability of the ingredients and their cost on the local markets (Manary, 2006). As an example, vegetal oil, peanut butter, and sucrose are relatively easy to find local resources, but powdered milk and CMW usually are imported, and the price can vary much depending on the market [4]. According to data reported by Valid International and by the Clinton Foundation for Malawi RUTF is due to the cost of the ingredients and, among these, the powdered milk contributes itself for the 42%. Powdered milk according to these data accounts consequently for about the 29% of the total RUTF cost. A breakthrough in the RUTF production to cut the cost down could be the replacement of the powdered milk in the RUTF recipe as suggested by UNICEF [4]. The United Nations suggestion to treat SAM with RUTF would require about 258,000 tonnes of therapeutic food per year. In 2007 the production has been of about 19,000 tonnes and the purchased quantity has been of about 8500 tonnes. This means that only 3% of the children with severe malnutrition condition has been treated with these therapeutic food [30]. The main problems that influence the market of the RUTF locally produced or imported could be indicated in the high cost of the ingredients due to the market prices always rising, the impossibility to improve the original patented recipe by Nutriset, and the difficulty to control the possible aflatoxins contamination of the local produced RUTF. The imported RUTF, e.g. Plumpy’ Nut, has an average cost of about 3€/kg, comparable to the cost of the same RUTF produced locally, about 2.60€/kg [4,30]. The RUTF market is actually controlled by the Nutriset, and for this reason the Médecins Sans Frontières Organization [MSF] asked the Nutriset to give manufacturing licenses to possible producers at favourable conditions [31], but to cut down the cost of this food alternative formulations are needed.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
Challenges to RUTF Production and Distribution in India
Severe acute malnutrition is almost always an illness that stems from the lack of sufficient food - few children get malnourished when sufficient quality food is available. The availability or the capability to produce RUTF does not address prevention of SAM or directly improve food security in these households. Irrespective of how reasonably indigenously produced RUTF is priced, it will remain beyond the purchasing power of most households where severe malnutrition occurs. For these children, RUTF will have to be provided by governments [local or central] or be channeled through nonprofit and aid agencies. Therefore, the treatment of SAM, condition arising from food insecurity, through commercial processed food is seen as a paradoxical, conflicting situation. It can be argued that some resources will be directed to procure RUTF that could possibly be used to improve access to food for these vulnerable sections of society through enhancements in employment, agricultural production, food rations or subsidized food. While this may be true in part, the treatment of millions of severely malnourished children, many out of whom die every day is as pressing a need as the prevention of further malnutrition through improved access to food.
These therefore, must not be viewed as competing activities, much like the treatment of any other illness [e.g. Tuberculosis, HIV etc.] while concerted efforts are made towards preventing it. Varied interpretations of the Supreme Court ruling on hot cooked meals have also led to confusion. The Supreme Court had ruled that hot cooked meals must be provided for children through the Integrated Child Development Scheme [ICDS] [17,32]. This cannot be directly interpreted to preclude the use of food based therapeutic interventions for children who suffer from illnesses other use of RUTF for children with SAM. Uncertainty about the support for Community management of SAM based on RUTF will deter food companies from investing or venturing into RUTF production.
- Review Article
- Abstract
- Introduction
- Ideal Characteristic Properties of RUTF
- Ingredients
- Antioxidants
- Prevention of oxidation
- Composition of RUTF
- Safety
- Product Shelf Life
- Secondary Packaging (Carton)
- Labelling [27]
- Cost and Sustainability
- Challenges to RUTF Production and Distribution in India
- References
References
- World Health Organization (1999) "Management of Severe Malnutrition: A manual for physicians and other senior health workers,” WHO, Geneva, Switzeland.
- Food and Agriculture Organization (FAO) (2010) The state of food insecurity in the world. Rome, Italy.
- WHO (2005), UNICEF & SCN, "Meeting report: Informal Consultation on Community Based Management of Severe Malnutrition in Children.”
- UNICEF (2009) A supply chain analysis of Ready-to-Use therapeutic food for the horn of Africa. A study commissioned by the United Nations Children's Fund, UNICEF Press.
- World Health Organization (2007) WHO Community-based management of Severe Acute Malnutrition. Geneva, Switzerland.
- & WHO (2006) UNICEF & SCN, "Informal consultation on community- based management of severe malnutrition in children,” Food and Nutrition Bulletin 27(3).
- World Health Organization (2009) "United Nations Children's Fund - WHO Child growth standards and the identification of severe acute malnutrition in infants and children. A Joint Statement by the World Health Organization and the United Nations Children's Fund.
- WA Awad, K Ghareeb, J Bohm (2012) Occurrence, health risks and methods of analysis for aflatoxins and ochratoxin A, Journal of Veterinary Animal Science 2(1-10).
- Espin JC, Garcia-Conesa MT, Tomas-Barberan FA (2007) Nutraceuticals: Facts and fiction. Phytochemistry 68(22-23): 2986-3008.
- Wang L, Weller CL (2006) Recent advances in extraction of nutraceuticals from plants. Trends in Food Science & Technology 17(6): (300-312).
- Das L, Bhaumik E, Raychaudhuri U, Chakraborty R (2012) Role of nutraceuticals in human health, J Food Sci Technol 49(2): 173-183.
- Go VLW, Harris D M, Srihari P (2012) Global overview of the role of nutraceuticals in cancer, Nutraceuticals and Cancer 1: (1-12).
- RUTF product specifications (2012) Odile Caron Coordinator for food quality assurance MSF International Office, UNICEF SD, RUTF pre-bid conference.
- World Health Organization (1999), "Management of severe malnutrition. A manual for physicians and other senior health workers, Geneva: World Health Organization, Switzerland.
- Nestel P, Briend A, de Benoist B, Decker E, Ferguson E, et al. (2003) Complementary food supplements to achieve micronutrient adequacy for infants and young children. J Pediatr Gastroenterol Nutr 36(3): 316-328.
- McClements DJ, Decker EA (2000) Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. Journal of Food Science 65(8): 12701282.
- Manary MJ (2006) Local production and provision of Ready-to-Use therapeutic food (RUTF) spread for the treatment of severe childhood malnutrition. Food Nutr Bull 27(3): S83-S89.
- FAO, WHO. Codex Alimentarius, FAO/WHO food standards.
- Turner PC, Sylla A, Gong YY, Diallo MS, Sutcliffe AE, et al. (2005) Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community-based intervention study, Lancet 365 (9475): 1950-1956.
- Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A (2004) Home- based therapy for severe malnutrition with ready-to-use-food. Arch Dis Child 89: 557-561.
- Centers for Disease Control and Prevention (CDC) (2004) Outbreak of aflatoxin poisoning eastern and central provinces, Kenya January-July 2004, MMWR Morb Mortal Wkly Rep 53(34): 790-793.
- Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, et al. (2004) Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa, Environ Health Perspect 112(13): 1334-1338.
- Fellows P (2004) Local production of RUTF. In: Khara T and Collins S (Eds.) Community-based therapeutic care (CTC). Emergency Nutrition Network, Supplements 2: 33-35.
- Official Methods of Analysis of AOAC international (2000) (17th edn), AOAC INTERNATIONAL, Gaithersburg, MD, USA.
- Arya SP, Mahajan M, Jain P (1998) Photometric methods for the determination of Vitamin C. Analytical Sciences 14: 889-895.
- 26 Website for Codex Alimentarius.
- ASEAN Manual of Food Analysis (2011) Regional Centre of ASEAN Network of Food Data System Institute of Nutrition, Mahidol University Thailand.
- Community-Based Management of Severe Acute Malnutrition, A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children's Fund.
- UNICEF Technical Requirements for RUTF Products (2010) Giorgia Paiella Medicine and Nutrition Center UNICEF Supply Division, Consultation with RUTF Suppliers Copenhagen.
- Nutriset (2010).
- Médecins Sans Frontières, MSF (2010).
- Deshpande M, Dasgupta R, Baru R, Mohanty A (2008) "The case for cooked meals concerns regarding the proposed policy shifts in the mid-day meal and ICDS programs. Indian Pediatr 45: 445-449.






























