Optimizing the Extraction of Procyanidins Oligomers through Decamer
Alyson E Mitchell1*, Danielle Robertson1 and Eunmi Koh2
1Department of Food Science and Technology, One Shields Avenue, University of California, USA
2Department of Food and Nutrition, Seoul Women's University, Korea
Submission: September 28, 2017; Published: December 14, 2017
*Corresponding author: Alyson E Mitchell, Department of Food Science and Technology, One Shields Avenue, University of California at Davis, Davis CA 95616, USA, Tel: 530-752-7926; Fax: 530-752-4759; Email: aemitchell@ucdavis.edu
How to cite this article: Alyson E M, Danielle R, Eunmi K. Optimizing the Extraction of Procyanidins Oligomers through Decamer. Nutri Food Sci Int J.2017; 4(3): 555636. DOI: 10.19080/NFSIJ.2017.04.555636
Abstract
The extraction of procyanidins from a food is influenced by food matrix, extraction solvent, sample particle size, sample to solvent ratio, as well as other factors. Many of these parameters can easily be controlled in a laboratory to improve or target the extraction of low or high molecular weight oligomers. As various oligomers have differing biological and functional qualities, preferred extraction of one group of oligomers may be a desired target. In the following study, we describe the influence of solvent polarity and composition, solvent-solute ratio and particle size on the extraction of procyanidin oligomers monomer through decamer. The solvents evaluated included dimethyl sulfoxide (DMSO), acetone and methanol. Reversed-phase high-performance liquid chromatography (HPLC) and a diol-stationary phase were used to qualitatively compare extraction efficiencies of the procyanidin oligomers. Results indicate that optimal extraction conditions for total procyanidins were achieved using 70-80% DMSO; a solute-solvent ratio of 3g to 15mL of solvent and a particle size <0.99mm. Although 70-80% DMSO results in the highest total concentrations of procyanidin oligomers extracted, several characteristics of using DMSO make using 70% acetone a highly efficient, perfectly suitable substitute.
Keywords: Cocoa procyanidins; Theobroma cacao; Extraction; Optimization; Acetone; Methanol; Dimethyl sulfoxide (DMSO)
Introduction
Proanthocyanidins, or condensed tannins, are complex oligomeric compounds composed of flavan-3-ol subunits which contribute significantly to the organoleptic properties of plant-derived foods and beverages [1]. These secondary plant metabolites are of great interest for their numerous reported benefits to human health [2-5]. Cocoa (Theobroma cacao) is a rich source of procyanidins; cocoa procyanidins are composed exclusively of catechin and epicatechin subunits and exists as monomers through dodecamers linked through the C4-C6 and/or C4-C8 positions (B-type interflavan bonds) [6]. The procyanidins have differing health promoting properties based upon their size as this can influence bioavailability. Therefore, it is important to understand the main parameters that influence their extraction from foods (eg. solvent choice, solvent-to-solute ratio, particle size, etc.).
The extraction of the range of oligomeric procyanidins from food matrices is complicated by food-matrix interactions (eg. complexation with proteins, carbohydrates, etc.) and because the solubility of the oligomers vary with increasing size [7]. Solvents frequently used to extract procyanidins include: water, methanol, ethanol, acetone, ethyl acetate, isopropanol, DMSO and their combinations [8]. Currently, there is no consensus on the best way to maximize the range procyanidins oligomers extracted from a food. However, several parameters and methods for extracting cocoa procyanidins are compared and assessed for optimal procyanidin oligomer yield through decamer [8]. Maximum recoveries were achieved using dried, non-roasted unfermented or under-fermented cocoa beans and by using a mixed aqueous/organic extraction solvent acidified to a pH of 2-4 [3]. Though no one solvent is unanimously agreed on for maximum extraction, most studies have relied on 70% (by volume) acetone [9-12]. Studies, such as those by Kahkonen et al., [13] and Heinonen et al., [14] evaluated different extraction methods using different solvents. Both studies determined that aqueous acetone is better than aqueous methanol for extracting phenolics in berries and apples. One survey on the extraction of grape seed phenolics used water, absolute or 75% ethanol, absolute or 70% acetone, methanol, n-butanol, diethyl ether and ethyl acetate. Methanol was the best solvent for extraction of catechin and epicatechin, but the 70% acetone solution was the best solvent for extraction of procyanidins [15]. A review of the extraction and analysis of phenolics in food concluded that acetone: water (70:30v/v) was the best solvent for extraction of proanthocyanidins from rapeseed/canola, beach pea, and blueberries [16].
There is no consensus on solute-to-solvent ratio or particle size, but ratios commonly used range from 0.1 - 0.3g: 1mL, and cocoa samples are often ground to ~90|im before lipid removal [9-12]. Methods for fractionation and purification of cocoa procyanidins have changed over the years, but Kelm et al. [9] shows that using a diol stationary phase column operating in normal phase mode gives the best separation and isolation of procyanidin oligomers, allowing efficient quantification. Reports on the levels of procyanidins in natural cocoa powder vary widely based on extraction methods and chromatographic conditions, ranging from 40.8 + 8.3mg/g [17] to 97.76 + 5.32mg/g [11], expressed as the sum of all oligomers, with respect to nonfat cocoa solids. Epicatechin is the main polyphenol in unfermented beans [18], composing 2-4% of the dry mass of defatted cocoa powder, while catechin makes up only 0.05-0.1% [19]. The proportion of other oligomers varies based on extraction method and chromatography conditions, so there are no clear guidelines as to how to target specific oligomers for extraction.
The objective of this study is to provide conclusive details on the optimal parameters for extracting low molecular weight procyanidins oligomers (<pentamer), high molecular procyanidin oligomers (pentamer through decamer) and total procyanidins from cocoa. The three main solvents evaluated included acetone, methanol and DMSO. The composition ([solvent/water v/v]) of each solvent was evaluated at 50%, 60%, 70%, 80%, 90% and 100%. The influence of solvent- solute ratio was investigated by comparing five solute-solvent ratios (i.e. 3g to 8,10,12,15 and 20mL) on the extraction of the procyanidin oligomers. Lastly, the influence of particle size was assessed using three different groups (i.e. <1.37mm; 0.991.17mm; and <0.99mm).
Materials and Methods
Chemicals
All solvents (DMSO, acetone, methanol, acetonitrile, and acetic acid) were HPLC analytical grade, purchased from Sigma-Aldrich (Milwaukee, WI). Epicatechin was purchased from Sigma-Aldrich (Milwaukee, WI). Deionzied, distilled water was purified using a Milli-Q water purification system (MIlli-Q, Bedford, MA).
Samples
Unfermented cocoa seeds (of Brazilian origin) were provided by Mars Inc. Beans were hulled by hand, ground into fine powder using a commercial mill and kept in sealed plastic bags set inside an ice chest at room temperature until analyzed. The powder was sifted through several Tyler Standard Screen sifters (WS Tyler, Mentor, OH) of various size to create three groups with homogeneous particle sizes of <0.99 mm; 0.99 - 1.17 mm; and <1.37mm.
Procyanidin extraction
Dried, milled cocoa powder (100g of ground cocoa) was defatted with 450mL hexane three times. Each time, samples were stirred by hand at a consistent, medium pace for 10min and solvent decanted. The lipid-free solids were air-dried overnight, yielding ~60g fat-free cocoa material. A 3g subsample was extracted twice using 10mL of one of the main extraction solvents: dimethyl sulfoxide, acetone or methanol. For each of the three main extraction solvents, six different solvent concentrations (50%, 60%, 70%, 80%, 90% and 100% [solvent: water (v/v)]) were investigated. All solutions were acidified with 1% acetic acid by volume. After mixing the subsample with the solvent on a stir plate for 10 min, the subsample was transferred to a 50mL centrifuge tube and centrifuged at 4000rpm for 10min at room temperature. The subsample was then decanted and vacuum filtered, thoroughly, then the solids were transferred back to a flask, and the extraction repeated. The supernatant recoveries for each subsample were combined, volumized with its respective solvent to 25mL, then filtered through 0.45|im HN-syringe filters. Each sample was diluted to the proper detection range with mobile phase prior to HPLC injection. To investigate the effects of particle size, a 3g subsample from each of the three groups (i.e. <0.99mm; 0.99 -1.17mm; and <1.37mm) was extracted twice with 10mL aqueous, acidified 70% acetone. A3g subsample was also extracted twice with 8mL, 10mL, 12mL, 15mL or 20mL aqueous, acidified 70% acetone to determine the influence of solvent-to-solute ratio. All samples were extracted identically with the exception of those used for solute-solvent ratio comparisons, which were diluted with 70% acetone instead of mobile phase prior to HPLC injection. Also, the 15mL and 20mL subsample groups were volumized with 70% acetone to 50mL instead of 25mL.
Normal phase HPLC analysis of cacao procyanidin extracts
Chromatographic conditions used to separate and assess procyanidin content are described in Kelm et al., [9]. Each procyanidin fraction was analyzed using a Waters 2690 HPLC (Waters Corporation, Milford, MA) interfaced to a Shimadzu 496 Spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD). Eluent was monitored by fluorescence detection with excitation at 276nm, emission at 316nm and Gain set to 10. The column used was a 250mm x 4.6mm i.d., 5μm Develosil diol (Phenomenex, Torrence, CA). The chromatographic mobile phase consisted of (A) acetonitrile: acetic acid (98:2v/v) and (B) methanol: water: acetic acid (95:3:2 v/v/v). Each extraction recovery was volumized to 25mL (unless otherwise noted) and filtered through 0.45|im Millex-FN syringe filter (Millipore, Bedford, MA) prior to analysis. Relative quantification of each oligomeric group was achieved by using external standardization against authentic epicatechin [4].
Results and Discussion
The resulting concentrations of the individual procyanidin oligomers through decamer extracted using each solvent and each aqueous: solvent ratio is given in Tables 1-3. The results for DMSO extractions, shown in Table 1, indicate that 70% DMSO optimizes procyanidin extraction, resulting in the highest total procyanidin extraction efficiencies per gram of However, 70% DMSO is more efficient for trimer through fat-free cocoa (56.3mg/g). For targeting low molecular weight decamer, making it the most efficient solvent for both low procyanidins oligomers (<pentamer), 80% DMSO optimizes molecular weight oligomers (<pentamer) and high molecular extraction for the monomer (27.3mg/g) and dimer (8.0mg/g). weight oligomers (pentamer through decamer).
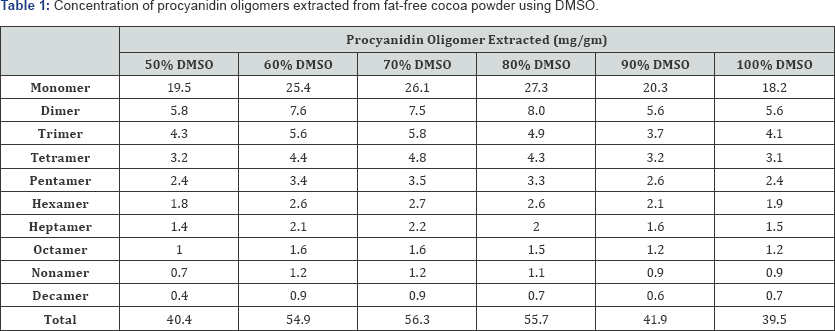
*Bold, underlined entries denote the highest value for each oligomer.

*Bold entries denote the highest value for each oligomer.

* Bold entries denote the highest value for each oligomer.
The results for acetone extractions, shown in Table 2, indicate that 70% acetone is optimal for extraction of total procyanidin per gram of fat-free cocoa (46.8mg/g). It appears that 70% acetone extracts more of the low molecular weight oligomers (<pentamer), whereas the 80% acetone improves the extraction efficiencies for pentamer-decamer. The procyanidins extracted with the 100% acetone showed limited solubility, and drop out of solution without constant agitation.
The results for methanol extractions (Table 3) indicate that as the concentration of acidified methanol increases, so does the ability to extract the larger oligomers. Specifically, 70% methanol is optimal for extracting monomer through trimer, whereas a 90% solution is optimal for extracting trimer through hexamer, and a 100% solution maximized the extraction of oligomers greater than heptamer. Overall, 70% methanol is optimal for extraction of total procyanidin per gram of fat-free cocoa (36.8mg/g).
Based on the trends in procyanidin extraction, it appears the 50% DMSO solvent is too polar to allow optimal solvation of the amphiphilic procyanidins. The dielectric constant for water is 80, but for DMSO it is 48. As the proportion of water in the extraction solvent decreased, the extraction efficiency improved, to a point. Extractions with a solution greater than 80% DMSO show poor extraction efficiency, though the procyanidins were still completely soluble.
Both acetone and methanol have dielectric constants much lower than that of water (21 and 33, respectively, versus 80) so the extraction trends, though subtle, are most likely a response to the decreasing polarity of the extraction solution. With the acetone extractions, efficiency increased with decreasing proportion water (decrease in polarity), but the poor solubility of procyanidins in solutions more than 80% acetone prevented any further increase in extraction efficiency. With methanol extractions, there were no solubility issues, and the 100% methanol solution is both the least polar and most efficient methanol solvent for targeting higher weight oligomer extraction. The fact that the more concentrated methanol solvent was optimal for the higher weight oligomers indicates the important relationship between solvent polarity and degree of polymerization: with higher weight oligomers, hydrophobicity increases, thus strong solvent polarity becomes more of a deterrent.
Figure 1 demonstrates the maximal amount of each oligomer extractable from cocoa using the optimum solvent concentration, per oligomer, for each of the three main extraction solvents. Total procyanidin extraction is represented as "oligomer 0". Overall, 80% DMSO is the optimal solvent for targeting low molecular weight procyanidin extraction (47.8mg/g), but 70% DMSO is optimal for higher weight procyanidin extraction (8.6mg/g). If 70% DMSO was used for total procyanidin extraction, the resulting concentration of procyanidins per gram fat-free cocoa (56.3mg/g) would still be more efficient than any optimized extractions with either acetone (46.8mg/g) or methanol (36.8mg/g) (Figure 1). Nevertheless, all three optimal extractions surpass the largest sum of oligomers 1-10 indicated by Gu et al. [17], 31.86mg/g.
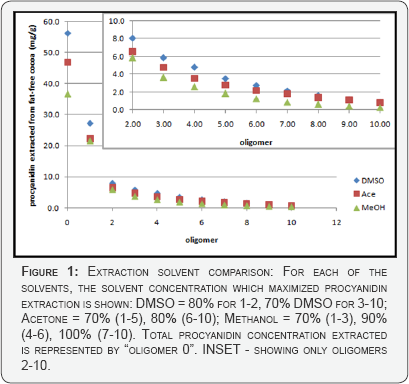
The ability of DMSO to provide optimal extractions is most likely due to its moderate polarity, but stereochemistry also plays a role in solvating ability. DMSO is trigonal pyramidal while acetone is planar. The lone pair of electrons at the apex of the DMSO pyramidal structure plays a role in solute-solvent interaction, giving DMSO unique capabilities as a solvent [20]. However, there are some real drawbacks to using DMSO instead of acetone, solvent removal aside. DMSO is more permeable, capable of carrying procyanidins through the skin where these antioxidants may show pro-oxidant characteristics and pose harm to those who do not use caution. DMSO adheres to the glass walls of a flask more than acetone, making the transfer of the cocoa powder from a flask to its centrifuge tube more difficult. Most of all, the centrifugation and decanting steps of this extraction protocol are more challenging when using DMSO because the density of DMSO causes the smaller cocoa powder particles to float on top of the supernatant containing the procyanidin extracts. When using acetone, the cocoa powder forms a nice pellet, so decanting and vacuum filtration are much easier. Due to its affordability, easy accessibility, and common use in other published studies, acetone was chosen over DMSO as the main extraction solvent further studies.
The effects of solute: Solvent ratios were assessed, and the resulting concentrations of procyanidin oligomers extracted are shown in Table 4. Values are normalized with respect to the values obtained using 70% acetone and a default ratio of 3g: 10mL. As expected, a smaller solute: solvent ratio results in greater extraction of the procyanidins (Table 4). Simply by increasing the solvent volume by 20% from the default 3g: 10mL ratio, overall extraction increases by 22.6%. Doubling the solvent volume to 20mL increased overall extraction by 29.1%, which is only a 6.5% bump in procyanidins extracted with a 80% bump in solvent volume used. Ultimately this trend shows that increasing the solvent volume may provide only slight gains in procyanidins extracted in exchange for significant waste of solvent resources.
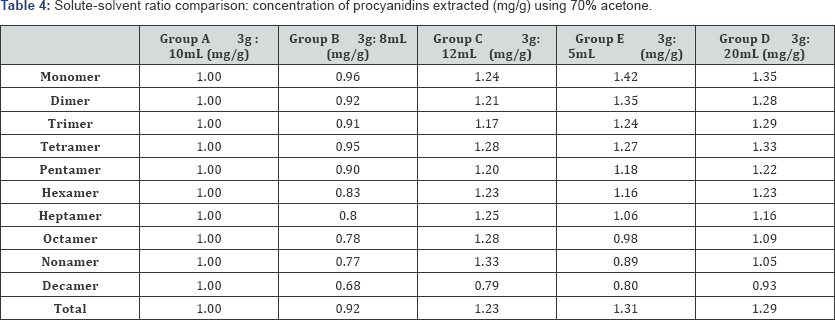
*Values shown are normalized with respect to 3g : 10 mL ratio.
Conversely, increasing the solute-solvent ratio (decrease in solvent volume) shows only marginal decreases in procyanidin extraction. Decreasing the solvent volume by 20% led to a mere 8.1% loss in total procyanidins extracted per gram of fat-free cocoa. Since a minor decrease in solvent used leads to only minor decrease in procyanidins extracted, this finding has positive implications for conservation of resources. However, the viscosity of the solute-solvent solution becomes problematic when the solvent volume is decreased more than 20%.
There is an obvious and predictable trend between solute- solvent ratio and procyanidin concentration extracted. With a smaller ratio (a larger solvent volume) the solubility of the cocoa particles increases. More solute is exposed to the solvent, and thus more procyanidins can be extracted. While viscosity affects the lower limit of solvent volume used, asymptotic behavior between solvent volume and concentration extracted exposes the upper limit. The increase in concentration of total procyanidins extracted between the 12mL group and the 15mL group is ~8%, while the difference between the 15mL group and the 20mL group is insignificant, within the margin of error.
The influence of particle size was assessed using three different groups (i.e. <0.99mm; 0.99mm to 1.17mm; and <1.37mm) and the resulting concentrations of procyanidins extracted are detailed in Table 5. Values are shown normalized to the <1.37mm group. As expected, the group with smallest particle size (<0.99mm) had the highest total concentration of procyanidins extracted (49.8mg/g). The >0.99mm to 1.17mm group had the lowest concentration (34.1mg/g) and the <1.37mm group had a concentration in the mid-range (46.8mg/g). This order of efficiency does not change whether one is targeting lower molecular weight oligomers or higher molecular weight oligomers: with all three groups, concentration decreases with increasing oligomeric size.
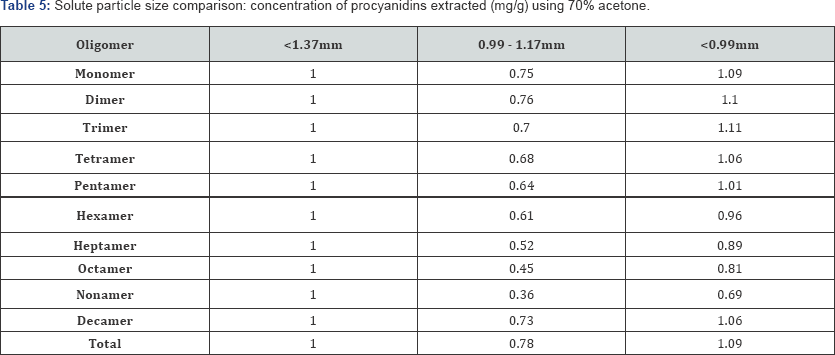
*Values are shown after normalization with respect to default group 1.37mm > 0.1mm.
With a smaller particle size, there is more solute surface area exposed to the extraction solvent, and therefore more solute-solvent interaction. The <1.37 mm group extracts more than the 0.99-1.17mm group because the former consists of all particle sizes smaller than <1.37mm (including those <0.99mm). In the 0.99mm-1.17mm group, small particles (<0.99mm) are not present to increase extraction recovery. Clearly, the smaller particle size provides better salvation potential, and thus more efficient extraction. However, these results illuminate a very important detail. One of the largest challenges of this very study was in obtaining consistent data. These results indicate that even small variations in particle size may have a significant impact on the concentration of procyanidins recovered.
Based on the results of all extraction methods examined, a final, optimized extraction was performed using 70% acetone, solute-solvent ratio 3g: 15mL, and particle size <0.99mm.
Though the oligomer distribution is equivalent, the total concentration of this optimized method (55.2mg/g) surpasses almost all extractions discussed thus far, with the exception of the 3g-20mL subsample, and is within the margin of error of even the optimized 70% and 80% DMSO subsamples (56.3mg/g and 55.7mg/g, respectively). For targeting the lower molecular weight oligomers (<pentamer) the optimized method extracts roughly the same concentrations as 70% DMSO and 80% DMSO, but70% DMSO is significantly more efficient for targeting higher oligomers (pentamer through decamer). If one chooses to forgo the troubles associated with using DMSO, using 70% acetone with the optimal particle size and solute-solvent ratio will result in approximately the same total concentration of procyanidins. Aqueous, acidified 70% acetone is already a popular choice, but this study shows that just by optimizing the solute: solvent ratio and particle size,total procyanidin extraction improves 1.2-fold.
Conclusion
In conclusion, procyanidin extractions are optimized by using a 70% acetone, a solute: solvent ratio of 3g-15mL and particle size <0.99mm. While using 70% DMSO enables higher concentrations of the higher oligomers, all aspects of using DMSO must be considered, in which case 70% acetone is a highly efficient, suitable substitute that enables essentially the same total concentration of procyanidins extracted.
References
- Lea AGH, Arnold GM (1978) The phenolics of ciders: Bitterness and astringency. Journal of the Science of Food and Agriculture 29(5): 478483.
- Ramljak D, Romanczyk LJ, Metheny-Barlow LJ, Thompson N, Knezevic V, et al. (2005) Pentameric procyanidin from Theobroma cacao selectively inhibits growth of human breast cancer cells. Mol Cancer Ther 4(4): 537-546.
- Kenny TP, Keen CL, Jones P, Kung HJ, Schmitz HH, et al. (2004) Cocoa procyanidins inhibit proliferation and angiogenic signals in human dermal microvascular endothelial cells following stimulation by low- level H2O2. Exp Biol Med (Maywood) 229(8): 765-771.
- Kondo K, Hirano R, Matsumoto A, Igarashi O, Itakura H (1996) Inhibition of LDL oxidation by cocoa. Lancet 348(9040): 1514.
- Keen CL, Holt RR, Oteiza PI, Fraga CG, Schmitz HH (2005) Cocoa antioxidants and cardiovascular health. Am J Clin Nutr 81(1): 298S-303S.
- Porter LJ (1988) In: The Flavanoids, JB. Harborne (Ed), Chapman and Hall Ltd, New York, USA, pp. 21.
- Haslam E (1998) Practical Polyphenols: From Molecular Recognition and Physiological Action. Cambridge University Press: Cambridge, UK.
- Hammerstone JF, Chimel MJ (2001) An Improved Method For Extracting Cocoa Procyanidins International Patent WO/2001/093690.
- Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, Schmitz HH (2006) High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J Agric Food Chem 54(5): 1571-1576.
- Adamson GE, Lazarus SA, Mitchell AE, Prior RL, Cao G, et al. (1999) HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J Agric Food Chem 47(10): 4184-4188.
- Gu L, Kelm M, Hammerstone JF, Beecher G, Cunningham D, et al. (2002) Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J Agric Food Chem 50(17): 4852-4860.
- Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH (1999) Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem 47(2): 490-496.
- Kahkonen MP, Hopia AI, Heinonen M ( 2001) Berry phenolics and their antioxidant activity. J Agric Food Chem 49(8): 4076-4082.
- Heinonen IM, Meyer AS, Frankel EN (1998) Antioxidant Activity of Berry Phenolics on Human Low-Density Lipoprotein and Liposome Oxidation. J Agric Food Chem 46(10): 4107-4112.
- Kallithraka S, Garcia-Viguera C, Bridle P, Bakker J (1995) Survey of solvents for the extraction of grape seed phenolics. Phytochemical Analysis 6(5): 265-267.
- Naczk M, Shahidi F (2004) Extraction and analysis of phenolics in food. J Chromatogr A 1054(1-2): 95-111.
- Gu L, House SE, Wu X, Ou B, Prior RL (2006) Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J Agric Food Chem 54(11): 4057-4061.
- Kim H, Keeney PG (1984) (-)-Epicatechin Content in Fermented and Unfermented Cocoa Beans. J Food Science 49(4): 1090-1092.
- Niemenak N, Rohsius C, Elwers S, Ndoumou DO, Reinhard L (2004) Comparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. J Food Composition and Analysis 19: 612-619.
- Dimethyl Sulfoxide (DMSO): a Dipolar, Aprotic Reaction Solvent (2007) In: DMSO Product Information Bulletin #123B, GC Company, Gaylord Chemical Company: Bogalusa, LO, USA.






























