- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Prospects of Medicated Feed in Aquaculture
Amit Ranjan*, NP Sahu*, Subodh Gupta and Md Aklakur
Central Institute of Fisheries Education, India
Submission: September 08, 2017; Published: October 25, 2017
*Corresponding author: Amit Ranjan, Central Institute of Fisheries Education, Versova, Mumbai, India, Email: amitranjanfcri@gmail.com NP Sahu, Central Institute of Fisheries Education, Versova, Mumbai, India Ali.shahsavari2@gmail.com
How to cite this article: Amit R, NP Sahu, Subodh G, Md Aklakur. Prospects of Medicated Feed in Aquaculture. Nutri Food Sci Int J. 2017; 3(4): 555617. DOI:10.19080/NFSIJ.2017.03.555617.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Abstract
The use of pharmaceutics in aquaculture is in practice to control bacterial infections however, side effects are inevitable with excessive use. The use of feeds as a vehicle for animal’s medication is an old practice. Medicated feeds are prepared by the addition of a pharmaceutical premix to a homogenized or extruded diet and sometimes sprayed or top-coated onto feed. The successful administration of a medicated feed is largely dependent on the level of feeding of the infected population. Beside it in aquaculture, the amount of feed taken by individual animal is uncertain and a fixed ration according to total biomass of the pond has to be given. Therefore, a precise pharmacological aspect such as the bioavailability of the drug, concentrations at which it accumulates in the host tissues and the elimination rate should be taken into account with a holistic knowledge on level at which side effect and toxicity appears before making medicated feeds. Medicated feeds should be used according to manufacturer’s instructions, with particular attention to withdrawal periods and feeding rate and ration. The treatment should always be recommended dose for that species and should be fed for recommended days. Therefore, species and pathogen specific use of pharmaceutical moieties are need of time. That must address the safe and hazard free treatment of culture animal, with no or minimum residue in flesh and environment. Considering these aspects delivery of the medicament in feed, its form, dose, feed inclusion and feeding ration have to be technically implicated.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Introduction
Today aquaculture contributes to about half the total volume of global fish supplies and expected to increase further. The high density in aquaculture is a risk factor for disease outbreaks [1]. A typical example for loss due to diseases is of Chile, where the production of Atlantic salmon was dramatically reduced because of disease [2]. It is estimated that up to 40% of tropical shrimp production is lost annually, mainly owing to viral pathogens for which standard preventative measures such as vaccination or other medicament are not available [3]. As intensification is growing the use of antimicrobial and additives has been profound.
The use of antimicrobial drugs in aquaculture has well-known positive effects on the control of bacterial infections; however, several side effects that affect both the fish and the environment are associated with excessive use [4]. If one takes into account that 70 to 80% of the antibiotics administered to fish as medicated pelleted feed are released into the aquatic environment via urinary and fecal excretion and/or as unused medicated food [5,6]. It is not hard to imagine the extent to which antibiotics can affect the aquatic habitat. The effects of antibiotics on the environment are mainly due to the unregulated dumping in pond and overuse of these drugs by the aquaculture industry and the presence of drug residues in fish products [7]. Unfortunately, there are very limited studies conducted to analyse the side effects of antibiotic on fish themselves in immersion and through in feed formulation, but surely the immersion application has no or very poor control of the amount and effective dose of administration . This produces non target and hazardous effects. Which is the major threat to environment and potential microbial resistance and it need to be discouraged.
The use of antibiotics in aquaculture can cause microbial resistance and there is fear that this may spread to bacteria of relevance for terrestrial animals, including humans [8]. A reduction in the use of antibiotics is possible; an example is Norway, where since 1987 there has been a decrease in the use of antibiotics in aquaculture [9,10]. Simultaneously, the Norwegian production of farmed fish continuous to increase [11]. The main reason for this is the application of effective vaccines. The placing of the locality and coordination between farms to prevent transmission of infectious disease was also important. While the second approach can be that if we are forced to use antimicrobials for treatment a proper toxicity and pharmacological studies must be ensure to safe and hazard free use of these moieties at low dose. Similarly other effective biological means of disease control has to be explored example; species combination, vaccine, phytobiotics, bioflocs, probiotics and prebiotics. And most of these can be administered through feed. Over the years salmon lice have been an increasing problem in salmon farming and efforts have been made to establish effective alternatives to veterinary drugs. The use of cleaner fish in co-culture with salmon to remove salmon lice has proved to be effective [12]. While vaccine, phytobiotics, probiotics and prebiotics can be delivered through feed as a vehicle for medication through feed to cultured animal either as prophylactic or therapeutic way possible.
The use of feeds as a vehicle for medication of farm animals is an old practice [13]. Antimicrobials are by far the most important VMPs (Veterinary Medicinal Product) currently used for the production of medicated feed [14]. From the start, concern had been expressed that the continuous use of specific antibiotics in feeds would eventually result in an increase of drug resistant strains of pathogenic microorganisms [14]. Medicated feed is a mixture of large quantities of animal feed and a veterinary premix (medicinal product containing one or more active substances).These feeds have undergone extensive animal, human-food, and environmental testing prior to approval for use in fish. Unfortunately, there are very few drugs approved by the FDA for use in fish [15]. Compounding this issue can be the improper use of antibiotics which has resulted in development of many antibiotic resistant strains of bacteria. Therefore, medicated feeds should only be used when absolutely necessary and according to label or veterinarian instructions to avoid the development of the resistance in pathogen.
Feeding lower concentrations of antibiotics or decreasing the number of days the drug is fed can allow bacterial pathogens to develop a resistance to the antibiotic. If this occurs, the antibiotic would likely not be able to control certain infections that may occur later at a fish farm or hatchery. Once a bacterial disease is diagnosed and the appropriate medicated feed is determined, the feed should be used immediately. In food fish or ornamental aquaculture, many of the bacterial diseases of fish can be successfully treated with medicated feeds, and it is usually the preferred method of treatment. However, care must be taken because some of the causes of disease such as stress can lead to treatment failures or the recrudescence of disease after the completion of treatment [16]. Fish in ponds are best treated using oral medications. However, sick fish may not eat and even feed with medicament will not have the good acceptance, so withholding food for 12-24 hours may increase the acceptance of a medicated feed [16].
The use of antibacterial agents included in the feed remains largely unrestricted in aquaculture in several countries with high and growing aquaculture production [17,18]. A relatively small number of antibacterial agents are registered for use in aquaculture in Canada, the United States and Europe. These include amoxicillin, florfenicol and various agents in the tetracycline, quinolone, fluoroquinolone and sulphonamide groups [19-21]. While several countries don’t have regulation on use of antimicrobial in farm and Safety. This concern is understandable in view of the fact that drug resistant organisms have developed in human medicine. Medicated feed is frequently recommended to control bacterial disease outbreaks in cultured fish. Medicated feeds are commercially prepared, and contain an antibiotic to control specific bacterial infections by either killing the bacteria or preventing the bacteria from reproducing. Many bacterial diseases of cultured fish can be successfully treated with medicated feeds that contain U.S. Food and drug administration (FDA) approved antibacterial drugs.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Methods for Mixing Medication into Feed Depend on Feed Type
For dry pellets, the medication can be dispersed in water or oil in advance, and then the solution is poured over the feed. For moist pellets, the medicine should be well mixed with the other feed material and then extruded. In both cases, it is necessary to make sure the proper amount of medication is uniformly included and mixed in the final feed. Drying the surface of pellets or coating them with oil will decrease leaching of the medication. The medicated feed should be offered quickly to the entire net pen. If fed slowly, only active fish will consume the feed while sick and inactive fish will be devoid of feed so to ensure throughout acceptance the feed should be given in optimum ration and must have two or more feed frequency.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Storage of Medicated Feed
As with all fish food, medicated feed should be stored in a cool and dry place. If available, a freezer is ideal for storing fish medicated feed for extended periods provided it does not get moist or wet. Antibiotics and essential nutrients will deteriorate rapidly in a warm, moist environment. Excessive decomposition of antibiotics as a result of improper storage can result in unsuccessful treatment. Any unused medicated feed, stored at room temperature, should be discarded after 3 to 4 months [22].
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Mode of Administration Route
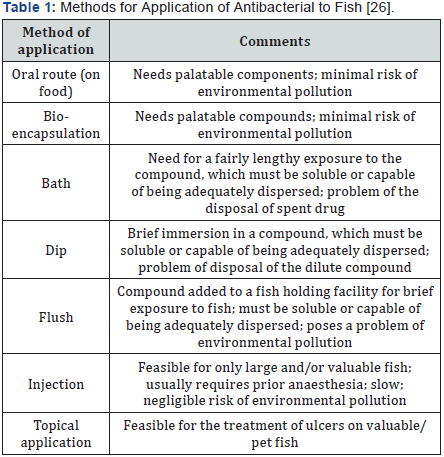
In aquaculture the pharmaceutics used to treat any microbial infection are generally administered by either oral or water immersion or bath or else through injection [23-25] but it is not possible to inject all individual as it is time consuming and labour intensive process so medicated feed becomes a cost effective easy and principal route. Various methods for the application of antibacterial used in aquaculture are given below (Table 1).
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Pharmaceuticals Incorporation into Feed
The daily dose of medicinal active ingredient should be contained in a quantity of feed corresponding to at least half the daily feed ration distributed. And the daily dose shall be precisely based on per kg weight of the animal. There are several methods for incorporating this dose into the feed stuff. The premix can be incorporated at the beginning of the manufacturing line (pelleted medicated feeds) or it can be added at the end of the pelleting process or after granulation or extrusion. In this case the premix is poured or sprayed on the surface of the pellets (surface-coated medicated feeds).
In order to achieve optimal homogeneity of the drug concentration, the ideal way to mix the drug is to add it at the beginning of the premix process and added to the blend together with other micro-ingredients, just prior to pelleting. Obviously, this can only be done at the feed mill, both on press granulation lines or on extrusion lines. However, the pelleting process involves high temperature and humidity conditions and hence this method can be used only with heat stable compounds.
On the other hand, the major drawback of this "initial incorporation" is the risk of carry over along the whole manufacturing line and of subsequent cross contamination of blank feeds with medicinal drugs. An intermediary option would have to a specific pelleting line fully dedicated to medicated feeds, but this is quite an exceptional situation, almost restricted to Norway [27,28]. In any case, this method can only be considered for very large batches of medicated feeds because the whole production line must be thoroughly cleaned after producing a batch of medicated feed.
Surface coating is the only suitable way to medicate feeds with products that don’t resist extrusion (especially heat labile compounds). It is also the only affordable way to produce small volumes of medicated feeds. This can be done either at the feed mill or on-farm. At the feed mill, the drug can be added after extrusion and pre-cooling, in the fat coater. Modern vacuum coaters allow the addition and adequate mixing of very small quantities of additives in liquid or powder forms which can be a good mean of medicament application as well.
On fish farms, the mixing is generally done in a concrete mixer: the pellets are loaded first and the powdered drug is poured and thoroughly mixed to the feed, followed by a binding agent. In most cases this binding agent is an edible vegetable or fish oil such as sunflower oil or cod liver oil. The medicated feed is then allowed to stay until the pellets have absorbed the added oil. The biggest obstacle to surface coating is that it is difficult to achieve a sufficient homogeneity of the mixture. This is the reason why administrative authorities in most countries require it to be made only in registered facilities, even if it is a simple concrete mixer (samples must be taken and analysed in order to check the homogeneity of the mix) [28]. Other important disadvantages include leaching and palatability of the moieties from the pellets.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Leaching
Leaching of drug into the water will occur with all kinds of medicated feeds, but is especially high with surface coated pellets. The extent of leaching depends on various factors including the water solubility of the chemical, the time during which the feed remains in the water before the fish eat the pellets, and the size of the pellets (ratio of surface area to weight). The smaller the pellets the higher will be the leaching losses. Apart from it water temperature has impact on leaching as study by Rigos showed that pellets (4.5 x 6mm) oil-coated with oxolinic acid (OA) and oxytetracycline (OTC) were considerably affected by leaching at 16°C (losses of 55.5% and 42.5% for OA and OTC, respectively) and at 24°C (32% for OA and 47% for OTC).Significantly fewer losses occurred with "on line" mixed pellets (5% for OA and 6.5% for OTC at 16°C; 10% and 20% at 24°C, respectively). The reason why high temperatures had a strong influence on the leaching of both drugs in mixed pellets but not in the surface coated ones was not explained.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Palatability
Depressed palatability is an important drawback of medicated feeds that can originate from the chemical itself, from the excipients or from the binders used to medicate the feed [28]. Bearing in mind that already diseased fish have the tendency to stop feeding, this problem can be of crucial importance for the success of treatments. Several fish species, particularly carnivorous and selective feeders are very reluctant to the chemical and medicament. Even Mediterranean species like sea bass sometimes hardly consume feed if it is not attractive enough. For example, in the same study, Rigos et al. showed that surface coated oxytetracycline was highly repellent for sea bass and depressed feed consumption by 90%.
A way to obviate leaching and palatability is to top coat the medicated feed with a special binder designed to overcome these problems. A binder based on an alginate matrix, TOPGEL® (PROVIMI-VETCARE), was initially developed for shrimp feeds. As such, it has high binding properties in order to keep the drug intact after several hours in the water. It also contains strong attractants and has found interesting applications in fish feeds. Another binder MEDITAK® Oil, made from a mixture of stabilised poly-unsaturated fatty acids, was also put on the market in 1996 [24].
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Administration of Medicated Feed
The administration of medicines via the feed is generally the adopted method for treating a large number of fish, whenever possible, because it is a much less wasteful way of administration than medication through the water. The most obvious limit to in-feed medication is that the fish to be treated must actually be feeding. Thus, it has no application to egg and sac-fry, nor for most reared species, because, except for Salmonids, the larvae and young fry need to be fed on live preys. Furthermore, fish suffering almost any kind of disease have a tendency to cease feeding. Thus in-feed medication is seen more as a prophylactic method than a therapeutic one: the goal is to medicate still healthy fish but that are in contact with diseased ones to prevent them contracting or expressing the disease. Already diseased fish will generally die irrespective of treatment [28].
Medicated feeds are prepared by the addition of a small amount (premix) of the antimicrobial to a homogenized and extruded diet or sometimes sprayed or top-coated onto feed. The successful administration of a medicated feed is largely dependent on the level of feeding of the infected population. Principles of clinical pharmacology such as the bioavailability of the drug, concentrations at which it accumulates in the host tissues and the elimination rate should be taken into account with medicated feeds. Medicated feeds should be used according to manufacturers’ instructions, with particular attention to withdrawal periods.
Prior to and during medication, fish culturists should review all husbandry and environmental factors that may have contributed to the disease outbreak and correct them to prevent the disease from continuing or re-occurring. Once a bacterial infection has been diagnosed in fish, an approved antibiotic feed can be determined. The treatment should always be the maximum recommended dose for that species and should be fed for the total number of days recommended (even if the fish appear to have recovered before the end of the treatment period).Information on types and amounts of therapeutic agents used in aquaculture throughout the world is not easily available, since only a limited number of nations provide reliable, detailed and accessible statistics on consumption of these drugs.Table 2

- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Economic Feasibility of Medicated Feed
Economics and other factors help to determine the appropriateness of using medicated feed. If the cost of the treatment is more than the cost of the fish, it does not make economic sense to treat the fish. When possible expensive treatments should be avoided unless they are likely to save money for the producer. A good example is the treatment strategy for Enteric Septicemia of Catfish (ESC) caused by Edwardsiellaictaluri. This disease occurs when temperatures are between 68 and 82°F (20 and 28°C) when the bacteria are in their optimum growth range. Fish dying from ESC will usually stop dying as temperatures rise above 82°F (28°C) or fall below 68°F (20°C) [30].
Medicating fish just before temperatures are forecast is often not advised, because the disease stops on its own due to the high temperatures. Using this type of strategy can save a significant amount of money on medicated feed purchases. All of the approved antibiotics for use in food fish are Type A medicated feeds. Producers may purchase Type A premixes only if they hold a valid feed mill license [29]. Everyone involved in producing medicated or non-medicated feed, whether at a commercial off-farm plant or with an on-farm mill or grinder/ mixer, must comply with the GMPs [29].
Finally, the efficacy of an oral medication also depends on the drug concerned not being digested and transformed into inactive metabolites before absorption. So such interaction of drug and physiology must be understood for cost effective use of medicated feed. The preferred dosage form for aquaculture medicated feeds is a powdered pre-mixture including the active compound and one or more excipients that can act as active carriers or as diluents for the active drug. Targeted dose of premix shall be according to the active compound available in the premix rather than the excipients.
Medicated feed is administered to fish based on body weight to be treated. Standard units of treatment are given in grams of active ingredient per 45kg (100lb) of fish or in milligrams active ingredient per kilogram (mg/kg) of body weight per day for a defined number of days. Drugs are incorporated into feed at a concentration that delivers a desired dose per unit of fish weight per day and fish are fed at a specific feeding rate (percentage of body weight divided into a defined number of feedings per day). Prophylactic use of drugs in feed for short periods of time or continuous feeding at low dosage is not advised because these practices are not favoured by FDA and they enhance drug resistance of bacteria and is not economical in long term.
The incorporation of an antibacterial in the feed is usually via a powdered premix in conjunction with a binder, such as gelatine (up to 5%), fish or vegetable oil [23].The dosage required for treatment with a medicated feed depends upon the original level of active ingredient/kg fish body weight. The dosage rates used in medicated feeds will vary according to the specific antibacterial used, but usually the rate is based on a number of grams per 100kg of fish per day.
When drugs are fed to fish it is essential to know the weight of fish to be treated, treatment rate (weight of drug fed per unit weight per day), and concentration of active ingredient in the drug. Following Formula is used to calculate how much drug to feed per day. The exact dosage will also require the number and average weight of the fish to be treated, as well as a daily feeding rate and consideration of whether the fish are marine or fresh water species. It is also important that treated fish must not be harvested for food use until a specified withdrawal period has elapsed [16].
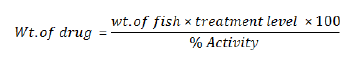
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Bioavailability of Medicated Feed
One problem for the treatment of marine species is that antibacterial have been shown to be less effective in seawater, which is related to their reduced bioavailability, e.g., tetracycline has a low bioavailability in fish (< 10%) due to binding with sea-water-borne divalent cations such as Mg2+ and Ca2+. It is noteworthy that non-bioavailable tetracyclines contaminate the environment [31]. It is usually best to use a feed that has enough medication so that feeding at a rate of 1% of body weight per day will give the needed dosage [32].
The bioavailability (F %) of a drug represents the fraction of an oral dose which is effectively absorbed into the circulatory system. It is determined by comparing blood levels following single oral and intravenous administration of the drug. For premixes, bioavailability should be determined by administration of a complete medicated feed prepared following the procedure recommended by the manufacturer.
The blood concentrations are estimated by the AUC (Area under the concentration/time curve) and F is calculated according to the following equation:
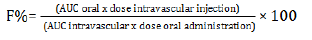
F%=((AUC oral x dose intravascular injection))/( (AUC intravascular x dose oral administration))×100
Of particular concern for sea farming is the reduced bioavailability of many antibiotics in seawater compared to freshwater. Notably, quinolones and tetracyclines are known to bind with seawater borne divalent cations Mg2+ and Ca2+ ions, especially Mg2+ [33]. Normal seawater concentrations of Mg2+ have a market effect on both antibacterial activity and uptake by fish of various antibiotics. Barnes et al. [34] found the minimum inhibitory concentration (MIC) of oxolinic acid against Aeromonassalmonicidato be increased 40- to 60-fold with seawater Mg2+.Of course, such an effect is particularly important when the feed is medicated by surface oil coating.
Whatever the bioavailability of the chemical used, an important point is that in-feed medication is prophylactic rather than therapeutic and this has profound implications on the desired kind of pharmacokinetic profiles [24]. High concentrations of drug are not necessarily needed in the organs and tissues affected by the disease but rather at normal portals of entry of the pathogen. The major portals of entry for pathogens are the gills and the gut, and to a lesser extent the skin. Seawater f ish drink continually, so the gut wall is an important portal of entry for many marine pathogens. If entry is through the gut wall, then even the portion of the drug which will stay in the gut lumen without being absorbed can play an important local role to prevent ingress of pathogens.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Pharmacokinetics and Pharmacodynamics of Medicated Feed
Pharmacological research on aquaculture drugs has focused mainly on a few antibiotics widely used in aquaculture and, consequently, the development and commercialisation of new medicines for the treatment of aquatic diseases are rather scarce. There is an increasing demand for better knowledge of medicinal products and the availability of quality pharmaceutical and biological products suitable both for the prevention and treatment of diseases of cultured fish. In-feed medication has largely benefited from the progress made by the pharmaceutical industry on modern formulations, as well as some innovative oral delivery systems, such as bioencapsulation and protected micro-diets. Despite the fact that over 100 aquatic species are being farmed at a significant level around the world, most pharmacokinetic studies have been performed on only a few species, most notably in salmonid fish, as well as some other Coldwater and or temperate species. However, there are considerable inter-specific variations in the pharmacokinetics of antibacterial compounds, so speciesspecific information is always needed before determining a suitable product to be used.
The principal objectives of pharmacokinetics study involves with the absorption, distribution and elimination (metabolism and excretion) (basic pharmacokinetic studies) and/or to compare the bioavailability of the active ingredient from two or more product formulations and/or routes of administration (bioequivalence studies). Other important aspects of pharmacokinetic studies include the determination of withdrawal periods and the estimation of residues introduced into the environment.
Fish that are eating less need a higher percentage of the drug in their diet, but there are limits on the legally allowable amount as well as practical considerations, since some drugs are unpalatable at high doses (e.g., many antibacterial) [32,35]. Drug dosage regimens also are host-dependent. Fish species reared in warm water may absorb, metabolise and excrete drugs at a different rate (often faster) than those in cold water. The salinity of the holding water also affects drug kinetics. Fish kept in saltwater drink the water while freshwater fish do not. Thus, antibacterial in the gastrointestinal tract of fish species held in saltwater may bind cations, which can reduce their uptake [31,36]. This is especially true for antibacterial – such as the tetracyclines – that have low bioavailability even in freshwater. The half-lives of drugs in fish are highly dependent upon the dosage regimen, the route and the temperature. Temperature is a very important factor in deciding on the dose and treatment intervals [31].
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Detoxification Metabolism in Fish
Liver is the primary organ for the detoxification of drugs in fish. Similarities exist in the metabolism of drugs by fish and mammals [37]. The metabolism of aquaculture antibacterial by the cytochrome P450 system could affect their activation, tissue distribution and elimination rates, and determine the persistence of residues as well as the length of the withdrawal period before the fish can be used for human consumption [38].
For study of drug metabolisation and detoxification mechanism Cytochrome P450 are well recognised. Cytochrome P450 (CYP) enzymes are members of the hemeprotein super family, and are involved in the mono-oxygenation reactions of a wide range of endogenous and exogenous compounds (Uno et al. 2012).They unmask or add polar groups on the drug, thus increasing its solubility and deliver it as a readily excretable metabolite. Over the last few years, the focus has come upon the Characterization of CYP genes in fish. In Japanese puffer fish (Takifugurubripes), 54 P450s encoding genes have been identified. All over the world, 137 P450s encoding genes have been isolated and identified. These genes are classified into 18 CYP families: namely, CYP1, CYP2, CYP3, CYP4, CYP5, CYP7, CYP8, CYP11, CYP17, CYP19, CYP20, CYP21, CYP24, CYP26, CYP27, CYP39, CYP46 and CYP51. Among these, eight CYP families: namely, CYP1, CYP2, CYP3, CYP4, CYP11, CYP17, CYP19 and CYP26 families are studied in detail (Uno et al. 2012) [39].
Compounds that are intended to treat a particular pathological condition must reach therapeutic level in target tissue in a manner so that the population of microbes which we are targeting could be eliminated. Concentration and effectiveness of that particular compound in various tissues could be measured by the application of pharmacokinetics and pharmacodynamics with the help of various bio analytical instruments viz. HPLC-MS.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Impact of Medicated Feed
Orally administered drugs enter the environment associated with organic material such as uneaten medicated pellets and faeces and as a water-soluble fraction if eliminated via gills and urine. Whereas the water-soluble fraction is diluted in the surrounding water, much of the drug associated with organic material will settle on the seabed or on the bottom of the pond. The amount of drug reaching the sediment depends on the fraction of uneaten pellets and the pharmacokinetic properties (absorption rate, metabolism and elimination pathway) of the drug in the fish [40]. If the absorption and metabolism are low, most of the consumed drug will be eliminated as the parent compound via the liver and bile to the intestine and, if readily associated with particles rich in organic content, the faecal particles may contain concentrations of the drug higher than the original pellets. Once reaching the sediment, factors such as water solubility, affinity for organic particles, photo-stability and microbial and chemical degradation determine the persistence of the drug in the sediment [40]. Drugs associated with small slow-sinking organic particles may be transported a long distance before settlement. Following medication at a nearshore cage operation, organic material collected 1.1 km away from the fish farm using sediment traps contained detectable drug residues [41].
A large difference in stability among antibacterial agents was observed when found in marine sediments. Oxytetracycline, oxolinic acid, flumequine, and sulfadiazine were stable, whereas sulfadimethoxine was partially degraded and ormethoprim, trimethoprim and furazolidone were completely degraded after 180 days [42]. In field studies and in tanks with running water, a gradual disappearance of oxytetracycline, oxolinic acid and flumequine from the sediment was observed, due to a slow release into the water (Hansen et al. 1992; Samuelsen et al. 1994) [42]. In comparison, furazolidone and florfenicol were degraded in the sediment and had completely disappeared within days [43,44].It has been estimated that approximately 80% of the antimicrobials used in aquaculture enter the environment with their activity intact [45].
During cage operations, wild fish may consume excess pellets and are therefore exposed to medicines. Under Norwegian conditions saith (Pollachiusvirens) are especially known to search for food close to fish farms, and flumequine muscle concentration reaching 12.51 mg/g have been found. This by far exceeds the concentrations tolerated in food for human consumption [46]. In a field study of medication with teflubenzuron in a commercial salmon farm, drug residues in wildfish varied greatly between species and individuals within a species [41]. In most of the wild fish, the residue was low but a few individuals contained high concentrations. Therefore, for a few individuals of wild fish waste pellets furnish a larger part of the diet, whereas for most of the population waste feed is less important.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
Conclusion
Large quantities of antibacterial are used in aquaculture in some countries, often without professional consultation or supervision. Considering the rapid growth and importance of the aquaculture industry in many regions of the world and the wide spread, intensive, and often unregulated use of antibacterial agents for animal production, additional efforts are required to prevent the development and spread of the technological characteristics of oral medicine products are of utmost importance to ensure the effectiveness of in-feed medication in the aquaculture industry. Also, safer, more effective medicines are necessary, along with improvements in husbandry and management which will reduce the need for those medicaments. Furthermore an accurate knowledge, of the species-specific pharmacological peculiarities and of the impact of the environment (seawater) on the physical properties of drugs is of equal importance. Pharmacokinetics and pharmacodynamics studies are therefore essential before predicting the success of an oral treatment.
- Research Article
- Abstract
- Introduction
- Methods for Mixing Medication into Feed Depend on Feed Type
- Storage of Medicated Feed
- Mode of Administration Route
- Pharmaceuticals Incorporation into Feed
- Leaching
- Palatability
- Administration of Medicated Feed
- Economic Feasibility of Medicated Feed
- Bioavailability of Medicated Feed
- Pharmacokinetics and Pharmacodynamics of Medicated Feed
- Detoxification Metabolism in Fish
- Impact of Medicated Feed
- Conclusion
- References
References
- Interact AE (2010) Host density thresholds and disease control for fisheries and aquaculture.
- Asche F, H Hansen, R Tveteras, S Tveterås (2009) The salmon disease crisis in Chile. Marine Resource Economics 24(4): 405-411.
- Stentiford GD, Neil DM, Peeler EJ, Shields JD, Small HJ, et al. (2012) Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J Invertebr Pathol 110(2): 141-157.
- Bowker J, J Trushenski, M Tuttle-Lau, D Straus, M Gaikowski, et al. (2011) Guide to using drugs, biologics, and other chemicals in aquaculture.” American Fisheries Society, Fish Culture Section, Bethesda, Maryland.
- Martinsen B, Horsberg TE (1995) Comparative single-dose pharmacokinetics of four quinolones, oxolinic acid, flumequine, sarafloxacin, and enrofloxacin, in Atlantic salmon (Salmo salar) held in seawater at 10 degrees C. Antimicrobial agents and chemotherapy 39(5): 1059-1064.
- Smith P, OB Samuelsen (1996) Estimates of the significance of out-washing of oxytetracycline from sediments under Atlantic salmon sea-cages. Aquaculture 144(1-3): 17-26.
- Saglam N, ME Yonar (2009) Effects of sulfamerazine on selected haematological and immunological parameters in rainbow trout (Onchorhynchus mykiss, Walbaum, 1792). Aquaculture Research 40(4): 395- 404.
- FAO/OIE/WHO (2006) Antimicrobial Use in Aquaculture and Antimicrobial Resistances.
- Grave K, Lingaas E, Bangen M, Rønning M (1999) Surveillance of the overall consumption of antibacterial drugs in humans, domestic animals and farmed fish in Norway in 1992 and 1996. J Antimicrob Chemother 43(2): 243-252.
- Grave K (2012) Usage in animals. In: Norstr€om, M & SkovSimonsen G (Eds.), NORM/ NORM-VET 2012. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway; Chapter V. National Veterinary Institute and Norwegian Institute of Public Health, Oslo, Norway. ISSN: 1502-2307.
- Directorate of Fisheries, Bergen, Norway (2014) Key Figures for the Norwegian Aquaculture Industry.
- Skiftesvik AB, RM Bjelland, CM Durif, IS Johansen, HI Browman (2013) Delousing of Atlantic salmon (Salmo salar) by cultured vs. wild ballan wrasse (Labrusbergylta). Aquaculture 402: 113-118.
- Hanson LE (1963) Feed additives, rationale for medicated feeds. J Agric Food Chem 11(5): 365-367.
- Borràs S, R Companyó, M Granados, J Guiteras, AM Pérez-Vendrell, et al. (2011) Analysis of antimicrobial agents in animal feed. Trends in Analytical Chemistry 30(7): 1042-1064.
- Companyó R, M Granados, J Guiteras, M Prat (2009) Antibiotics in food: Legislation and validation of analytical methodologies. Analytical and bioanalytical chemistry 395(4): 877-891.
- Rodgers CJ, MD Furones (2009) Antimicrobial agents in aquaculture: Practice, needs and issues. Options Méditerranéennes 86: 41-59.
- Antimicrobial drug use in aquaculture (2013) Reimschuessel R, et al. (Ed.), Antimicrobial Therapy in Veterinary Medicine, (5th edn), pp. 645- 661.
- FAO (2005) Responsible Use of Antibiotics in Aquaculture. Fisheries Technical Paper 469, Rome.
- Defoirdt T, Sorgeloos P, Bossier P (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14(3): 251-258.
- Lunestad BT, O Samuelsen, Ø Lie (2008) Veterinary drug use in aquaculture. Improving farmed fish quality and safety 97-127.
- Exposure assessment of veterinary medicines in aquatic systems (2008) Metcalfe C, et al. (Eds.), Veterinary medicines in the environment pp. 123-145.
- Ruth FF and P Reed (1991) Use of medicated feed in channel catfish. Florida Cooperative Extension Service Publication VM70, Institute of Food & Agricultural Sciences, University of Florida, Gainesville, FL.
- Shao ZJ (2001) Aquaculture pharmaceuticals and biologicals: current perspectives and future possibilities. Adv Drug Deliv Rev 50(3): 229- 243.
- Treves-Brown, KM (2001) Applied Fish Pharmacology. In: G Poxton Michael (Eds.), Aquaculture. The Netherlands: Kluwer Academic Publishers.
- Zounková R, Klimešová Z, Nepejchalová L, Hilscherová K, Bláha L (2011) Complex evaluation of ecotoxicity and genotoxicity of antimicrobials oxytetracycline and flumequine used in aquaculture. Environ Toxicol Chem 30(5): 1184-1189.
- Haya K, L Burridge, I Davies, A Ervik (2005) A Review and Assessment of Environmental Risk of Chemicals Used for the Treatment of Sea Lice Infestations of Cultured Salmon. In Environmental Effects of Marine Finfish Aquaculture, edited by Barry Hargrave, Springer Berlin / Heidelberg pp. 305-340.
- Heidenreich E (1997) Carry over and cross contamination. Paper presented at the 3rd East/West Feed Industry Conference, Prague, Czech Republic.
- Daniel P (2009) Drugs and chemicals in aquafeeds: The problems and solutions. Options Méditerranéennes A 86: 85-94.
- Kelly AM (2013) Medicated feed for food fish. SRAC Publication-Southern Regional Aquaculture Center 473: 1-6.
- Durborow RM, R Francis-Floyd (1996) Medicated feed for food fish. Southern Regional Aquaculture Center 473.
- Toutain PL, Ferran A, Bousquet-Mélou A (2010) Species differences in pharmacokinetics and pharmacodynamics. In: Comparative and veterinary pharmacology . Handb Exp Pharmacol 199: 19-48.
- Noga EJ (2010) Fish Disease Diagnose and Treatment. 2nd (edn.), Iowa, Wiley -Blackwell, USA.
- Smith P, T Niland, F O’Domhnaill, G O’Tuathaigh, M Hiney (1996) Influence of marine sediments and divalent cations on the activity of oxytetracycline against Listonellaanguillarum. Bulletin of European Association of Fish Pathologists 16(2): 54-57.
- Barnes AC, TS Hastings, SGB Amyes (1995) “Aquaculture antibacterials are antagonized by seawater cations “. Journal of Fish Diseases 18(5): 463-465.
- Winton JR (2001) In: Fish Hatchery Management, In: G Wedemeyer (eds.) American Fisheries Society, Bethesda, USA: Pp. 559 - 640.
- Smith PR., A Le Breton, TE Horsberg, F Corsin (2009) Guidelines for antimicrobial use in aquaculture. In: Luca Guardabassi, Lars B Jensen & Hilde Kruse (eds.) In: Guide to antimicrobial use in animals, pp. 207- 218.
- Sekkin S, C Kum (2011) Antibacterial drugs in fish farms. In: Faruk Aral & Zafer Doğu(eds.), In: application and its effects. INTECH Open Access Publisher.
- Moutou KA, MD Burke, DF Houlihan (1998) Hepatic P450 monooxygenase response in rainbow trout (Oncorhynchus mykiss (Walbaum)) administered aquaculture antibiotics. Fish Physiology and Biochemistry 18(1): 97-106.
- Uno T, Ishizuka M, Itakura T (2012) Cytochrome P450 (CYP) in fish. Environ Toxicol Pharmacol
- Yu D, Yi X, Ma Y, Yin B, Zhuo H, et al. (2009) Effects of administration mode of antibiotics on antibiotic resistance of Enterococcus faecalis in aquatic ecosystems. Chemosphere 76(7): 915-920.
- Samuelsen OB, Lunestad BT, Hannisdal R, Bannister R, Olsen S, et al. (2015) Distribution and persistence of the anti-sea lice drug teflubenzuron in wild fauna and sediments around a salmon farm, following a standard treatment. Sci Total Environ 508: 115-121.
- Samuelsen OB, BT Lunestad, A Ervik, S Fjelde (1994) Stability of antibacterial agents in an artificial marine aquaculture sediment studied under laboratory conditions. Aquaculture 126(3): 283-290.
- Samuelsen OB, Solheim E, Lunestad BT (1991) Fate and microbiological effects of furazolidone in a marine aquaculture sediment. Sci Total Environ 108(3): 275-283.
- Hektoen H,Berge JA, Hormazabal V, Yndestad M (1995) Persistence of antibacterial agents in marine sediments. Aquaculture 133(3-4): 175- 184.
- Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, et al. (2013) “Antimicrobial use in aquaculture re‐examined: its relevance to antimicrobial resistance and to animal and human health.” Environ Microbiol 15(7): 1917-1942.
- Ervik A, B Thorsen, V Eriksen, BT Lunestad, OB Samuelsen (1994) Impact of administering antibacterial agents on wild fish and blue mussels Mytilus edulis in the vicinity of fish farms. Diseases of Aquatic Organism 18: 45-51.






























