Functional Properties of a Strawberry Ice Cream Based on Donkey Milk and Lactic Acid Bacteria
Flavio Tidona1*, Giovanna Cortellino2, Ichrak Charfi3, Anna Rizzolo2 and Domenico Carminati1
1Centro di Ricerca Zootecnia e Acquacoltura (CREA-ZA), Italy
2Centro di ricerca Ingegneria e Trasformazioni agroalimentari (CREA-IT), Italy
3Department des science et technologie alimentaires, Ecole Superieure des Industries Alimentaires de Tunis, Tunisia
Submission: September 06, 2017; Published: September 25, 2017
*Corresponding author: Flavio Tidona, Consiglio per la ricerca in agricoltura e l'analisi dell'economia agraria (CREA)-Centro di Ricerca Zootecnia e Acquacoltura (CREA-ZA), via Lombardo 11, 26900 Lodi, Italy, Tel: +39-0371-45011; Fax: +39-0371-35579; Email: flavio.tidona@crea.gov.it
How to cite this article: Tidona F, Cortellino G, Charfi I, Rizzolo A, Carminati D. Functional Properties of a Strawberry Ice Cream Based on Donkey Milk and Lactic Acid Bacteria. Nutri Food Sci Int J. 2017; 3(3): 555613.DOI:10.19080/NFSIJ.2017.03.555613
Abstract
Donkey milk was used as unconventional ingredient for products of new generation. Fresh strawberries and donkey milk were used to develop an ice cream with a high functional profile. The ice cream was supplemented with two strains of lactic acid bacteria showing probiotic properties, Lactobacillus plantarum 998 and Bifidobacterium adolescentis ATCC15703. The final product had very low fat levels and achieved an overrun of 24.7±1.2%. Changes of the main nutritional factors were investigated during 4 months of storage at -20°C. Remarkably, the content of vitamin C decreased whereas total phenols and the antioxidant capacity resulted stable, with no significant changes. In conclusion, we have developed a new ice cream proved to be a good carrier of viable probiotic bacteria (over 9 log CFU/g) resistant throughout storage.
Keywords: Functional ice cream; Donkey milk; strawberries; Lactobacillus plantarum; Bifidobacterium adolescentis
Abbreviations: LAB: Lactic Acid Bacteria; TA: Total Titratable Acidity; SSC: Soluble Solids Content; TBC: Total Bacterial Counts; TP: Total Phenols Amount; GAE: Gallic Acid Equivalent; TAN: Total Anthocyanins Amount; Pg3GE: Pelargonidin 3-Glucoside Equivalents; DPPH: 2,2-Diphenyl-1-Picrylhydrazyl; EPR: Electron Paramagnetic Resonance
Introduction
In recent years, probiotic dairy products are considered the major branch of the functional food Industry, with a continuously expanding market. Most of microorganisms currently used as probiotics belong to Lactobacillus and Bifidobacterium. Their consumption bears multiple benefits such as the inhibition of bacterial pathogens, protection against gastrointestinal disorders, improvement of lactose tolerance, enhance calcium absorption, vitamins synthesis and stimulation of the immune system [1]. Currently, several functional dairy products have been proposed: yogurts, fermented milks and beverages. In addition, the development of frozen desserts and ice creams with added prebiotics proved to be a successful application, suitable as vehicle of probiotic bacteria [2-3]. Recently, donkey milk has recently gained a growing interest due to its composition, similar to human milk, and it shows a good tolerability and palatability [4]. The attractive nutritional features and health-promoting properties of donkey milk meet the consumer's demand for natural and functional foods [5]. So far, donkey milk is not commonly exploited as a commercial food product. Few attempts were performed to produce fermented products using donkey milk, such as fermented milks, yogurt and probiotic yogurt, all showing promising possibilities for new dairy applications [6-8]. Strawberries, which are rich in phenolic compounds, are commonly added to dairy products to increase their functionality and antioxidant capacit y.
The aim of this work was to manufacture an innovative ice cream that combines the nutritional properties of donkey milk, the positive properties of fresh strawberries and beneficial lactic acid bacteria, namely Lactobacillus plantarum 998 and Bifidobacterium adolescentis ATCC 15703. Rheological parameters such as hardness, nutritive factors like vitamin C, total anthocyanins, antioxidant capacity and the viability of lactic acid bacteria after processing and during storage were evaluated.
Materials and Methods
Organic whole pasteurized and lyophilized donkey milk (mean composition: dry matter 8.8%, fat 0.3%, proteins 1.9%, lactose 7.0%), supplied by a specialized donkey breeding farm (Azienda Agricola Montebaducco, Salvarano di Quattro Castella, Reggio Emilia, Italy), was used. According to supplier's instructions, donkey milk was reconstituted by dissolving 11 g of powder in 100 ml of sterile distilled water. Strawberries at commercial maturity (6.32% w/w soluble solids content, 6.02±0.16 meq/100 g f.w. total titratable acidity) were purchased at local market. Before use, strawberries were dipped for 15min in a commercial chorine sanitizing solution (Amuchina, ACRAF SpA, Roma, Italy), and then gently rinsed. Lactobacillus plantarum 998 and Bifidobacterium adolescentis ATCC 15703 were cultivated in 500ml of MRS broth (Merck SpA, Vimodrone, Italy) at 30°C for 20 h and MRS broth with 0.1% (w/v) of cysteine at 37°C for 20 h, respectively. LAB cells were recovered by centrifugation (7000 rpm for 10 min at 4°C), washed with Ringer solution (Oxoid SpA, Rodano, Italy), suspended in 20 ml of reconstituted donkey milk and immediately freeze dried. Before processing, the lyophilized cells were re-suspended in 20 ml of sterile distilled water.
Processing
Ice cream was prepared using 200 g of homogenized strawberry, 93 g of sucrose and 200 ml of reconstituted donkey milk, including the 20 ml of LAB cell suspension. Three batches were processed using a semi-professional ice cream machine (Gaggia, Italy). Each batch was shared in six polyethylene glasses and then stored at -20°C. At each storage time (0, 2 and 4 months) three glasses (one for each batch) were analyzed.
Product characterization
Physico-chemical analyses for the strawberry homogenate, total titratable acidity (TA) was determined according to the official method (DM, AOAC, 1985) whereas soluble solids content (SSC) was measured using a refractometer (RFM81, Bellingham-Stanley Ltd., England). The pH of donkey milk, strawberry homogenate and the final blend before processing was measured.
The following parameters were evaluated for ice cream
Dry matter (AOAC, 950.15), protein content (ISO 8968-1), fat content (ISO1211, 2010). Total sugar content and single sugar composition was determined by HPLC according to Bouzas [9]. All the analysis were done in triplicate. The energy content was calculated using the Atwater factors: 4 kcal/g for protein and carbohydrate and 9 kcal/g for fat. The overrun of the ice cream samples was calculated according to Akin [10]. Hardness was measured using an Instron Universal Testing Machine (model 4301, Instron Ltd., Great Britain). Immediately prior to starting the test, a sample was taken out the freezer, acquiring one measurement per single sample. The glass was completely surrounded by crushed ice to maintain the temperature of the sample during the analysis. The conditions for analysis were as follows: 2 mm diameter probe penetrated the ice cream to a depth of 5 mm at 120 mm/min, with 100 N load cell. The maximum force was recorded.
Microbiological analyses
The microbiological quality of the strawberries (before and after chlorine disinfection) and of the ice cream at the end of processing were evaluated by enumerating the following: total bacterial counts (TBC) on Plate Count Agar (PCA, Oxoid) incubated at 30°C for 72 h; Enterobacteriaceae on Violet Red Bile Glucose agar (VRBG, Oxoid) at 37°C for 24 h; yeasts and molds on Yeast Extract Glucose Chloramphenicol agar (YGC, Oxoid) at 28°C for 72 h. Analyses were carried out on 25 g of each sample and results were expressed in CFU/g, as means of duplicates. The survival of the added LAB strains was determined on the ice-cream sampled at the end of processing and after 2 and 4 months of storage at -20°C. Enumerations were carried out on MRS agar with 0.1% (w/v) cysteine (37°C, 24 h, anaerobiosis) for B. adolescentis ATCC 15703, and on MRS agar (30°C, 24 h, aerobiosis) for L. plantarum 998. The different colony morphology (L. plantarum: white colonies, round shaped; B. adolescentis: translucent colonies, irregular edges, bigger size) allowed to distinguish easily the two species.
Nutraceutical Evaluation
Ascorbic acid was determined according to Rizzolo [11] and the result was expressed as mg/100 g. The following nutraceutical evaluation was performed on ethanol acidified extract made by mixing 500 mg of lyophilized ice cream and 10 ml of extract mix (EtOH 95% and HCl 0.02 M; 1:1). The mixture was vortexed for 30 s, then centrifuged at 4°C at 10000 x g for 15 min and filtered on glass wool.
The most important nutraceuticals in strawberry belong to phenols, so this class of compounds was analysed by two spectrophotometric different assays
i) Total phenols amount (TP) was measured according to Folin-Ciocalteu method, as described by Singleton and Rossi [12]. Results are expressed as mg gallic acid equivalent (GAE) in 100 g.
ii) Total anthocyanins amount (TAN) was quantified by the pH-differential method described by Giusti & Wrolstad [13]. Results were expressed as mg pelargonidin 3-glucoside equivalents (Pg3GE) in 100g.
Two methods were also used to measure the antioxidant capacity of ice cream
i) An indirect method, measuring the scavenging activity on a stabilized artificial free radical, the 2,2-diphenyl-1- picrylhydrazyl (DPPH) [14] by spectrophotometer (Jasco V-630 Spectrophotometer,USA). The percent inhibition of DPPH was evaluated according to the formula: S = 100 -[(Af / Ai) * 100] where Af and Ai are the absorbance (517 nm) at the final and initial point, respectively.
ii) A direct method, measuring the scavenging activity on DPPH by electron paramagnetic resonance (EPR), using a MiniScope MS200 Magnettech spectrometer (Berlin, Germany). The scavenging activity of the extract was defined as: DPPH = 100 x (h0 - hX )/ h0 [%] where h0 is the height of the selected band in the EPR spectrum in the blank (reference), and hX the height of the same band in the EPR spectrum in the presence of the scavenging solution (test). The EPR conditions for the DPPH assay are reported in Picchi [15]. For both methods, units were quantified as mg Trolox equivalents (TE) in 100g.
Statistical Analysis
Analysis of variance and Tukey's multiple range test were used to determine statistically significant differences (p<0.05). Different letters corresponded to a significant difference. The software used was Statgraphics version 7 (Manugistic Inc., Rockville MD, USA).
Results and Discussion
Ice cream composition
Dry matter of the ice cream was determined 26.44±0.15 g/100g and the main component was the sucrose (19.79±0.03g/100g), which was added for ice-cream preparation. Other sugars were found and quantified, i.e. lactose 2.98±0.02g/100g, mainly from donkey milk, while glucose 1.13±0.01g/100 g and fructose 1.28±0.01 g/100 g was attributable to the strawberries. Consistent with the composition of donkey milk, protein content was low (1.19±0.02g/100g) and the lipids present only in trace (0.08±0.01g/100g) nutritionally, these ice cream samples can be classified as low-fat products and the energy provided by 100 g of this ice cream was 106.24±0.39 kcal, which was even lower from previously published low-calorie functional ice cream [2].
Physical-chemical properties of the ice cream
The newly generated ice cream was slightly acidic with a pH of 4.19, this was the result of the mixture of donkey milk (pH 7.09) and strawberries (pH 3.34). The overrun is a parameter used to indicate the amount of air incorporated during the manufacturing process, which defines the texture of the product, affecting both physical properties and storage stability. The overrun of our ice cream was 24.7±1.2%, higher than a sorbet, typically less than 20% [16], and similar to other functional frozen desserts [2,17]. The overrun was limited compared to other products with higher fat, protein and sugar content, or made with supplementation of technological additives (emulsifiers, stabilizers, anti-crystallizers etc) [18]. The addition of LAB was reported to be non-influential on the overrun [2].During storage at -20°C, the ice cream had a significant hardening, showing a fourfold increase of hardness after 4 months (Figure 1). However, the level of hardness might be considered acceptable within 2 months of storage since it is comparable to the hardness values (19 - 37 N) found in other reduced-fat ice creams [19].
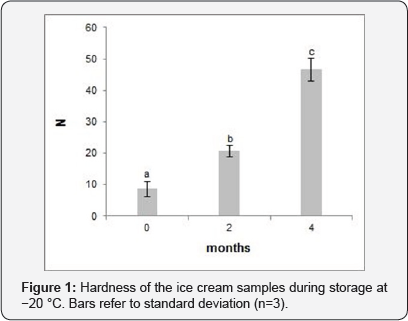
Microbiological quality
The safety of the commercial pasteurized donkey milk was previously demonstrated [8]. Fresh strawberries were characterized by a TBC of 3.51 log CFU/g, a yeasts count of 3.76 log CFU/g, whereas, Enterobacteriaceae and molds were about 1.0 log CFU/g. The disinfection treatment improved the microbiological quality of raw strawberries, determining more than 1 log reduction of bacterial load. As a whole, the final ice cream blend showed a TBC of 2.45 log CFU/g and yeasts at 2.01 log CFU/g. Enterobacteriaceae and molds were not detected (<1.0 log CFU/g).
Survival of lactic acid bacteria
The added LAB strains showed an extremely high survival rate (Figure 2). The incorporation of L. plantarum 998, previously selected by in vivo trials for potential probiotic characteristics [20], and B. adolescentis ATCC 15703, commercially used as probiotic in different applications [21], enabled to obtain more than 9 log CFU/g viable LAB cells in the ice cream. Bifidobacteria are reported to show a better survival rate than Lactobacilli [17] but in our case, both L. plantarum 998 and B. adolescentis ATCC 15703 did not show any significant reduction after 120 d of storage at -20°C. The potential therapeutic value of probiotic bacteria normally depends on the viability of probiotic bacteria which must be maintained throughout the product shelf-life and the gut environment [22]. International Dairy Federation (IDF) suggested that a minimum of 7 log probiotic bacterial cells per gram should be alive at the time of consumption of the product. This requirement was successfully achieved detecting a rarely high viability compared to other studies [10,17]. The strains employed in this work can be considered suitable candidates for probiotic application in functional ice creams.
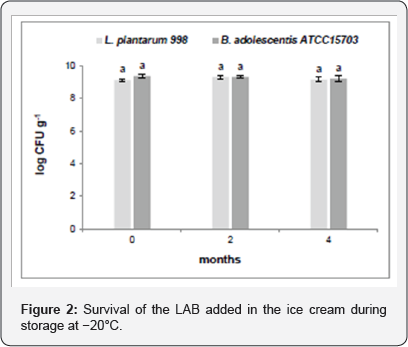
Nutraceutical Profile
It is important to understand the evolution of phytochemicals throughout storage of strawberry ice cream to predict the real benefits for the consumer. Ascorbic acid is an important attribute for nutritional labeling and it can restrict product's shelf life, being very susceptible to degradation [23]. The content of ascorbic acid showed a progressive and significant reduction and after 4 months of storage it approximately halved the initial content (Table 1). This result was in agreement with Dermensonlouoglou [23] who showed a 50% reduction in frozen strawberry stored at -16°C for 4 months. No data about changes of ascorbic acid content in dairy products made with strawberries are available. In fruits like strawberries, the main phenolic compounds detected are anthocyanins, flavonols and derivatives of hydroxycinnamic and ellagic acid. Anthocyanins are quantitatively the most important phenolic constituent and they are responsible for the red color of fresh fruits. However, color changes in strawberry occur during storage also in refrigerated conditions and the reduction of anthocyanins content was reported to be one of the causes for fruit browning [23]. In this study, anthocyanins showed only a slight, though statistically relevant (P < 0.05), reduction during storage without evident color changes. Oliveira [24] found similar results for strawberry yogurt kept for 28 d at 2°C. Total phenols resulted very stable, preserving the initial value till the end of storage as observed by Hipolito [25] in strawberry sorbets stored for 21 d. A valuable result was obtained regarding the antioxidant capacity of the ice cream that, even determined by two different methods (DPPH and EPR), was not only stable throughout the 4 months of storage but showed a tendency to slightly increase over time. These findings confirm a good interaction between the phenolic compounds derived from the strawberries and the viability of probiotic bacteria to gain antioxidant property of ice cream as previously reported [26].
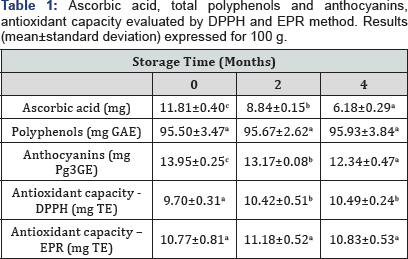
Different letters indicate statistically significant differences during storage.
Conclusion
The high nutritional profile and health-promoting properties of donkey milk were combined with the micronutrients of a fresh red fruit (strawberries) to produce an unconventional low-calories functional ice cream, which also proved to successfully deliver viable LAB with probiotic properties. Except for vitamin C, which gradually decreased, the nutraceutical value of the ice cream was stable throughout storage.
Acknowledgment
This work was partially supported by the Italian Ministry of Agriculture through the CANADAIR Project (D.M. MIPAF 27240/7303/2011).
References
- Giraffa G (2012) Selection and design of lactic acid bacteria probiotic cultures. Engineering in Lif Sciences 12: 391-398.
- Hashemi M, Gheisari HR and Shekarforoush S (2015) Preparation and evaluation of low-calorie functional ice cream containing inulin, lactulose and Bifidobacterium lactis. Int J Dairy Technol 68: 183-189.
- da Silva PDL, de Fatima Bezerra M, Olbrichdos Santos KM, Pinto Correia RT (2015) Potentially probiotic ice cream from goat's milk: Characterization and cell viability during processing, storage and simulated gastrointestinal conditions. Food Science and Technology 62: 452-457.
- Monti G, Viola S, Baro C, Cres F, Tovo PA, et al. (2012) Tolerability of donkey's milk in 92 highly-problematic cow's milk allergic children. J Biol Regul Homeost Agents 26 (3 Suppl): 75-82.
- Jirillo F, Jirillo E, Magrone T (2010) Donkey's and goat's milk consumption and benefits to human health with special reference to the inflammatory status. Curr Pharm Des 16: 859-863.
- Chiavari C, Coloretti F, Nanni M, Sorrentino E, Grazia L (2005) Use of donkey's milk for a fermented beverage with lactobacilli. Lait 85: 481490.
- Perna A, Intaglietta I, Simonetti A, Gambacorta E (2015) Donkey milk for manufacture of novel functional fermented beverages. J Food Sci 80(6): 352-359.
- Tidona F, Charfi I, Povolo M, Pelizzola V, Carminati D, et al. (2015) Fermented beverage emulsion based on donkey milk with sunflower oil. International Journal of Food Science and Technology 50: 26442652.
- Bouzas J, Kantt CA, Body felt F, Torres JA (1991) Simultaneous determination of sugars and organic acids in Cheddar cheese by High- Performance Liquid Chromatography. Journal of Food Science 56: 276278.
- Akin MB, Akin MS, Kirmaci Z (2007) Effects of inulin and sugar levels on the viability of yogurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice cream. Food Chemistry 104: 93-99.
- Rizzolo A, Brambilla A, Valsecchi S, Eccher-Zerbini P (2002) Evaluation of sampling and extraction procedures for the analysis of ascorbic acid from pear fruit tissue. Food Chemistry 77: 257-262.
- Singleton, VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolibdic- phosphotungstic reagents. American Journal of Enology and Viticulture 16, 144-158.
- Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-Visible spectroscopy. In Wrolstad RE, et al. (Eds.), Current protocols in food analytical chemistry. New York: John Wiley and Sons. P F1.2.1 - F1.2.13.
- Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology 28: 25-30.
- Picchi V, Migliori C, Lo Scalzo R, Campanelli G, Ferrari V, et al. (2012) Phytochemical content in organic and conventionally grown Italian cauliflower. Food Chemistry 130: 501-509.
- Goff HD and Hartel RW (2013) Ice cream. Seventh Edition, Springer® + Business Media New York.
- Aboulfazli F, Baba AS, Misran M (2016) Replacement of bovine milk with vegetable milk: Effects on the survival of probiotics and rheological and physicochemical properties of frozen fermented dessert. International Journal of Dairy Technology 69: 71-80.
- Akalin AS, Erisir D (2008) Effects of inulin and oligofructose on the rheological Characteristics and probiotic culture survival in low fat probiotic ice cream. J Food Sci 73: 184-188.
- Mahdian E, Karazhian R (2013) Effects of fat replacers and stabilizers on rheological, physicochemical and sensory properties of reduced-fat ice cream. Journal of Agricultural Science and Technology 15: 11631174.
- Zago M, Fornasari ME, Carminati D, Burns P, Suarez V, et al. (2011) Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol 28(5): 1033-1040.
- Wang J, Korber DR, Low NH, Nickerson MT (2015) Encapsulation of Bifidobacterium adolescentis cells with legume proteins and survival under stimulated gastric conditions and during storage in commercial fruit juices. Food Science and Biotechnology 24(2): 383-391.
- Kailasapathy K, Chin J (2000) Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol 78(1): 80-88.
- Dermesonlouoglou EK, Giannakourou M, Taoukis PS (2016) Kinetic study of the effect of the osmotic dehydration pre-treatment with alternative osmotic solutes to the shelf life of frozen strawberry. Food and Bioproducts Processing 99: 212-221.
- Oliveira A, Alexandre EMC, Coelho M, Lopes C, Almeida DPF, et al. (2015) Incorporation of strawberry preparation in yoghurt: Impact on phytochemicals and milk proteins. Food Chemistry 171: 370-378.
- Hipolito C, Ramalheira R, Beirao da Costa S, Moldao-Martins (2016) The effect of fruit cultivar/origin and storage time on sorbets quality. Food Science and Technology 68: 462-469.
- Sagdic O, Ozturk I, Cankurt H, Tornuk F (2012) Interaction between some phenolic compounds and probiotic bacterium in functional ice cream production. Food and Bioprocess Technology 5: 2964-2971.






























