Fish Oil Supplements in Lupus - Weighing the Evidence
Sirisha Gokaraju and Chandra Mohan*
Department of Biomedical Engineering, USA
Submission: August 28, 2017; Published: September 14, 2017
*Corresponding author: Chandra Mohan, MD, PhD, Department of Biomedical Engineering, University of Houston, 605 Cullen Blvd. Houston, Texas, USA -77204, Tel: 713-743-3709; Email: cmohan@central.uh.edu
How to cite this article: Gokaraju S and Mohan C. Fish Oil Supplements in Lupus - Weighing the Evidence. Nutri Food Sci Int J. 2017; 3(2): 555606. DOI: 10.19080/NFSIJ.2017.03.555606
Abstract
This review strives to examine the beneficial effects, or of lack thereof, of fish oil supplement use in systemic lupus erythematosus (SLE) patients. The majority of prospective studies reviewed in this article render support to fish oil supplement use in SLE patients, as evident by reduction in disease activity, inflammatory markers and increase in individual global well-being scores. The existing evidence also provides guidance with regards to future research.
Keywords: Lupus; Fish oil; Omega fish oil; Inflammation; Systemic lupus erythematosus; SLE; Western diet
Abbreviations: DHA: Docosahexaenoic Acid; DMARD: Disease Modifying Antirheumatic Drugs; EPA: Eicosapentaenoic Acid; HDL: High Density Lipoproteins; IL: Interleukin; LTB4: Leukotriene B4; NSAID: Nonsteroidal Anti-Inflammatory Drug; PhyGA: Physician's Global Assessment; SF-36: Short Form Survey (36 Items); SLAM-R: Systemic Lupus Activity Measurement - Revised; SLE: Systemic Lupus Erythematosus; VLDL: Very Low Density Lipoprotein; NS: Not Specified; ESR: Erythrocyte Sedimentation Rate; ↑: Enhanced/Increased; ↓: Reduced/ Decreased; ↔: No Change; NA: Not Applicable; HDL: High Density Lipoprotein; TG: Triglycerides; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; FSS: Fatigue Severity Scale; BILAG: British Isles Lupus Assessment Group; NA: Not Applicable; RBC: Red Blood Cell; fi3-FA: Omega-3 Fatty Acids; Gm: Grams
Introduction
Fish oil nutritional supplement use was brought to relevance by an epidemiological study [1] conducted from 1950-1974 in Greenland Eskimos. Low incidence of chronic medical problems such as diabetes mellitus, bronchial asthma, multiple sclerosis and psoriasis in Eskimos in comparison to their age and gender matched Western European cohorts were attributed to omega-3 fatty acids. Greenland Eskimo diet predominantly comprises of fish; fish oil, in turn, is known to be a rich source of omega-3 fatty acids (n-3). Also known as eicosanoids, the omega-3 fatty acids are polyunsaturated fatty acids (n-3 PUFA), and chief among them are Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Both EPA and DHA tip the scales for anti-inflammatory eicosanoids over pro-inflammatory omega-6 fatty acids (n-6 PUFA). The latter group of polyunsaturated fatty acids (n-6 PUFA) comprise of immunoactive eicosanoids such as prostaglandin E2 (PGE2), thromboxane B2 (TXB2), and leukotriene B4 (LTB4) [2].
Animal studies established a causal relationship between EPA and DHA and their ability to promote anti-inflammatory effects [3-5]. Subsequent clinical trials in humans confirmed the beneficial anti-inflammatory properties of the omega-3 fatty acids EPA and DHA in improving clinical outcomes in chronic medical illnesses such as coronary artery disease, obesity-related diseases and rheumatic diseases [6-10]. Rheumatic diseases, in particular, are widely recognized to have inflammatory pathophysiology at their core and thus represent ideal medical conditions to gauge clinical efficacy upon treatment with fish oil. In this review, we focus on available evidence in the literature with regards to clinical outcomes in systemic lupus erythematosus (SLE) patients treated with fish oil supplements [11-20]. SLE is one of the most common rheumatic diseases along with rheumatoid arthritis (RA) and osteoarthritis (OA), with prevalence rates ranging from 20 to 150 cases per 100,000 [21-23].
Impact of Fish Oil Supplementation in Systemic Lupus Erythematosus (SLE)
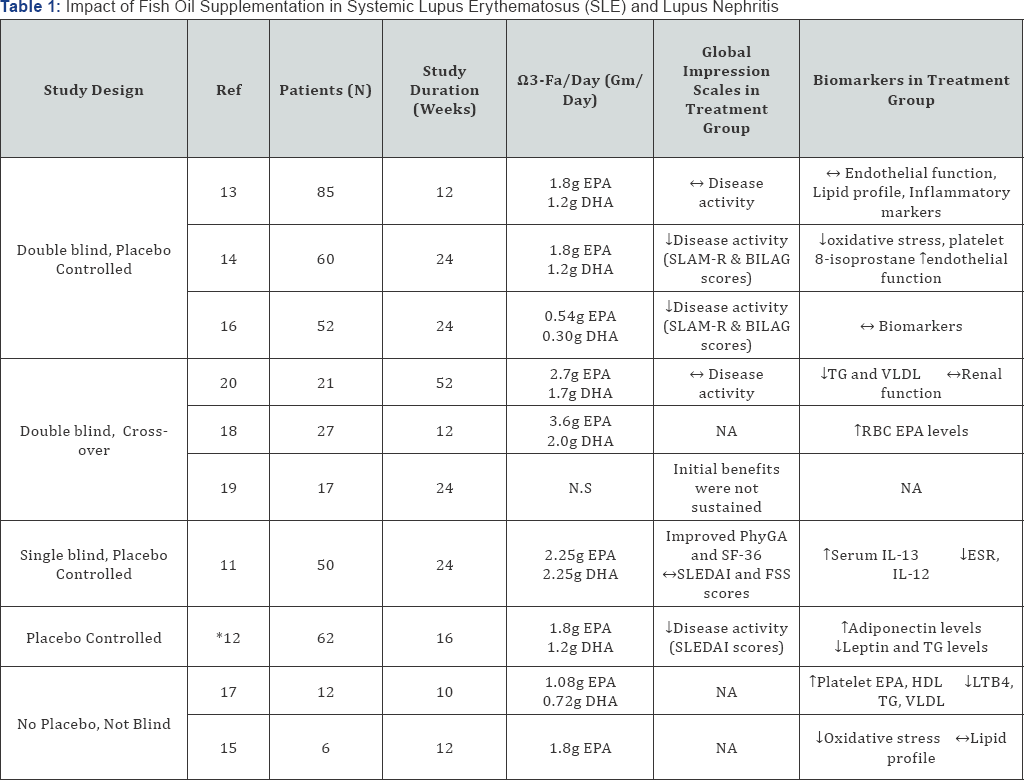
We have identified ten studies [11-20] that assessed the effects of fish oil supplementation on various clinical outcomes mostly in systemic lupus erythematosus (SLE) and in some lupus nephritis cohorts. The available evidence is tabulated in Table 1 based on the study design. Seven out of these ten studies exclusively included patients with SLE, two of the three remaining studies included only patients with lupus nephritis, while one study included both patients with SLE and Lupus nephritis. The number of patients included in these prospective studies ranged from 6 to 85 and the study duration ranged from 10-52 weeks. In the treatment group, the patients received anywhere from 0.54-3.24grams of EPA and 0.30-2.25grams of DHA per day. Also, in all the trials both treatment and control groups simultaneously continued treatment with non-steroidal anti-inflammatory drugs (NSAID) and disease modifying anti rheumatologic drugs (DMARD), except in the Lozovoy et al. [12] study, where the patients were treated with NSAIDs and anti-hypertensive's. Outcomes were measured using a range of global subjective rating scales, including the Physicians Global Assessment (PhyGA), Short Form Survey-36 (SF-36), Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), Fatigue Severity Scale (FSS), and Systemic Lupus Activity Measurement- Revised (SLAM- R). Objective outcome measures included a host of biomarkers such as serum interleukin (IL) levels, erythrocyte sedimentation rate (ESR), lipid profiles, 8-isoprostane levels (an indicator of oxidative stress), red blood cell EPA concentration, etc.
Five of these studies [11,12,14,16,18] showed that patients in the treatment group that received daily omega-3 fatty acid supplementation with EPA and DHA benefitted with reduced disease activity, further corroborated by improvement in various biomarkers assayed, as outlined in Table 1. The study by Bello et al. [13], likely due to its shorter trial duration, failed to show any significant benefit in the treatment arm. In another SLE-only patient study [19], the initial benefits observed three months from baseline were not sustained at six months from baseline. Three of the studies reviewed in this article [15,17,20] included patients with lupus nephritis. Lupus nephritis patients who received fish oil supplementation exhibited a reduction in oxidative stress, increase in platelet EPA levels, reduction in pro-inflammatory cytokines such as LTB4 and a favorable lipid profile.
Overview of Mechanism of Action
The fish oil supplements EPA and DHA modulate inflammatory pathways putatively via altering the levels of polyunsaturated fatty acids (PUFA) in the cell membranes. Although cell membranes contain both omega-6 fatty acids (n-6 PUFA) and omega-3 fatty acids (n-3 PUFA), if the ratio of n-3 PUFA to n-6 PUFA is not adequate, the arachnoid acid derivatives from n-6 PUFA have been documented to favor pro-inflammatory cytokine production [24-28]. Through experimental studies [29-33] it has been reported that cell membrane composition of omega-3 fatty acid (n-3 PUFA) can be increased by dietary intake of these fatty acids. Omega-3 fatty acids reinforce anti-inflammatory pathways via a host of mechanisms including alteration of membrane receptors and modifying the synthesis of lipid mediators, in addition to inhibiting production of pro-inflammatory cytokines such as Tumor Necrosis Factor (TNF) and Interleukin-1 (IL-1) [34].
Conclusion
Modern Western diet with high omega-6 fatty acid to omega-3 fatty acid ratio in excess of 15:1 over recommended ratios (2) have been shown to up regulate inflammatory pathways. This skewing can well promote a wide spectrum of chronic medical illnesses with inflammatory underpinnings, including the common rheumatic diseases. The preponderance of data in systemic lupuserythematosus (SLE) attests to the beneficial effects of fish oil supplements (omega-3 fatty acids) in moderating inflammatory pathways and improving clinical outcomes, with similar findings being noted in patients with lupus nephritis. However, the existing evidence also raises questions regarding the long-term benefits of fish oil supplement use. Further research is warranted to see if fish oil supplementation could reduce dependence on steroids, NSAID and other immunosuppressive agents that are invariably associated with side effects. Given that fish oil supplements are well tolerated by humans (except for rare gastrointestinal discomfort), studies are also warranted to establish the efficacy of long-term fish oil therapy over several years.
Conflict of Interest
All authors concur with the findings in this review. No financial support was received for this work, and none of the authors have any conflict of interest.
References
- Kromann N, Green A (1980) Epidemiological studies in the Upernavik district, Greenland: Incidence of Some Chronic Diseases 1950-1974. Acta Med Scand 208(1-6): 401-406.
- Wall R, Ross RP, Fitzgerald GF, Stanton C (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 68(5): 280-289.
- Peterson LD, Jeffery NM, Thies F, Sanderson P, Newsholme EA, et al. (1998) Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids 33(2): 171-180.
- Yaqoob P, Calder P (1995) Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell Immunol 163(1): 120-128.
- Chapkin RS, Akoh CC, Miller CC (1991) Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J Lipid Res 32(7): 1205-1213.
- Bucher HC, Hengstler P, Schindler C, Meier G (2002) N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 112(4): 298-304.
- Browning LM (2003) n-3 Polyunsaturated fatty acids, inflammation and obesity-related disease. Proc Nutr Soc 62(2): 447-453.
- James M, Proudman S, Cleland L (2010) Fish oil and rheumatoid arthritis: past, present and future. Proc Nutr Soc 69(3): 316-323.
- Boe C, Vangsness CT (2015) Fish Oil and Osteoarthritis: Current Evidence. Am J Orthop (Belle Mead NJ) 44(7): 302-305.
- Borges MC, Santos Fde M, Telles RW, Correia MI, Lanna CC (2014) Polyunsaturated omega-3 fatty acids and systemic lupus erythematosus: what do we know? Rev Bras Reumatol 54(6): 459-466.
- Arriens C, Hynan LS, Lerman RH, Karp DR, Mohan C (2015) Placebo- controlled randomized clinical trial of fish oil's impact on fatigue, quality of life, and disease activity in Systemic Lupus Erythematosus. Nutr J 14:82.
- Lozovoy MA, Simao AN, Morimoto HK, Scavuzzi BM, Iriyoda TV, et al. (2015) Fish oil N-3 fatty acids increase adiponectin and decrease leptin levels in patients with systemic lupus erythematosus. Mar Drugs 13(2): 1071-1083.
- Bello KJ, Fang H, Fazeli P, Bolad W, Corretti M, et al. (2013) Omega-3 in SLE: a double-blind, placebo-controlled randomized clinical trial of endothelial dysfunction and disease activity in systemic lupus erythematosus. Rheumatol Int 33(11): 2789-2796.
- Wright SA, O'Prey FM, McHenry MT, Leahey WJ, Devine AB, et al. (2008) A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis 67(6): 841-848.
- Nakamura N, Kumasaka R, Osawa H, Yamabe H, Shirato K, et al. (2005) Effects of eicosapentaenoic acids on oxidative stress and plasma fatty acid composition in patients with lupus nephritis. In Vivo 19(5): 879882.
- Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, et al. (2004) The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosus. J Rheumatol 31(8): 1551-1556.
- Clark WF, Parbtani A, Huff MW, Reid B, Holub BJ, et al. (1989) Omega-3 fatty acid dietary supplementation in systemic lupus erythematosus. Kidney Int 36(4): 653-660.
- Walton AJ, Snaith ML, Locniskar M, Cumberland AG, Morrow WJ, et al. (1991) Dietary fish oil and the severity of symptoms in patients with systemic lupus erythematosus. Ann Rheum Dis 50(7): 463-466.
- Westberg G, Tarkowski A (1990) Effect of Max EPA in patients with SLE. A double-blind, crossover study. Scand J Rheumatol 19(2): 137143.
- Clark WF, Parbtani A, Naylor CD, Levinton CM, Muirhead N, et al. (1993) Fish oil in lupus nephritis: clinical findings and methodological implications. Kidney Int 44(1): 75-86.
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, et al. (1998) Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41(5): 778-799.
- Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM (2007) Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum 56(6): 2092-2094.
- Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L, Cooper GS (2010) Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 39(4): 257-268.
- Dennis EA, Norris PC (2015) Eicosanoid storm in infection and inflammation. Nat Rev Immunol 15(8): 511-523.
- Kinsella JE, Lokesh B, Broughton S, Whelan J (1990) Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition 6(1): 24-44.
- Calder PC (2001) Polyunsaturated fatty acids, inflammation, and immunity. Lipids 36(9): 1007-1024.
- Moalli F, Cupovic J, Thelen F, Halbherr P, Fukui Y, et al. (2014) Thromboxane A2 acts as tonic immunoregulator by preferential disruption of low-avidity CD4+ T cell-dendritic cell interactions. J Exp Med 211(13): 2507-2517.
- Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST (2003) Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA 100(4): 751-1756.
- Simopoulos AP (2003) Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet 92: 1-22.
- James MJ, Gibson RA, Cleland LG (2000) Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 71(1 Suppl): 343S-348S.
- Simopoulos AP (1999) Essential fatty acids in health and chronic disease. Am J Clin Nutr 70(3 Suppl: 560S-569S.
- Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2(3): 355-374.
- Hedi H, Norbert G (2004) 5-Lipoxygenase Pathway, Dendritic Cells, and Adaptive Immunity. J Biomed Biotechnol 2004(2): 99-105.
- Renier G, Skamene E, DeSanctis J, Radzioch D (1993) Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice. Modulation of macrophage secretory activities. Arteriosclerosis, Thrombosis, and Vascular Biology 13(10): 15151524.






























