Effect of Silymarin as Natural Antioxidants and Antimicrobial Activity
Abdelazim SAA*
Department of Field Crops Technology, Agriculture Research Center, Egypt
Submission: January 17, 2017; Published: February 06, 2017
*Corresponding author: Abdelazim SAA, Field Crops Technology Department Food Technology Research Institute, Agriculture Research Center, Egypt, Email: abdelazim_73@yahoo.com
How to cite this article:Abdelazim S. Effect of Silymarin as Natural Antioxidants and Antimicrobial Activity. Nutri Food Sci Int J. 2017; 2(3): 555589. DOI: 10.19080/NFSIJ.2017.02.555589
Abstract
Effect of silymarin as natural antioxidants and antimicrobial activity was main target of the present study. Physicochemical properties of the sunflower oil were determined. The ratio was 4.234, 0.485 and 6.289 between (Σ PUFA / ΣSFA, MUFA / ΣPUFA and ΣUSFA / ΣSFA) respectively. Antioxidant activities of the silymarin were evaluated using Rancimat test at 100°C and calculated at 25°C using the temperature coefficient of 2.2 and the DPPH radical scavenging method. The addition of the different concentrations of silymarin to sunflower oil (100 to 500ppm) led to clear increment in relative stability % and increasing index of sunflower oil ( 137.589 to 144.899% and 37.589 to 44.899) compared with BHT (107.329% and 7.329) respectively. The DPPH radical scavenging activities (%) were increased with increasing silymarin concentration from 100 to 450ppm from70.27% to 86.77% respectively. Meanwhile the 500ppm of silymarin slightly lowered its value to 83.78% than that of 300 to 450ppm (86.77%) still higher scavenging activity (%) compared with BHT (72.78%) at 200ppm.
Antimicrobial activities of each concentration of silymarin were measured and the data indicated that the different concentrations of silymarin had exhibited antimicrobial activities against gram-positive bacteria (Bacillus subtilis ATCC 33221, Bacillus cereus ATCC 33018, Bacillus cereus NRRLB 3711 and Staphylococcus aureus ATCC 20231), gram negative bacteria (Escherichia coli ATCC69337, Escherichia coli 0157 and Pseudomonas aeruginosa, ATCC 9027 ), molds (Aspergillus niger NRRL326, Aspergillus flavus MERCN 101, Aspergillus parasiticus and Penicillium sp.) and yeast (Geotericum candidum NRRLY552 ) . No antimicrobial activity of silymarin concentrations was noticed against either Saccharomyces cerevisiae NRRLY 3034 or Candida lipolytica NRRLY1095. The antimicrobial activity of silymarin was found to be increased in parallel with increasing the concentrations against all tested microorganisms strains. It could be concluded that, the silymarin can be used as natural antioxidants and antimicrobial activity in food industry.
Keywords: Silymarin; Natural antioxidants; Antimicrobial activity; Rancimat DPPH; BHT; Sunflower oil
Introduction
Silymarin is a lipophilic extract from the seeds of milk thistle and is composed of three isomer flavonolignans, silybin, silydianin and silychristin. Silymarin was first isolated by. Silymarin content of milk thistle seed ranged from 4 to 6% based on dry weight. Silybin is the component with the greatest degree of biological activity and comprises 50-70% of silymarin. Seeds of milk thistle contain small amounts of flavonoids (taxifolin) and approximately 20-35% fatty acids and other polyphenolic compounds [1]. A number of other flavonolignans have also been found in the seeds including isosilybin, dehydrosilybin, desoxysilycristin, desoxysilydianin, silandrin, silybinome, silyhermin and neosilyhermin [2]. Silymarin has free radical scavenging. Properties and its ability to enhance endogenous anti-oxidant defense systems in vivo. Silymarin has been shown to inhibit the growth of human prostate, breast, and cervical cancer cells in test tubes.
Further in vivo studies are needed to determine whether milk thistle is safe or effective for people with these forms of cancer. Silymarin can protect liver, brain, heart and other vital intra organs from oxidative damage for its ability to prevent lipid peroxidation and replenishing the reduced glutathione levels [3]. silymarin have anti-inflammatory and anti-arthritic effects due to excellent antioxidant property, scavenging free radicals which act as pro-inflammatory agents. Silymarin was found to be more effective in cases of developing arthritis compared to developed arthritis. Silymarin and silibinin hinder inflammatory process by inhibiting neutrophil migration and kuppfer cell inhibition. They also inhibit the formation of inflammatory mediators' viz. prostaglandins and leukotriens and release of histamine from basophils. Therefore, milk thistle seed may possess anti allergic and antiasthmatic activities [4]. Silymarin is a strong antioxidant that has been proven to promote liver cell regeneration, to reduce blood cholesterol and to help prevent cancer [5].
The hepatoprotective action of silymarin is mainly through its anti-free radical and anticarcinogenic roles [6] but it has also been ascribed other actions includes antioxidant, anti-lipid peroxidative, antifibrotic, anti-inflammatory immunomodulatory and liver regenerating activity. Silymarin has clinical applications in alcoholic liver diseases, liver cirrhosis, Amanita mushroom poisoning, viral hepatitis, toxic and drug diseases of the liver, psoriasis and in neuroprotective and neurotropic activity [7]. Silymarin acts as a toxin blockade agent by inhibiting binding of toxins to hepatocyte cell membrane receptors .The toxicity of silymarin is very low, the oral 50% lethal dose being 10,000mg kg-1 in rats and the maximum tolerated dose being 300mg kg-1 in dogs [8]. Silymarin is not soluble in water and is usually administered in capsules as a standard extract (70-80% silymarin).
Formulation ofthe phytotherapic extract (silymarin) in self- emulsifying pellets gave rise to a simultaneous improvement of the oral biodiversity of its main active compounds [9]. Silymarin has been shown to reduce liver fibrosis up to 30-35%, and in few cases it has reversed the liver fibrosis [10]. Silymarin has potent antioxidant properties, reduces and suppress harmful effects of solar ultraviolet radiation (UVR) [11]. Silymarin is well tolerated in chronic HCV-infected patients. However, no evidence of salutary effects of oral silymarin has yet been reported based on intermediate endpoints (ALT and HCV RNA) in this population. Moreover, intravenous administration of silymarin should be further studied. [12].
Silymarin have the potential to protect the cell from genotoxic damage, due to a lower value DNA in tail. Effective prevention against ROS production [2]. Silymarin cream was a novel, effective and safe treatment modality for melasma especially inepidermal and mixed types in Fitzpatrick skin phototype III, IV and V as it showed a significant improvement ofmelasma lesion. It was as effective as 50% glycolic acid peeling in the treatment of melasma without postinflammatory hyperpigmentation that occurred by glycolic acid peeling [13]. Silymarin combined with grape seed extract had synergistic effect as antifibrotic therapy than single treatment with silymarin or GSE alone and GSE (200mg/Kg) combined with silymarin had a good resultant effect [14]. The main objective to investigate effect of silymarin as natural antioxidants and antimicrobial activity substance.
Materials and Methods
Materials
Sunflower oil: Refined bleached and deodorized sunflower oil (with no added antioxidants) was obtained from Cairo for Oils and Soap Company, Giza, Egypt. All the chemicals and reagents used were of analytical reagent grade and were purchased from Sigma (St. Louis, MO, USA) and Merck (Darmstadt, Germany) Company. Silymarin were purchased from Sigma (St. Louis, MO, USA) and Merck (Darmstadt, Germany) Butylated hydroxyl toluene (BHT): BHT and DPPH were purchased from Sigma Chemical Co. (St. Louis, Mo 63178, USA).
Microbiological evaluation
Microorganisms: some bacterial strains representing gram-negative bacteria Escherichia coli ATCC 69337, Escherichia coli 0157, Pseudomonas aeruginosa ATCC 9027, and gram-positive bacteria Bacillus subtilis ATCC 33221, Bacillus cereus ATCC 33018, Bacillus cereus NRRLB 3711 and Staphylococcus aureus ATCC 20231 in addition to 3 strains of yeasts Saccharomyces cerevisiae NRRLY 2034, Candida lipolytica NRRLY 1095 , Geotericum candidum NRRLY 552 , in addition to 3 strains of moulds Aspergillus flavus 101 MERCN , Aspergillus niger NRRL 326, Aspergillus parasiticus and Penicillium sp were obtained from MERCN Cairo (Microbial Collection Center, the Faculty of Agriculture , Ain Shams University) . These microorganisms were checked for their purities likewise they were reactivated to obtain active microorganisms.
Used media
Nutrient agar medium: The medium was obtained from El Nasr Pharmaceutical Chemical Co., Egypt and Malt agar medium which used for yeasts and moulds growth was obtained from Biolifes, Milano, Italy
Methods
Physicochemical properties of the Sunflower oil: The peroxide value, acid value, refractive was determined according to the method described in the AOAC (2005) [15].
Gas chomatography analysis Methylation of fatty acids
An aliquot of oil, about 10 mg, was dissolved in 2ml hexane and then 0.4 ml of 2N KOH in anhydrous methanol was added [16], after 3min, 3ml water was added. The organic layer, separated by centrifugation which was dried over anhydrous sodium sulfate, and then concentrated, with a N2 stream to around 0.5ml for GC analysis of fatty acids methyl esters (FAME) as described below.
Evaluation of antiox
idant properties of Silymarin on sunflower oil Determination of antioxidant activity of silymarin by Rancimat test
Sunflower oil (5g), was mixed separately with different concentrations of 0, 100, 150, 250, 300, 350, 400, 450 and 500 silymarin and 200ppm of BHT antioxidants and then exposed to the rancimat test using Metrohm Rancimat model 734 at 100°C and calculated at 25°C using the temperature coefficient of 2.2 for induction period at an airflow of 20L/ h. Measuring vessels, electrodes, connecting tubes and glassware were cleaned several times before the experiments [17]. The relative stability (%) and the increasing index were calculated using the following equations:

Determination of antioxidant activity of silymarin by (DPPH test)
The free radical scavenging activity of different concentration of silymarin was measured by the 2, 2-diphenyl-1 picryl hydrazil (DPPH) methods according to [18]. The scavenging activity was calculated using the folwwing formula:
DPPH scavenging activity % = (A blank -A sample / A blank) x 100
Antimicrobial activity of Silymarin
The effect of Silymarin on bacterial and yeast growth was studied by the Paper-Disc plate method according to Jiang, et al. [19] by measuring the inhibition zone in mm.
Statistical analysis
The collected data of biological assay and organoleptic evaluation were statistically analyzed by the least significant differences (LSD) at the 5% level of probability procedure according to the method of Snedecor & Cochran [20].
Results and Discussion
Physical and chemical properties of sunflower oil
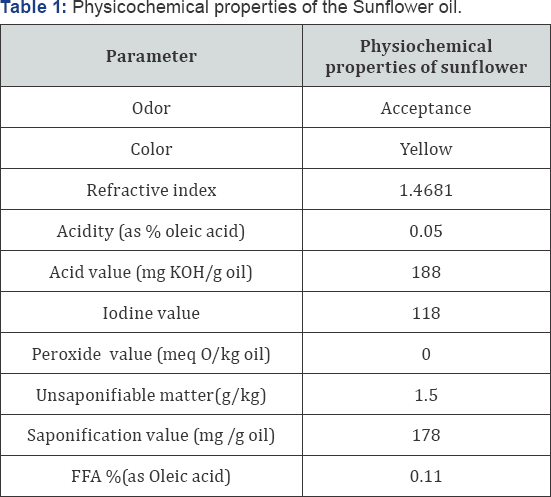
The physical and chemical properties of sunflower oil used in the present study were determined to define the identity of the oil, and insure that oxidative reaction has not begun. The obtained results are shown in Table 1. The acid values were 188mg KOH/g oil where the low acid values (188-194mg KOH/g oil) of the oil indicated its freshness state. Moreover, the physical and chemical properties of sunflower oil under study were found to be corresponding to the values approved by the Egyptian Standard (49- 1993), for sunflower seed oil (refractive index: 1.4681, acidity (0.05 as % oleic acid), peroxide value 0.0 equivalent of active oxygen/ kg. of oil, iodine value: 118, saponification value: 178, and unsaponifiable matter % not more than 1.5%). The above mentioned data revealed that no lipolysis and /or oxidative rancidity occurred in the oil under study. Therefore, the oil can be used in study and the previous obtained data were in harmony with the findings of Firestone, Hilali et al, Yunus et al. [21-23].
Fatty acids composition of sunflower oils
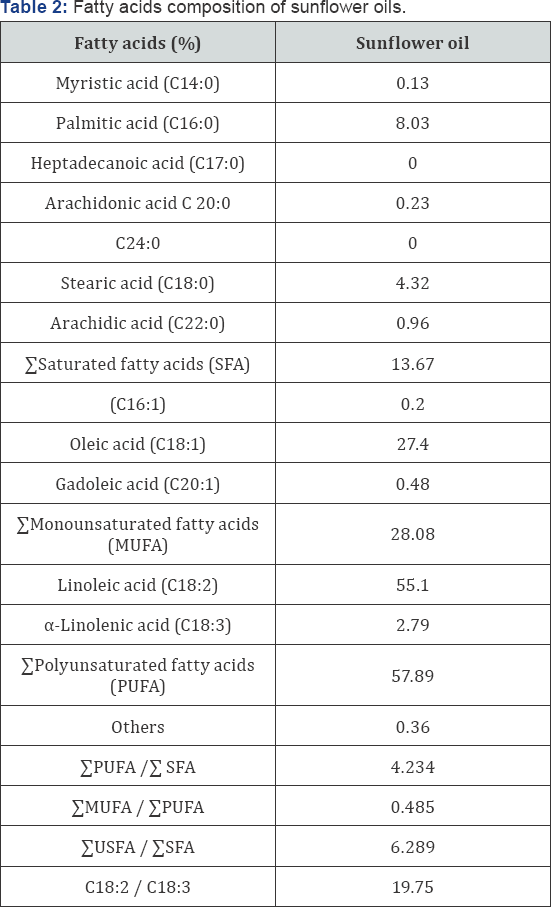
The fatty acids composition of sunflower oil show in Table 2. The results indicated that sunflower oil contains five saturated fatty acids, i.e., merestic (14:0), palmetic (16:0) , stearic acid (18:0) and arachidonic acid (C 20:0) in relative percentages of 0.13%, 8.03%, 4.32% and 0.96% respectively, comprising 13.67% of Saturated fatty acids (SFA) . Moreover, there monounsaturated fatty acids (MUFA), i.e. (16:1), (18:1) and (20:1) showed relative percentages of 0.20%, 27.4% and 0.48% resp., comprising 28.08% of Monounsaturated fatty acids (MUFA). Linoleic acid (C18:2) and a-Linolenic acid (C18:3) as resulted in a value of 55.1% and 2.79%, respectively, comprising 57.89 of Polyunsaturated fatty acids (PUFA).
In sunflower oil, the main fatty acid is Linoleic acid, followed by oleic, Palmitic and Stearic acids. Also, these results were confirmed by the ratio between ΣPUFA/ ΣSFA, MUFA/ΣPUFA and ΣUSFA/ ΣSFA, (4.234, 0.485 and 6.289) respectively which showed that the oil had a high TU/ TS ratio (6.289). This means that sunflower oil is distinguished by containing fatty acids profile with high degree of unsaturation such as C18:2 (essential fatty acid) Moreover, the obtained data was in harmony with the findings Table 2 the fatty acid composition of sunflower oil. These results are agreement with Firestone, Hilali et al. and Yunus et al. [21-23].
Effect of different concentrations of the silymarin on oxidative stability of sunflower oil
The antioxidant properties of the silymarin on refined sunflower oil were determined by Rancimat test at 100°C and calculated at 25°C using the temperature coefficient of 2.2. The obtained results are recorded in Tables 3 & 4. From these results, it could be observed that addition of silymarin exhibited antioxidant activities on fresh sunflower oil compared control sample. The data in Table 3 showed that silymarin at level 100 to 500ppm had highly antioxidant activity more than that of BHT at 200ppm or control sample. The induction period of sunflower oil in the presence of 100 to 500ppm silymarin increased the induction period 13.649 - 14.375 hr compared with that of 9.922 and 10.648 hr for control and BHT respectively.
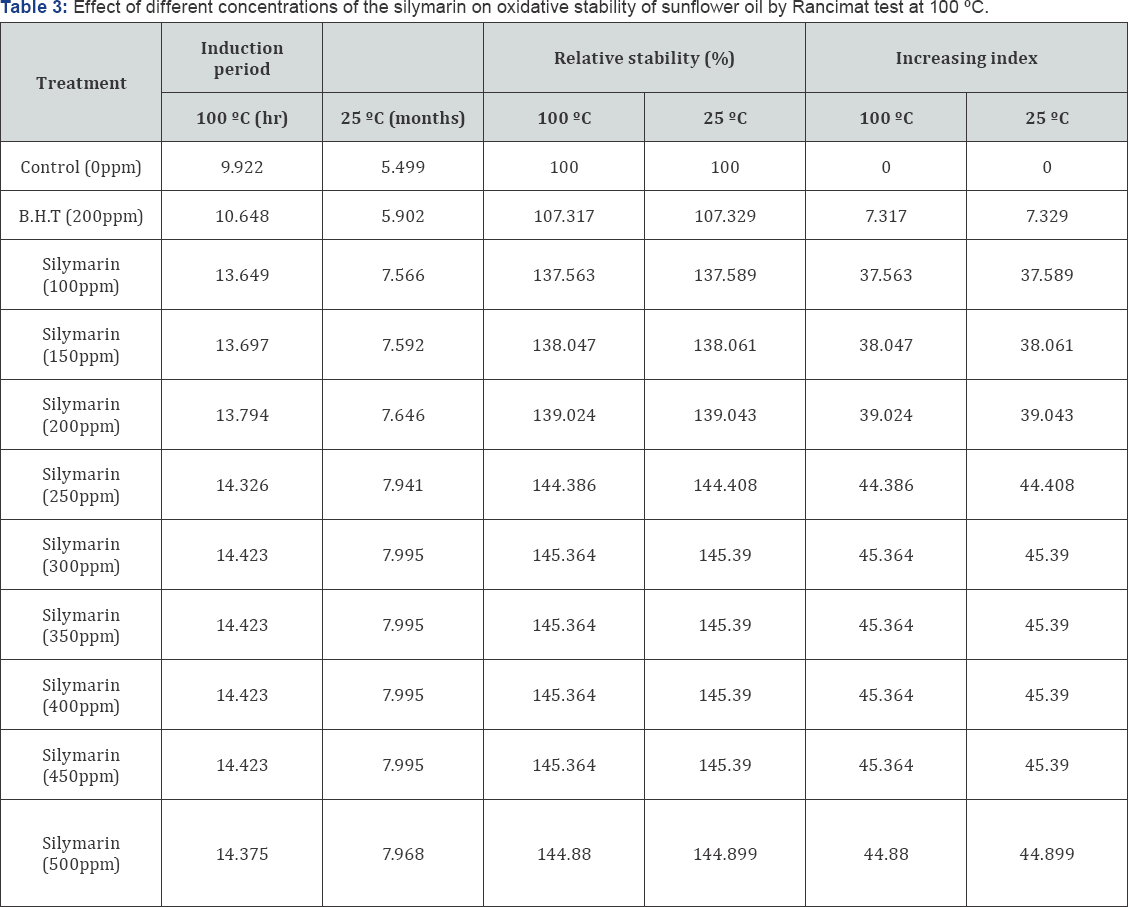
Relative stability (%) and induction index Table 3 in parallel with those of induction period for instance the relative stability increased gradually from 137.56 to 145.36% due to silymarin concentration from 100- 450ppm while a slight decrease was found due to 500ppm silymarin (144.88%) compared with BHT ( 107.317 %). Similar trend was found concerning increasing index which gradually increased from 37.56 to 45.36 due to 100-450ppm silymarin concentration with slight decreased due to 500ppm (44.88) compared with BHT (7.31) the calculated induction period related in 5-8 months as a shelf life (25°C) as a result of silymarin concentration. Finally, from these data, it could be concluded that these results of the antioxidant properties of the silymarin on refined sunflower oil were determined by Rancimat test at 100 °C and calculated at 25°C using the temperature coefficient of 2.2 are in agreement with those observed by antioxidant activity of silymarin by DPPH test .
Antioxidant activity of silymarin by (DPPH test)
The DPPH scavenging activity (%) of the silymarin
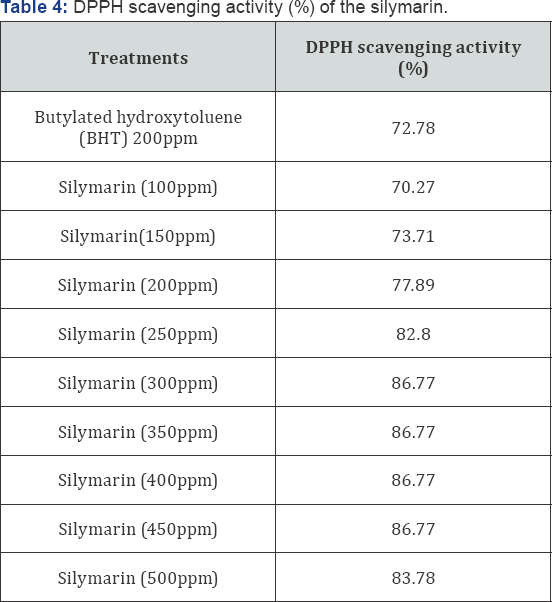
The Free radical-scavenging capacities of silymarin compare with Butylated hydroxytoluene (BHT) at 200 ppm were measured by DPPH assay at different concentrations are given in Table 4. The DPPH radical scavenging activities (%) were increased with increasing concentration of silymarin concentration from 100 to 450ppm from70.27% to 86.77% respectively. A high antioxidant activity was observed for silymarin (86.77%) at 300 to 450ppm compared with BHT (72.78%) at 200ppm. The concentration (500ppm) of silymarin (500ppm) resulted in lower value (83.78%) than concentration 300 to 450ppm (86.77%) but was also it had higher scavenging activity (%) compared BHT (72.78%) at 200ppm. On the other hand, found that no significant radical-scavenging capacities had noted for silymarin with 300 to 450ppm (86.77%). meanwhile that the silymarin 500ppm concentration slightly decrease of the DPPH scavenging activity to 83.78%.
From the above-mentioned data the that these results of the DPPH scavenging activity (%)of silymarin are in agreement with those observed by Rancimat test at 100°C and calculated at 25°C using the temperature coefficient of 2.2. Finally, from these data, it could be concluded that, the silymarin had a high antioxidant activity and considered as a good source of natural antioxidants. We can report the retarding of sunflower oil lipid oxidation using silymarin increased compared with butylated hydroxytoluene (BHT). It could be concluded that the silymarin prolongs stability of sunflower oil and it may be a potent antioxidant activity for its stabilization.
Evaluation of antimicrobial activity of silymarin
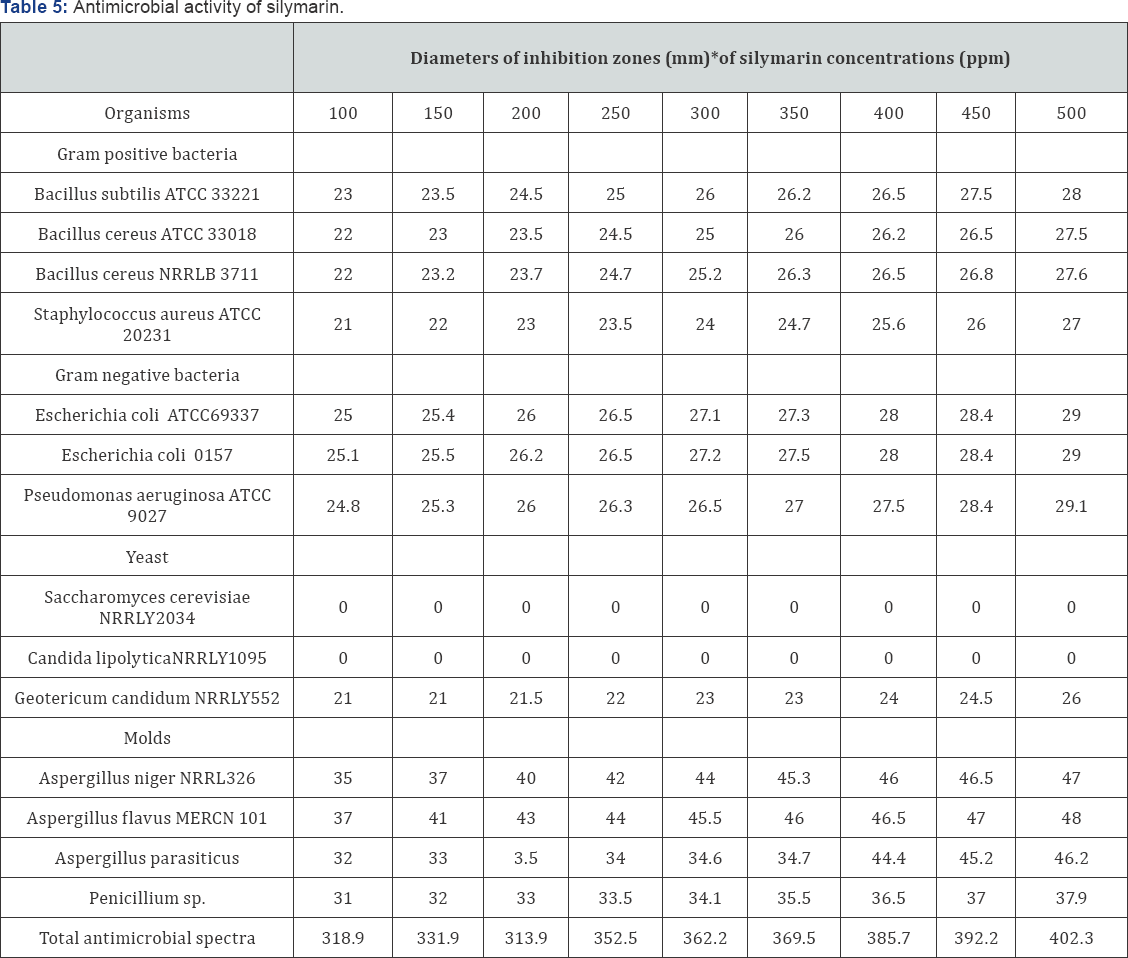
The antimicrobial activities of different concentrations of silymarin were determined against seven bacterial two yeast and four mold strains. The different concentrations of silymarin that used were 100,150,200,250,300,350,400,450 and 500ppm. The diameter of inhibition zones was taken as a criterion of the antimicrobial spectrum. The obtained results are presented in Table 5. Data indicated that the different concentrations of silymarin exhibited antimicrobial activities against gram-positive bacteria (Bacillus subtilis ATCC 33221, Bacillus cereus ATCC 33018,Bacillus cereus NRRLB 3711and Staphylococcus aureus ATCC 20231), gram negative bacteria (Escherichia coli ATCC69337, Escherichia coli 0157 and Pseudomonas aeruginosa, ATCC 9027), molds (Aspergillus niger NRRL326, Aspergillus flavus MERCN 101,Aspergillus parasiticus and Penicillium sp.) and yeast (Geotericum candidum NRRLY552 ).
No antimicrobial activity of the different concentrations of silymarin was noticed against either Saccharomyces cerevisiae NRRLY 3034 or Candida lipolytica NRRLY1095 while different concentrations of silymarin exhibited antimicrobial the concentrations against all tested microorganisms strains. The antimicrobial activity against Geotericum candidum NRRLY 552 (21-26mm inhibition zone). The antimicrobial activity of the different concentrations of silymarin was noticed increased with increased of the different concentrations of silymarin had higher effective on molds compared with that all tested bacterial strains (gram positive or negative gram). In general, data in Table 5, indicated that different concentrations of silymarin had the most powerful antimicrobial and molds spectra against all test microorganisms increased their antimicrobial spectra (318.9, 331.9, 313.9, 352.5, 362.2, 369.5, 385.7, 392.2 and 402.3 ) in parallel with the concentrations of silymarin from (100-500), respectively.
References
- Ramasamy K, Agarwal R (2008) Multitargeted therapy of cancer by silymarin. Cancer Lett 269(2): 352-362.
- Katerina T, Hana K, Klara P, Svatopluk B, Petr K, et al. (2014) Cytotoxicity and antioxidative effects of herbal and fruit extracts in vitro. FoodBiophysics 9(3): 267-276.
- Das SK, Mukherjee S, Vasudevan DM (2008) Medicinal properties of milk thistle with special reference to silymarin: An overview. Natural Product Rad 7(2): 182-192.
- Dixit N, Baboota S, Kohli K, Ahma S, Ali J (2009) Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J Pharmacol 39(4): 172-179.
- Vaknin Y, Hadas R, Schafferman D, Murkhovsky L, Bashan N (2008) The potential of milk thistle (Silybum marianum L.), an Israeli native, as a source of edible prouts rich in antioxidants. Int J Food Sci Nutr 59(4): 339-346.
- Shaker E, Mahmoud H, Mnaa S (2010) Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol 48(3): 803-806.
- Ghosh A, Ghosh T, Jain S (2010) Silymarin - a review on the pharmacodynamics and bioavailability enhancement approaches. J Pharm Sci Technol 2(10): 348-355.
- Abenavoli L, Capasso R, Milic N, Capasso F (2010) Milk thistle in liver diseases: past, present, future. Phytother Res 24(10): 1423-1432.
- Iosio T, Voinovic D, Perissutti B, Serdoz F, Hasa D, et al. (2010) Oral bioavailability of silymarin phyto complex formulated as self- emulsifying pellets, Phytomedicine.
- Haddad Y, Vallerand D, Brault A, Haddad PS (2011) Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid Based Complement Alternat Med 2011:nep164.
- Altaei T (2012) The treatment of melasma by silymarin cream. BMC Dermatol 12: 18.
- Zongguo Y, Liping Z, Yunfei L, Qingnian X, Xiaorong C (2014) Effects and Tolerance of Silymarin (Milk Thistle) in Chronic Hepatitis C Virus Infection Patients: A Meta-Analysis of Randomized Controlled Trials. BioMed Research International 9.
- Elfar NN, El-Maghraby GM (2015) Efficacy of Intradermal Injection of Tranexamic Acid, Topical Silymarin andGlycolic Acid Peeling in Treatment of Melasma: A Comparative Study. J Clin Exp Dermatol Res 6: 280.
- Nada SA, Gowifel AMH, El-Denshary EES, Salama AA, Khalil MG (2015) Protective Effect of Grape Seed Extract and/or Silymarin Against Thioacetamide-induced Hepatic Fibrosis in Rats. J Liver 4: 178.
- AOAC (2005) Association of Official of Analytical Chemists, Official Methods of Analysis. (18th Ed), Washington DC, USA.
- Cossignani L, Simonetti MS, Damiani P (2005) Biocatalyzed acidolysis of olive oil triacylglycerols with 9c, 11t and 10t, 12c isomers of conjugated linoleic acid. Eur Food Res Technol 220(3): 267-271.
- Farhoosh R, Tavassoli-Kafrani MH (2011) Simulataneous monitoring of the conventional qualitative indicators during frying of sunflower oil. Food Chem 125(1): 209-213.
- Kekuda PTR, Shobha KS, Onkarappa R (2010) Studies on antioxidant and anthelmintic activity of two Streptomyces species isolated from Western Ghat soils of Agumbe, Karnataka. Journal of Pharmacy Research 3(1) :26-29.
- Jiang L, Wang F, Han F, Prinyawiwatku W, No HK, et al. (2012) Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J Appl Microbiol 114(4): 956-963.
- Snedecor GW, Cochran WG (1980) "Statistical methods” Oxford and J.b.H publishing Co. (7th edition).
- Firestone D (2005) Olive oil: In Fereidoon Shahidi (Ed.), Bailey's Industrial Oil and Fat Products (6 volume) (6th edn), John Wiley & Sons, Inc.
- Hilali M, Charrouf Z, Soulhi AE, Hachimi L, Guillaume D (2005) Influence of origin and extraction method on argan oil physicochemical characteristics and composition. J Agric Food Chem 53(6): 2081-2087.
- Yunus WMM, Fen YW, Yee LM (2009) Refractive Index and Fourier Transform Infrared Spectra of Vigin Coconut oil and Virgin Olive OilAmerican Journal of Applied Sciences 6(2): 328-331.






























