Chemical-Bromatological Characterization of The Red Alga Chondracanthus Teedei Typical of The Sicilian Coasts (Màuru)
Anna De Angelis*
Dipartimento di Agricoltura, Alimentazione e Ambiente (Di3A), Università degli Studi di Catania, Italy
Submission: June 26, 2024; Published: July 05, 2024
*Corresponding author: Anna De Angelis, Dipartimento di Agricoltura, Alimentazione e Ambiente (Di3A), Università degli Studi di Catania, Italy
How to cite this article: Anna De Angelis*. Chemical-Bromatological Characterization of The Red Alga Chondracanthus Teedei Typical of The Sicilian Coasts (Màuru). Agri Res& Tech: Open Access J. 2024; 28(5): 556421. DOI: 10.19080/ARTOAJ.2024.28.556421
Summary
The present investigation shows the results of the analyses carried out on the red macroalga Chondracanthus teedei, widespread in a restricted area of eastern Sicily, where it is known by the dialect name of “Màuru”.
The data obtained show a chemical composition completely analogous to that highlighted in a previous research, that is, a high carbohydrate content (41.11% DM), a high protein content (37.57% DM), good nutritional value for a good ratio between essential and non-essential amino acids, a prevalence of sodium and potassium among the mineral macroelements, in the optimal ratio of 1.03, and iodine (1395 mg/Kg) among the microelements. The lipid fraction, poorly represented (1.70% DM), shows a composition in fatty acids completely comparable to the red algae belonging to the genus Asparagopsis.
In particular, a prevalence of saturated C14 (2.98% DM) and C16 (3.54% DM) and unsaturated C18:2 (6.92% DM) is highlighted; a prevalence of polyunsaturated (48.20% DM) compared to saturated (24.15% DM) and monounsaturated (17.83% DM); saturated fatty acids represent only 37% of the total unsaturated fatty acids. The red alga Chondracanthus teedei present along the coasts of eastern Sicily, has chemical-bromatological characteristics that make it particularly suitable for zootechnical use, representing a food with good nutritional value, from both an energy and protein point of view, and an ingredient with antimethanogenic action.
For these reasons, it would be desirable not only to maintain its presence through the improvement of the environmental ecosystem and to increase it through appropriate repopulation interventions, but also to characterize its carbohydrate fraction and bioactive compounds, mainly implicated in the antimethanogenic function.
Keywords: Chondracanthus teedei; Red algae; Chemical-bromatological composition, Lipid fraction; Bioactive compounds
Abbreviations: FA: Fatty Acids; SFA: Saturated Fatty Acids; MUFA: Monounsaturated Fatty Acids; PUFA: Polyunsaturated Fatty Acids; USFA: Unsaturated Fatty Acids; USFA: Unsaturated Fatty Acids; SFA: Saturated Fatty Acids
Introduction
There are two main categories of algae: macroalgae, sometimes called seaweeds, which mainly inhabit the littoral zone and can reach considerable sizes; and microalgae, which are characterized by their small size and are distributed throughout benthic and littoral environments, as well as throughout the ocean as phytoplankton [1-3].
Macroalgae are rich in a lot of nutrients, such as carbohydrates, proteins, amino acids, lipids, oils, minerals, vitamins and other bioactive compounds. For this reason, they have been a traditional food source in several regions, including Japan, Korea, China, and among coastal communities living in tropical locations [4]. Chondrocanthus Teedei, for example, until a few decades ago, was normally used as a food source by local populations and was identified with the dialect term “Màuru” to highlight its low fat content.
Macroalgae are used for various purposes including their application as soil improvers to improve its fertility, as a source of biomass for fuel production, for wastewater treatment and in the cosmetics industry [5-8]. Several researchers [1,2,9-13] have extensively examined the nutritional and biochemical attributes of various seaweed species. Seaweeds have a variety of chemical constituents, phytochemicals such as carrageenan, agar, and alginates, which have antioxidant, antimicrobial, antiviral, anti-inflammatory, and antitumor activities [9,14-16]. They possess a high concentration of essential nutrients and have been widely used for human nutrition, animal feed production, medicinal interventions and fertilizers [17,18].
In the livestock sector, algae are widely used and studied [2,19,20]. They are rich in bioactive components, including iodine, vitamins, minerals, polyunsaturated fatty acids (PUFAs), and phlorotannins [2,9,21-23]. The presence of bioactive components in algae offers potential advantages, such as inhibiting rumen proteolysis and combating harmful microbes in feed [24-28]. These benefits could improve and promote the production of rumen fermentation end products, leading to improved animal health, feed efficiency and production.
In addition, many algae, especially red algae (Asparagopsis taxiformis and Asparagopsis armata), contain secondary plant compounds, such as halogenated compounds and phlorotannins, with the potential to reduce rumen enteric methane (CH4) emissions by up to 99% when integrated into ruminant diets.
The aim of this work was to analyze a red alga, belonging to the Chondracanthus genus, Chondracanthus teedei, present on the coasts of eastern Sicily, to better define its bromatological characteristics. The poor availability of the species along the coasts, due to the changed environmental conditions, has limited the investigations to the main chemical constituents.
Materials and Methods
The chemical composition analyses were performed on dried samples of the red macroalgae Chondracanthus teedei according to the standard protocols of the Association of Official Analytical Chemists [29]. Moisture and ash content were determined at 105 °C for 24 h and 575 °C for 6 h, respectively; lipid content was analyzed by Soxhlet extraction with petroleum ether as solvent, and protein content of the dried samples was examined by the Kjeldahl method, using the corresponding red algal conversion factor of 4.59 following the instructions of Lourenço et al. [30]. Total carbohydrate content (g/100 g) was determined by difference as follows: 100-(g protein + g fat + g ash). Crude fiber, NDF, ADF and lignin were determined according to the Weende and Van Soest method; macronutrients, micronutrients and amino acids were determined by mass spectrometry and HPLC. Energy (kcal/100 g DM) was calculated according to Regulation (EU) No 1169/2011 as follows: 4 × (g protein + g carbohydrate) + 9 × (g fat). Fatty acids were determined with Agilent7890A gas chromatograph.
Results and Discussion
Due to the difficulty of finding red algae in the diffusion area of the Ionian coasts of eastern Sicily, there are no data in the bibliography on the chemical composition of the macroalgae Chondracanthus teedei, called “Màuru”.
The analyses conducted on dried samples revealed an overall chemical composition that was on average similar to other red macroalgae and to the results obtained in a previous test conducted on the same algae [31].
In particular, the carbohydrate component, prevalent compared to the other nutritional principles, was slightly higher than in the previous test (41.11% vs, 39.46%). It is interesting to note that algae often contain a variety of polysaccharides, with a composition that varies depending on the species. For example, red algae is rich in agar, agarose, agaropectin, carrageenan and porphyrian [32]. It is likely that the slightly higher total carbohydrate content is due to the season of collection of the samples in line with what has been highlighted by several authors on the significant seasonal variations in the chemical composition of red algae [33-35].
Not very different from the previous test were the contents of crude fiber (2.98% vs 3.19), ADF (11.1% vs 10.9%), NDF (33.09% vs 32.89%) and lignin (4.28% vs 4.08%). Algae contain significant amounts of structural fiber content for neutral detergent fiber (NDF) and acid detergent fiber (ADF) [2,9,36]. The cell wall composition of algae is mainly comprised of alginates [9,37], and red algae often have higher NDF levels than green and brown algae. Despite this structural distinction, digestive enzymes in ruminants possess the ability to hydrolyze and ferment the active polysaccharide constituents of algal polysaccharides [38].
The protein component (37.57% vs 38.3%), was only slightly lower than the results of the previous test and shows a level (~38%) that is comparable to that of high-protein plant diets such as soy and soy flour [9,10,39,40]. It seems that the protein content in algae is also subject to variations based on specific species and seasonal factors [41].
Ash (19.62% vs 20.68%); lipids continue to be scarce and not very dissimilar compared to previous data (1.70% vs 1.36%) (Table 1).
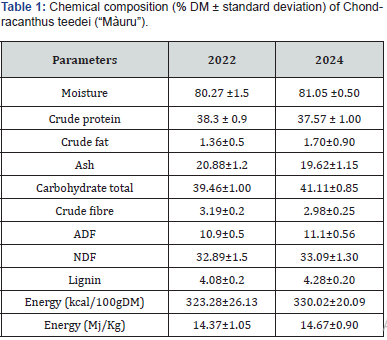
The amino acid content (Table 2) shows similar results to the previous test. In particular, a high percentage of essential amino acids (45.5% vs 46.6% of the total); among the essential amino acids, the most represented were, once again, leucine (7.1% vs 6.9%), valine (6.5% vs 7.4%), and phenylalanine (4.5% vs 4.8%). Among the non-essential amino acids (52.4% of the total) aspartic acid (13.5% vs 13.9%), glycine (9.2% vs 9.4%) and glutamic acid (8.4% vs 8.7%). Some authors note seasonal variations in the amino acid content probably due to the intensity of sunlight and the amount of nitrogen [42], others to the harvest season and the species [41,43,44].
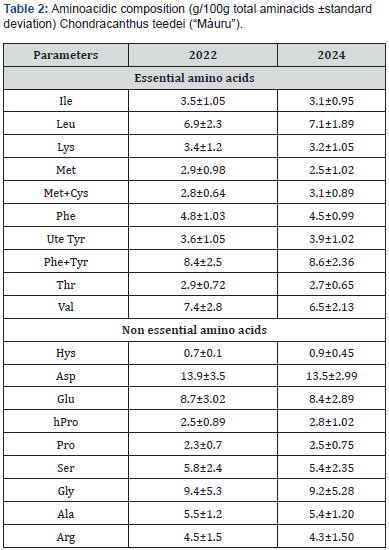
The content of macro and microelements also shows a distribution very similar to that shown in the previous test (Table 3). Among the macroelements, sodium and potassium are abundant (32946 vs 32030 and 32040 vs 31890 mg/kh DM), followed by magnesium (6689 vs 6759), calcium (4690 vs 4630) and phosphorus (2002 vs 1950). Among the microelements, iodine is the most represented (1395 vs 1305), iron (127 vs 140), zinc (123 vs 110) and copper (3.60 vs 3.56) show levels similar to those found by other authors in various marine algae [45,46]. The abundance of iodine could create nutritional problems due to goiter effects; However, some primitive sheep, native to the Scottish island of North Ronaldsay in the Orkneys of Scotland, rely on natural seaweed as their main food, which constitutes over 80% of their sustenance, and are able to efficiently excrete excess iodine [47,48].
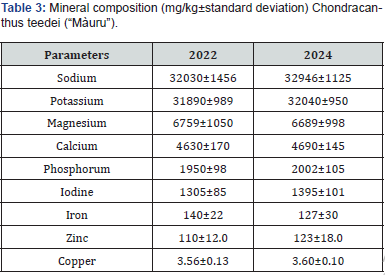
The lipid content of seaweed has attracted considerable attention in recent years due to the presence of useful fatty acids. Seaweed is generally composed of polyunsaturated fatty acids (PUFA; C18, C22), with species-specific variations. The distribution of unsaturated fatty acids (USFA) and saturated fatty acids (SFA) varies among seaweed species [9]. Table 4 shows the fatty acid content of Chondracanthus teedei and compares it with other red algae species. The table shows an acid composition completely comparable to the red algae belonging to the Asparagopsis genus.
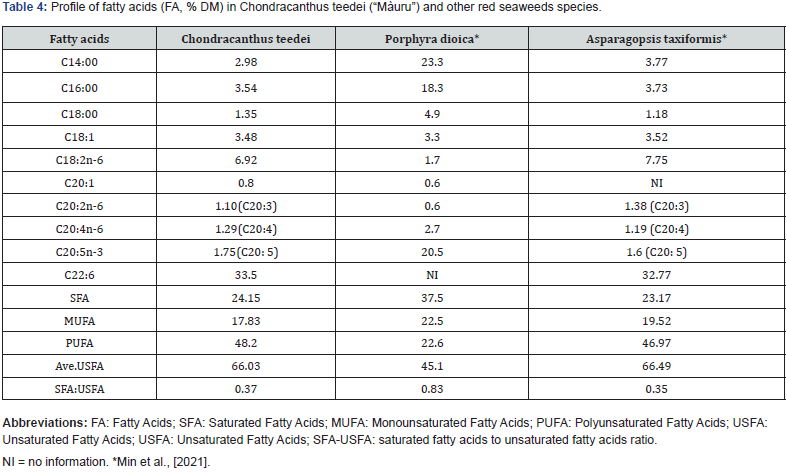
In particular, there is a prevalence of saturated C14 (2.98) and C16 (3.54) and unsaturated C18:2 (6.92); a prevalence of polyunsaturated (48.20) compared to saturated (24.15) and monounsaturated (17.83); saturated fatty acids represent only 37% of the total mono- and polyunsaturated.
In many forms of algae, in particular those belonging to the red and brown variants, a considerable amount of PUFA is found, including omega-3 and omega-6, i.e. compounds that positively influence the function of the immune system, as well as improving the quality of meat and milk and play a significant role in promoting animal health [9].
Conclusion
Chondracanthus Teedei is a red algae present in the coastlines of the eastern coast of Sicily, called by the dialect term “Màuru”, known by the local population for having been, until a few decades ago, a popular and tasty food. The persistent and continuous alteration of the ecosystem environment, over the years, has drastically reduced the presence of the species, to the point of making it difficult to find.
The results of the investigation conducted on limited samples of algae confirmed what was highlighted in a previous similar test [31], reiterating a high content of total carbohydrates and a good protein content, comparable to that of soy, also due to a favorable composition of essential amino acids. Among the macroelements, sodium and potassium are abundant and among the microelements, iodine.
Furthermore, gas chromatographic investigations have highlighted a composition of the lipid fraction, which is low in percentage terms, of good nutritional value, because it is rich in polyunsaturated fatty acids, with a presence of saturated fatty acids equal to only 37% of the total unsaturated fatty acids, and completely comparable to that of other species of red algae. The well-known versatility of use of red algae, as highlighted by the results of extensive research conducted over the years on the subject, requires its preservation through the restoration of environmental ecosystem conditions and its increase through the implementation of repopulation plans.
In particular, the chemical-bromatological characteristics of red algae, also highlighted by this investigation, stimulate their use in the livestock field, both as an alternative protein source and as a food with good nutritional value in general, in terms of energy, due to the high carbohydrate component, and quality, due to a favorable content of amino acids and fatty acids.
Further investigations could concern both the composition of the carbohydrate fraction and the presence of bioactive compounds, implicated, according to the prevailing bibliography, in the anti-methanogenic action of red algae, which, in addition to improving the performance of ruminants, would contribute to the mitigation of the much vaunted greenhouse effect.
Acknowledgment
The author wishes to thank the fishermen of the province of Catania (Sicily, Italy) for providing samples of Chondrachantus teedei.
References
- Morais T, Inácio A, Coutinho T, Ministro, Cotas J, et al. (2020) Seaweed potential in the animal feed: a review. J Mar Sci Eng 8: 559.
- Makkar HP, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, et al. (2016) Seaweeds for livestock diets: a review - Anim. Feed Sci Technol. 212: 1-17.
- Hasan MR, Chakrabarti R (2009) Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture: A Review - FAO Fisheries and Aquaculture Technical, FAO, Rome, Italy.
- McHugh DJ (2003) A guide to the seaweed industry - FAO Fisheries Technical. Food and Agriculture Organization (FAO) of the United Nations.
- DeSosa LL, Navarro-Fernández CM, Panettieri M, Madejón P, Pérez-de-Mora A (2023) Application of seaweed and pruning residue as organic fertilizer to increase soil fertility and vine productivity. Soil Use Manag 39(2): 794-804.
- Farghali M, Mohamed IMA, Osman AI, Rooney DW (2023) Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: a review. Environ Chem Lett 21: 97-152.
- Pagels F, Arias A, Guerreiro A, Guedes AC, Moreira MT (2022) Seaweed cosmetics under the spotlight of sustainability. Aust J Psychol 2: 374-383.
- Thakur N, Salama E, Sharma M, Sharma P, Sharma D, Li X (2022) Efficient utilization and management of seaweed biomass for biogas production. Mater Today Sustain 18: 100120.
- Min BR, Parker D, Brauer D, Waldrip H, Lockard C, et al. (2021) The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: challenges and opportunities. Anim Nutr 7(4): 1371-1387.
- Cian RE, Drago SR, Sanchez de Medina F, Martínez-Augustin O (2015)Proteins and carbohydrates from red seaweeds: evidence for beneficial effects on gut function and microbiota. Mar Drugs 13(8): 5358-5383.
- Angell AR, Angell SF, de Nys R, Paul NA (2016) Seaweed as a protein source for mono-gastric livestock. Trends Food Sci Technol 54: 74-84.
- Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23: 543-597.
- Heiba HI, Al-Easa HS, Rizk AFM (1997) Fatty acid composition of twelve algae from the coastal zones of Qatar - Plant Foods Hum Nutr 51(1): 27-34.
- Neethu P, Suthindhiran K, Jayasri M (2017) Antioxidant and antiproliferative activity of Asparagopsis taxiformis. Pharmacognosy Res 9(3): 238.
- Kolanjinathan K, Ganesh P, Saranraj P (2014) Pharmacological importance of seaweeds: a review. J Fish Mar Sci 6: 1-15.
- Pal MC, Kamthania A, Kumar (2014) Bioactive compounds and properties of seaweeds-a review. Open Access J Sci 1(4): 1-17.
- Nunes N, Valente S, Ferraz S, Barreto M, Pinheiro de Carvalho M (2018) Nutraceutical potential of Asparagopsis taxiformis extracts and assessment of a downstream purification strategy. Heliyon 4(11): e00957.
- Cardozo KH, Guaratini T, Barros MP, Falcão VR, Tonon AP, et al. (2007) Metabolites from algae with economic impact. Comp Biochem Physiol Part C: Toxicol Pharmacol 146: 60-78.
- Allen VG, Pond KR, Saker KE, Fontenot JP, Bagley CP, et al. (2001a) Tasco: influence of brown seaweed on antioxidants in forages and livestock a review. J Anim Sci 79: 21-31.
- Allen VG, Pond KR, Saker KE, Fontenot JP, Bagley CP, et al. (2001b) Tasco forage: III. Influence of a seaweed extract on performance, monocyte immune cell response, and carcass characteristics in feedlot-finished steers. J Anim Sci 79: 1032-1040.
- Antaya NT, Soder KJ, Kraft J, Whitehouse NL, Guindon NE, et al. (2015) Incremental amounts of Ascophyllum nodosum meal do not improve animal performance but do increase milk iodine output in early lactation dairy cows fed high-forage diets. J Dairy Sci 98(3): 1991-2004.
- Cvetkovic B, Brouk MJ, Shirley JE (2004) Impact of dried seaweed meal on heat-stressed lactating dairy cattle. In: Dairy Day (Report of Progress 941). Kansas state university agricultural experiment station and cooperative extension servic, p. 59-61.
- Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. Round FE, Chapman DJ (Eds.), Progress in Phycological Research Bristol, UK, pp. 129-241.
- Zhou M, Hünerberg M, Chen Y, Reuter T, McAllister TA, et al. (2018) Air-dried brown seaweed, Ascophyllum nodosum, alters the rumen microbiome in a manner that changes rumen fermentation profiles and lowers the prevalence of foodborne pathogens. Appl Environ Sci.
- Huang Q, Liu X, Zhao G, Hu T, Wang Y (2018) Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr 4(2): 137-150.
- Belanche E, Jones I, Parveen CJ, Newbold (2016) A metagenomics approach to evaluate the impact of dietary supplementation with Ascophyllum nodosum or Laminaria digitata on rumen function in RUSITEC fermenters. Front Microbiol 7: 299.
- Wang Y, Xu Z, Bach S, McAllister T (2008) Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on ruminal digestion of forage and concentrate diets in vitro. Anim Feed Sci Technol 145: 375-395.
- Connan S, Goulard F, Stiger V, Deslandes E, Gall EA (2004) Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot Mar 47: 410e6.
- AOAC (2019) Official method of AnalysisTM, (21st Edition).
- Lourenço SO, Barbarino E, De-Paula JC , Pereira LOSP, Marquez UML (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol Rese 50(3): 233-241.
- A De Angelis (2022) A Red Seaweed from Eastern Sicily: Chondracanthus Teedei, called “Màuru” - Agri Res & Tech: Open Access J 26(5): 556353.
- Xie XT, Cheong KL (2022) Recent advances in marine algae oligosaccharides: structure, analysis, and potential prebiotic activities. Crit Rev Food Sci Nutr 62(28): 7703-7717.
- Galland-Irmouli AV, Fleurence J, Lamghari R, Lucon M, Rouxel C, et al. (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10(6): 353-359.
- Davies SJ, Brown MT, Camilleri M (1997) Preliminary assessment of the seaweed Porphyra purpurea in artificial diets for thick-lipped grey mullet (Chelon labrosus). Aquaculture 152(1-4): 249-258.
- Marsham S, Scott GW, Tobin ML (2007) Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem 100(4): 1331-1336.
- Lahaye M (1991) Marine algae as sources of fibres: determination of soluble and insoluble dietary fibre contents in some ‘sea vegetables’. J Sci Food Agric 54(4): 587-594.
- Rogers HJ, Perkins HR (1968) Microbial cell walls and membranes. Rogers HJ, Perkins HR (Eds.), Cell Walls and Membranes, F. N. Spon Ltd., London pp. 564.
- Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, et al. (2010) Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 464(7290): 908-912.
- Norziah MH, Ching CY (2000) Nutritional composition of edible seaweed Gracilaria changgi. Food Chem 68(1): 69-76.
- Kuiken K, Lyman CM, Bradford M, Trant M, Dieterich S (1949) Essential amino acid composition of soy bean meals prepared from twenty strains of soy beans. J Biol Chem 177(1): 29-36.
- Pirian K, Jeliani ZZ, Sohrabipour J, Arman M, Faghihi MM, et al. (2018) Nutritional and bioactivity evaluation of common seaweed species from the Persian Gulf. Iran J Sci Technol Trans A: Sci 42: 1795-1804.
- Levavasseur G, Dion P (1988) Stratégie de purification in situ d’une rhodophycée source potentielle de protéines: Palmaria palmata (L.). Kuntze O, Delepine R, Gaillard J, Morand P (Eds.), Colloque Valva, CNRS-IFREMER Publication, Paris, France, pp. 181-184.
- Gaillard C, Bhatti HS, Novoa-Garrido M, Lind V, Roleda MY, et al. (2018) Amino acid profiles of nine seaweed species and there in situ degradability in dairy cows - Anim. Feed Sci. Technol 241: 210-222.
- Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10(1): 25-28.
- Filippini M, Baldisserotto A, Menotta S, Fedrizzi G, Rubini S, et al. (2021) Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 263: 127983.
- Hamid N, Ma Q, Boulom S, Liu T, Zheng Z, et al. (2015) Seaweed minor constituents, Tiwari BK, Troy DJ (Eds.), Seaweed sustainability. Food and non-food applications, Academic Press pp. 193-242.
- Haywood S, Britt D (2008) Scientific Endeavour: story of north Ronaldsay’s sheep. Vet. Times 1: 1-9.
- Hansen H, Hector B, Eldmann J (2003) A qualitative and quantitative evaluation of the seaweed diet of north Ronaldsay sheep. Anim Feed Sci Technol 105: 21-28.






























