A Red Seaweed from Eastern Sicily: Chondracanthus Tedeei, called “Màuru”
A De Angelis*
*Dipartimento di Agricoltura, Alimentazione e Ambiente - Università degli Studi di Catania, Italy
Submission: July 12, 2022; Published: July 29, 2022
*Corresponding author: A De Angelis, Dipartimento di Agricoltura, Alimentazione e Ambiente - Università degli Studi di Catania, Italy
How to cite this article: JA De Angelis. A Red Seaweed from Eastern Sicily: Chondracanthus Tedeei, called “Màuru”. Agri Res & Tech: Open Access J. 2022; 26 (5): 556353. DOI: 10.19080/ARTOAJ.2022.26.556353
Abstract
Much of the research conducted in recent decades has involved the identification of alternative foods capable of filling the growing demand for proteins and reducing the phenomenon of greenhouse gases, also thanks to their content of antimethanigenic substances. For this reason, many researchers have focused their attention on algae in general and red algae in particular. This note reports the results of the analyzes carried out on the red macroalga Chondracanthus tedeei, widespread in a restricted area of eastern Sicily, where it is known by the dialectal name of “Màuru”, to highlight its main bromatological characteristics. The results obtained show a good carbohydrate and protein content and a high ash content (39.46, 38.3 and 20.68%, respectively) on dry matter (DM) basin. The protein is of good value for having a percentage content of essential amino acids, similar to that of egg; the ashes have an optimal sodium and potassium content (~ 1: 1) and a high iodine content. Although the results obtained are comparable to those found in other red algae, of the same species and of other species, further investigations would be desirable, both to highlight any seasonal and / or environmental variability, and to determine the content in other interesting bioactive substances, such as, for example, fatty acids, antimethanigenic substances, heavy metals.
Keywords: Chondracanthus tedeei; Red algae; Bromatological characteristics
Introduction
In recent decades, the demand for food protein sources has visibly increased, especially in developed countries; global protein demand is expected to double between 2020 and 2050 [1]. A solution to the problem could be to resort to alternative protein sources such as insects which have high protein content and provide essential amino acids; the total protein level varies according to the stage of life and varies between approximately 50 and 80%. Due to relatively low consumer acceptance, incorporating insect proteins into food products still poses a significant challenge [2,3]. The increase in animal husbandry cannot be a viable solution due to their negative impact on the environment, including, among others, the production of greenhouse gases [4]. According to the 2019 FAO report, animal farms contribute about 14% to the production of greenhouse gases [5]. In particular, most greenhouse gas emissions from livestock production are in the form of methane (CH4), which is produced largely through enteric fermentation, representing a loss in food energy consumption, and to a lesser extent, the decomposition of manure [6]. For this reason, many studies have been carried out to reduce enteric emissions of CH4 through the use of food additives, dietary manipulation and the quality of forage [7].
Another alternative protein source is represented by algae. Algae have positive health effects such as immune regulation, radiation protection, skin whitening effect, blood pressure, fat, sugar reduction, Alzheimer’s disease alteration and delay, promotion bowel health, reducing the risk of osteoporosis and cardiovascular disease [8,9]. The beneficial properties of algae depend on the presence of bioactive compounds with anti-inflammatory, antimicrobial, antioxidant and antitumor characteristics [10,11].s
The edible aquatic macroalgae are classified into three main groups based on the composition of photosynthetic pigments present, red seaweed (Rhodophyta), brown seaweed (Ochrophyta, Phaeophyceae), and green seaweed (Chlorophyta). Many studies have involved red algae. Red algae have a higher protein content (10-40%) than green or brown algae [12]. The most active proteins are lectin and phycobiliprotein. Lectins are glycoproteins with biological control functions; phycobiliproteins are chromoproteins with anti-inflammatory, hepatoprotective and antioxidant functions [13] (Pooja, 2020).
They have a good content of essential amino acids even if some authors find a lack of methionine, cystine and lysine [14]. They possess water-soluble pigments, used as food dyes [15], polysaccharides with gelling properties such as agar, carrageenan and alginates applied in the food, biomedical, pharmaceutical and biotechnological industries [16]. Some red algae have antimethanogenic properties due to their ability to synthesize halogenated analogues of CH4, such as bromoform and dibromochloromethane, within specialized glandular cells as a natural defense mechanism [17].
Several studies have been conducted on the antimethanogenic power and on the mechanisms of action of synthetic additives, nitro and halogenated compounds, and natural halogenated compounds synthesized from algae [18-26]. Other studies, conducted in Australia, tested the antimethanigenic power, both in vitro and in vivo, of red algae [27-32]. The aim of the present was to analyze a red alga belonging to the Chondracanthus species, Chondracanthus tedeei, present in the coasts of eastern Sicily, known by the dialectal name of “Màuru” [33].
Chondracanthus derives from the Greek words chondrus (cartilage) and akanthos (thorn, alluding to its thorny aspect); and Teedei derives from the name of its collector, the British Tedde [34]. This edible seaweed once grew luxuriantly on the coasts of the Ionian coast; today, unfortunately, finding it represents a real stroke of luck: due to the pollution of the seas, this alga is no longer able to grow, precisely because it finds no conditions for survival except in clear and uncontaminated waters. Thanks to the help of the fishermen in the area, it was possible to find a suitable quantity to carry out the analyzes that are the subject of this study.
Materials and Methods
Analyzes relating to the chemical composition were carried out on dried samples of the red macroalgae Chondracanthus tedeei according to the standard protocols of the Association of Official Analytical Chemists [35]. Moisture and ash content were determined at 105 °C for 24 hours and 575 °C for 6 hours, respectively; the fat content was analyzed by Soxhlet extraction with petroleum ether as a solvent and the protein content of the dried samples was examined by the Kjeldahl method, using the corresponding red algae conversion factor of 4.59 following the instructions of [36]. The total carbohydrate content (g / 100 g of body weight) was determined by difference as follows: 100- (g protein + g of fat + g of ash). Crude fiber, NDF, ADF and lignin were determined according to the Weende and Van Soest method; macroelements, microelements and amino acids were determined by mass spectrometry and HPLC. The energy (kcal / 100 g of DM) was calculated according to Regulation (EU) no. 1169/2011 [37] as follows: 4 × (g proteins + g carbohydrates) + 9 × (g fat).
Result and Discussion
Due to the difficulty of finding red algae in the distribution area of the Ionian coasts of eastern Sicily, no data on the chemical composition of the macroalga Chondracanthus tedeei, called “Màuru”, is found in the bibliography. The analyzes carried out on the dried samples revealed an overall chemical composition on average similar to other red macroalgae. In particular, the percentage composition of the dry substance sees the carbohydrate component prevail (39.46%), represented by 3.19% by crude fiber, followed by the protein component (38.3%), only slightly lower, and by the ashes (20.68%); lipids are not very present (1.36%). Bastos et al. [38], finds on Chondracanthus tedeei native to Brazil a decidedly lower protein content (14.66%), a slightly higher ash content (28.68%) and a similar fiber and lipid content, respectively equal to 2 , 21% and 1.82% (Table 1).

Morgan et al. [39] , analyzing the red macroalga Palmaria palmata, found very wide variations in the values relating to the various chemical constituents: from 73 to 89% of humidity and, on a dry basis, from 12 to 37% of ash, from 8 to 35 % crude protein, 38 to 74% carbohydrates and 0.2 to 3.8% lipids. They attribute some of the variations to different seasonal and nutritional conditions. Other authors also find significant seasonal variations in the chemical composition of red algae [40-42]. The amino acid content (Table 2) shows a high percentage of essential amino acids (46.6% of the total) and the profile of essential amino acids is close to that of egg; this, in accordance with what Dupin et al. and Friedman [43,44], allows us to consider the seaweed protein of good nutritional value.
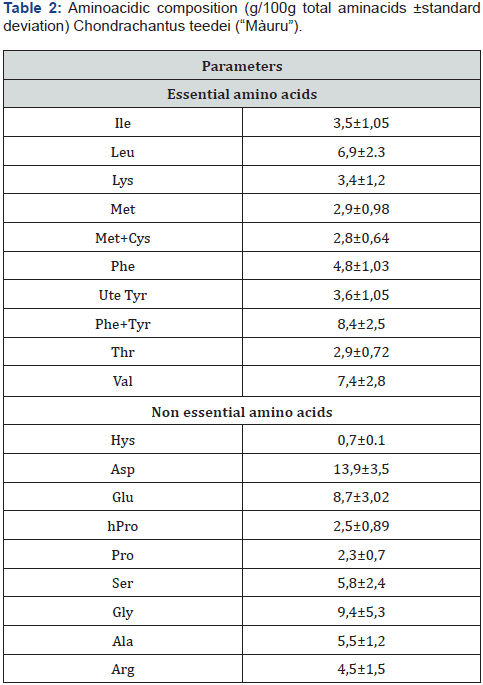
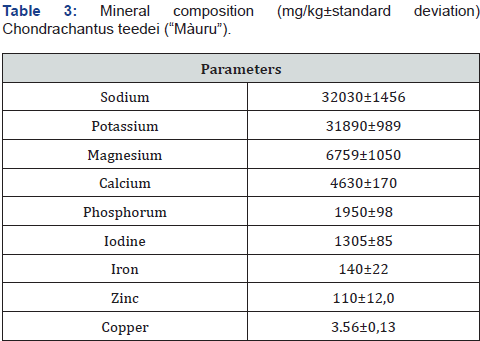
Among the essential amino acids, the most represented were valine (7.4%), leucine (6.9%) and phenylalanine (4.8%). Among the non-essentials, aspartic acid (13.9%), glycine (9.4%) and glutamic acid (8.7%). Some authors note seasonal variations in the amino acid content, probably due to variations in environmental conditions such as the intensity of sunlight and the amount of nitrogen [45]. Among the macroelements potassium and sodium abound with values of 32030 and 31890 mg / kh DM; their ratio of 1 makes this alga suitable for consumers with high blood pressure problems [46] (Table 3).
Among the microelements, iodine is the most represented (1305 mg / kg DM), but, unlike sodium and potassium, the abundance of iodine could create dietary problems to have goitrogen effects. Iron, zinc and copper show levels similar to those found by other authors, with values of 140, 110 and 3.56 respectively (Table 3), as found in various marine algae [47,48]. Despite this, it is known that various factors, including the species they belong to and the geographical area of diffusion, can greatly influence the mineral and vitamin content of the [49].
Conclusion
The use of brackish water in semi-arid to arid Small Island Developing States (SIDS) is a matter of adaptation and resilience. Irrigation with desalinated, blended, brackish water can be an alternative. This study gave indications that these waters, when combined can increase the availability of quality water, improve yield and agricultural productivity. Reducing the salinity of the electrical conductivity of brackish water, T4 (ECw 5.75) to T3 (ECw 3.5 dS/m) and T2 (ECw 2.5 dS/m,) by reverse osmosis desalination, increased tomato crop yield to more than 3 times in T3 (4.8 to 16.56 ton/ha ) and to more than 5 times in T2 (4.8 to 23.38 ton/ha). The studies give hints that moderately sensitive (MS) and moderately salinity tolerant (MT) crops can be grown using blended water. Mixing 50% saline water with 50% desalinated water raised the levels of salts and other essential minerals satisfactorily, while producing water with a salinity of ECw = 3.25 dS/m. Likewise, economic and environmental benefits of reducing irrigation water salinity from ECw 5.75 to ECw 2.5 dS/m in blended water T2 proved consistent in both tomato and sweet potato, blended option II, i.e., T2 treatment. Comparison of yields for sweet potato showed that irrigation with blended water maintained yields 62 and 75% for treatments T3 and T2 respectively, compared to irrigation with fully desalinated water (T1=14, 8 ton/ha) [10-12].
However, the economic and environmental costs of using desalinated water in production may not be the most attractive in the short to medium term. Desalinated water can be used to alleviate the salinization of soils by washing them or to increase the availability of water for irrigation by mixing it with brackish water. The precipitation that occurred during the trials contributed to leaching salts to deeper areas. The negative effects of using desalinated water on tomato production can be mitigated by irrigating with blended water (T2 and T3). In this research, the results show that irrigating salinized, light textured soils with desalinated water (T1) has detrimental effects on tomato plant productivity, quality and average weight of its fruit. The treatment (T1) proved to be the most effective on sweet potato crops and their tubers. The use of pure brackish water, treatment (T4), is not recommended for most horticultural crops, because besides the negative impact on irrigated soils, it endangers their salinity and consequently their fertility. Based on the results of this research it is concluded that several issues still need to be investigated, namely: (i) the effect of desalinated water and water movement in light and heavy textured soils along the soil profile; (ii) the amount of fertilizer needed under different levels of water and soil salinity; (iii) air, water and soil temperature and their effect on soil electrical conductivity; (iv) the influence of pH on results and electrical conductivity, soil texture its influence on the amount of available moisture; (v) cultural practices and water management, namely losses and waste of water in irrigation among others. Salinity is only one component of water quality assessment [13,14].
Once much appreciated by consumers of a restricted area of eastern Sicily, Chondrachantus teedei, known by the dialectal name “Màuru”, is now hardly available due to an ever smaller presence along the coasts of the distribution range, mainly due to changes undergone over the years by the chosen environment, probably due to various forms of pollution. This greatly limited the investigation possibilities of this research, the results of which concerned only some parameters of the chemical composition of the alga. Like red algae of the same species and of other species, Chondrachantus teedei has a composition in macronutrients characterized, on average, by a good level of total carbohydrates and protein and a high ash content; in particular, the protein is of good nutritional value due to its high content of essential amino acids; the ashes have a balanced ratio (equal to 1) between the most represented macroelements, ie sodium and potassium, and high quantities of iodine, between the microelements. The restoration of the good conditions of the natural habitat of Chondrachantus teedei could allow a greater presence of the alga in the distribution area and create the conditions for further desirable more in-depth investigations on the species. These investigations could concern: 1) the acidic characterization of the lipid component; 2) the presence of bioactive compounds; 3) the presence of heavy metals; 4) the detection of seasonal and / or environmental variations on nutritional characteristics.
References
- Pam IB, Lasika Senaratne LS, Stube AE, Brackenridge A (2020) Protein demand: review of plant and animal proteins used in alternative protein product development and production. Anim Front 10(4): 53-63.
- Gravel A, Doyen A (2019) The use of edible insect proteins in food: challenges and issues related to their functional properties. Innov Food Sci Emerg Technol 59: 102272.
- De Castro RJS, Ohara A, Aguilar JG, Dos S, Domingues MAF (2018) Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci Technol 76: 82-89.
- Bleakley S, Hayes M (2017) Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 6(5): 33.
- Gerber PJ, Steinfeld H, Henderson B, Mottet A, Opio C, et al. (2013) Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities. FAO, Rome, Italy.
- Moraes LE, Strathe AB, Fadel JG, Casper DP, Kebreab E (2014) Prediction of enteric methane emissions from cattle. Glob Change Biol 20(7): 2140e2148.
- Hristov AN, Oh J, Firkins JL, Dijkstra J, Kebreab E, et al. (2013) Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J Anim Sci 91(11): 5045-5069.
- Déléris P, Nazih H, Bard JM (2016) Seaweeds in Human Health. Seaweed in Health and Disease Prevention. Elsevier Science, Netherlands, pp. 319-367.
- Yimin Q (2020) Health benefits of bioactive seaweed substances. Handbook of Algal Science, Technology and Medicine, Elsevier Science, United Kingdom, pp. 455-466.
- Domínguez H (2013) Algae as a source of biologically active ingredients for the formulation of functional foods and nutraceuticals. Functional Ingredients from Algae for Foods and Nutraceuticals, United Kingdom, Elsevier Science pp. 1-19.
- Ganesan AR, Tiwari U, Rajauria G (2019) Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Science and Human Wellness 8(3): 252-263.
- Barral BM, Flórez FN, Domínguez H, Torres MD (2020) Tailoring hybrid carrageenans from Mastocarpus stellatus red seaweed using microwave hydrodiffusion and gravity. Carbohydr Polym, 248: 116830.
- Pina AL, Costa AR, Lage YMA, López HJ (2014) An evaluation of edible red seaweed (Chondrus crispus) components and their modification during the cooking process. LWT - Food Sci Technol 56(1): 175-180.
- Wong KH, Cheung PCK (2000) Nutritional evaluation of some subtropical red and green seaweeds Part I. Proximate composition, amino acid profiles and some physico-chemical properties. Food Chem 71(4): 475-482.
- Dumay J, Morançais M, Munier M, Guillard C, Fleurence J (2014) Phycoerythrins: valuable proteinic pigments in red seaweeds. Adv Bot Res 71: 321-343.
- Thiruchelvi R, Jayashree P, Mirunaalini K (2020) Synthesis of silver nanoparticle using marine red seaweed Gelidiella acerosa -A complete study on its biological activity and its characterization. Mater Today Proc 37: 1693-1698.
- Paul NA, Cole L, de Nys R, Steinberg PD (2006) Ultrastructure of the gland cells of the red alga Asparagopsis armata (Bonnemaisoniaceae). J Phycol 42(3): 637-645.
- Dijkstra J, Bannink A, France J, Kebreab E, van Gastelen S (2018) Short communication: antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J Dairy Sci 101(10): 9041e9047.
- Tomkins NW, Colegate SM, Hunter RA (2009) A bromochloromethane formulation reduces enteric methanogenesis in cattle fed grain-based diets. Anim. Prod. Sci. 49(12): 1053-1058.
- Kinley RD, de Nys R, Vucko MJ, Machado L, Tomkins NW (2016) The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim Prod Sci (56): 282e289.
- Duin EC, Wagner T, Shima S, Prakash D, Cronin B, et al. (2016) Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc Natl Acad Sci 113(22): 6172e6177.
- Smith EL, Mervyn L, Johnson AW, Shaw N (1962) Partial synthesis of vitamin B12 coenzyme and analogues. Nature 194(4834): 1175-1175.
- Wood JM, Kennedy FS, Wolfe RS (1968) Reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B12. Biochemistry 7(5): 1707-1713.
- Johnson ED, Wood AS, Stone JB, Moran ET (1972) Some effects of methane inhibition in ruminants (steers). Can J Anim Sci 52(4): 703-712.
- Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer RK (1997) Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation. Science 278(5342): 1457e1462.
- Liu H, Wang J, Wang A, Chen J (2011) Chemical inhibitors of methanogenesis and putative applications. Appl. Microbiol. Biotechnol 89(5): 1333e1340.
- Machado L, Magnusson M, Paul NA, de Nys R, Tomkins N (2014) Effects of Marine and Freshwater Macroalgae on In-Vitro Total Gas and Methane Production. PloS One (9)1.
- Machado L, Tomkins N, Magnusson M, Midgley D, deNyes R, et al. (2018) In vitro response of rumen microbiota to the antimethanogenic red macroalga Asparagopsis taxiformis. Microb Ecol 75(3): 811-818.
- Kinley RD, Vucko MJ, Machado L, Tomkins NW (2016) In vitro evaluation of the antimethanogenic potency and effects on fermentation of individual and combinations of marine macroalgae. Am J Plant Sci (7): 2038e2054.
- Roque BM, Salwen JK, Kinley R, Kebreab E (2019) Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J Clean Prod (234): 132-138.
- Lanigan GW (1972) Metabolism of pyrrolizidine alkaloids in the ovine rumen IV. Effects of chloral hydrate and halogenated methanes on rumen methanogenesis and alkaloid metabolism in fistulated sheep. Aus J of Ag Res 23(6): 1085-1091.
- Ungerfeld EM, Rust SR, Boone DR, Liu Y (2004) Effects of several inhibitors on pure cultures of ruminal methanogens. J. of Applied Microb 97(3): 520-526.
- Pérez JL, Hernández I, Vergara IJ, Brun FG, León Á (2018) Those curious and delicious seaweeds. A fascinating voyage from biology to gastronomy. Servicio de Publicaciones de la Universidad de Cádiz, Cádiz, pp. 384.
- Pereira L, Silva P (2021) A concise review of the red macroalgae Chondracanthus teedei (Mertens ex Roth) Kützing and Chondracanthus teedei var. lusitanicus (J.E. De Mesquita Rodrigues) Bárbara & Cremades. Journal of Applied Phycology 33: 111-131.
- AOAC (2019) Official method of AnalysisTM, 21st
- Lourenço SO, Barbarino E, De Paula JC, Pereira LOSP, Marquez UML (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycological Research 50(3): 233-241.
- European Union (2011) Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011. Off J Eur Union 54: 1-46.
- Bastos E, Felix M, Rover T, Horta PA, Hayashi L, et al. (2012) Caracterização nutricional das macroalgas Chondracanthus teedi (Gigartinaceae, Rhodophyta) e Kappaphycus alvarezii (Solieriaceae, Rhodophyta) do sul do Brasil. XIV Congresso Brasileiro de Ficologia. Universidade Federal de Santa Catarina, Brazil.
- Morgan KC, Wright JLC, Simpson FJ (1980) Review of chemical constituents of the red alga Palmaria palmata (dulse). Econ Bot 34(1): 27-50.
- Galland IAV, Fleurence J, Lamghari R, Lucon M, Rouxel C, et al. (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10(6): 353-359.
- Davies SJ, Brown MT, Camilleri M (1997) Preliminary assessment of the seaweed Porphyra purpurea in artificial diets for thick-lipped grey mullet (Chelon labrosus). Aquaculture 152(1-4): 249-258.
- Marsham S, Scott GW, Tobin ML (2007) Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem 100(4): 1331-1336.
- Dupin H, Giachetti I, Abraham J (1992) Apports conseillés en proteins. In: Dupin H, Abraham J, Giachetti I (Eds.), Apports Nutritionnels Conseillés, Lavoisier, Paris, France , pp. 18-26.
- Friedman M (1996) Nutritional value of proteins from different food sources: A review. J Agric Food Chem 44 (1): 6-29.
- Levavasseur G, Dion P (1988) Stratégie de purification in situ d’une rhodophycée source potentielle de protéines: Palmaria palmata (L.). In: Kuntze O, Delepine R, Gaillard J, Morand P (Eds.), ColloqueVALVA, CNRS-IFREMER Publication, Paris, France, pp. 181-184
- Morrissey E, Giltinan M, Kehoe L, Nugent A, McNulty B, et al. (2020) Sodium and potassium intakes and their ratio in adults (18–90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 12(4): 938.
- Filippini M, Baldisserotto A, Menotta S, Fedrizzi G, Rubini S, et al. (2021) Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 263: 127983.
- Hamid N, Ma Q, Boulom S, Liu T, Zheng Z, et al. (2015) Seaweed minor constituents. B.K. Tiwari, D.J. Troy (Eds.), Seaweed sustainability. Food and non-food applications, Academic Press, pp. 193-242
- Kraan S (2013) Pigments and minor compounds in algae. In: Domínguez H (Ed.), Functional Ingredients from algae for foods and nutraceuticals, Woodhead Publishing Limited, UK, pp. 205-251.






























