Sustainable Control Strategies for Mycotoxigenic Fungi and Their Metabolites in Food Safety: Review
Karen Cristina Saez-Gomez1, Teresa Soledad Cid-Pérez2,3, Fabiola Avelino-Flores1,5, Esperanza Duarte-Escalante4, Raúl Ávila-Sosa1,3 and Ricardo Munguía-Perez1,2*
1Posgrado en Ciencias Ambientales, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla, Ciudad Universitaria, Puebla, México
2Laboratorio de Micología, Centro de Investigaciones en Ciencias Microbiológicas, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla. Cd. Universitaria, Puebla, México
3Departamento de Bioquímica-Alimentos, Facultad de Ciencias Químicas, Benemérita Universidad Autónoma de Puebla. Cd. Universitaria, Puebla, México
4Unidad de Micología, Facultad de Medicina, Departamento de Microbiología y Parasitología, Universidad Nacional Autónoma de México, Mexico City, Mexico
5Laboratorio de Patogenicidad Microbiana, Centro de Investigaciones en Ciencias Microbiológicas, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla. Cd. Universitaria, Puebla, México
Submission: February 01, 2024; Published: February 20, 2024
*Corresponding author: Ricardo Munguía-Pérez, Posgrado en Ciencias Ambientales, Instituto de Ciencias, Laboratorio de Micología, Centro de Investigaciones en Ciencias Microbiológicas, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla. Cd. Universitaria, Puebla, México
How to cite this article: Karen Cristina Saez-Gomez, Teresa Soledad Cid-Pérez, Fabiola Avelino-Flores, Esperanza Duarte-Escalante, Raúl Ávila-Sosa, et al. Sustainable Control Strategies for Mycotoxigenic Fungi and Their Metabolites in Food Safety: Review. Agri Res& Tech: Open Access J. 2024; 28(2): 556402. DOI: 10.19080/ARTOAJ.2024.28.556402
Abstract
Mycotoxins, toxic secondary metabolites, contaminate 60-80% of agricultural crops. Their consumption through diet can affect human and animal health, causing cancer, teratogenesis, mutagenesis and immunosuppression. It is a relevant issue worldwide, because it represents a challenge that puts food safety at risk. The aim of this work was to review the main strategies for the sustainable control of mycotoxigenic fungi and their metabolites in food safety. Systematic research and a bibliographic analysis were performed using the following databases: SCOPUS, Science Direct, Web of Science and MDPI. Scientific articles, review articles and pages of organizations with worldwide intervention were consulted. The inclusion criteria included the keywords: mycotoxigenic fungi, mycotoxins, agriculture, biocontrol, and health. They also included bibliographic citations mainly from 2012 to 2022, although there are citations prior to this year that are relevant. This review summarizes alternatives found in research works, such as: immunoassays, biosensors, membranes, matrices, biological treatments, ozone, and UV radiation, among others. In conclusion, detection methods must be fast, inexpensive, sensitive, and selective, which guarantee food safety.
Keywords: Mycotoxins; Fungi; Agricultural crops; Control Strategies; Food Safety
Abbreviations: FAO: Food and Agriculture Organization; ITS: Internal Transcribed Spacer Regions; SERS: Surface Enhanced Raman Scattering; LOD: Limit of Detection
Introduction
Extreme events caused by climate change, economic or geopolitical shocks, and pest or disease epidemics can induce, spread, and prolong food insecurity. These direct and indirect effects lead to reductions in the availability of, and access to, healthy and nutritious food [1,2]. The magnitude, extent, and complexity of the threats posed by extreme events to global food security can further create cascading and systemic impacts that are difficult to predict or plan and prepare for [3,4]. The volume and quality of crops is mainly affected by pests, diseases, factors such as temperature, soil salinity, soil nutrient deficiency, and drought [5]. So too, the increase in the proliferation of mycotoxigenic fungi and their metabolites can have serious side effects on agri-food crops. According to the FAO (Food and Agriculture Organization of the United Nations) around 25% of food crops worldwide are affected by mycotoxigenic fungi, generating food losses of 1 billion tons per year [6]. Global climate change is expected to cause some species of mycotoxigenic fungi to dominate others, increasing mycotoxin production [7]. The consumption of these metabolites through diet generates a wide range of disorders, from gastroenteritis to cancer in humans and animals, which is why it is considered a public health problem in the world [8]. Due to this, there is a growing interest in new legislation that restricts the trade of chemical pesticides. The above encourages the development of new fungal pesticides that are friendly to the environment and do not generate toxic residues [9]. Because sustainable measures are necessary to control mycotoxigenic fungi and their metabolites. The aim of these paper was to review the main sustainable control strategies for mycotoxigenic fungi and their metabolites in food safety. To fully achieve this purpose, the following topics are addressed:
i. We describe the relationship between global warming and the development of mycotoxigenic fungi in crops.
ii. We present and discuss the main mycotoxins.
iii. We explain some novel control strategies to prevent fungal development and toxin production in food safety.
Mycotoxigenic fungi in crops, global warming
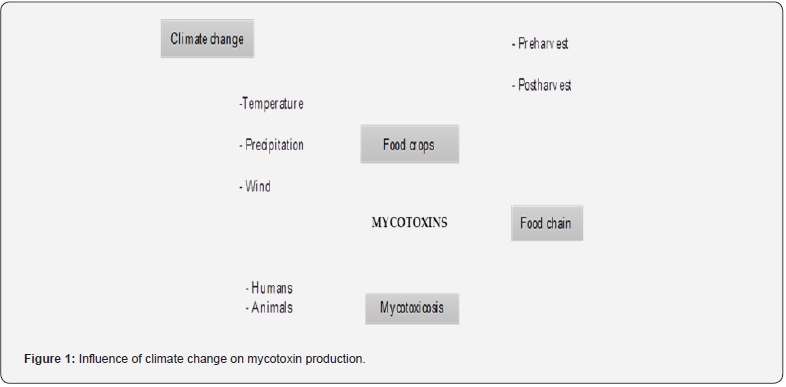
The agricultural system and a wide variety of foods for human and animal consumption are susceptible to contamination by mycotoxigenic fungi of the Aspergillus, Penicillium and Fusarium genera, which can cause severe damage to farmers and ranchers (Table 1) [10]. Although fungi can colonize cereals before or after harvest, colonization and proliferation depend on environmental and ecological conditions, because the resulting mycotoxin production will be different (Figure 1) [11]. Climate change has influenced the increase in temperature, as well as the variation in rainfall, modifying the scarcity of species even in cold regions [12]. For example, the current level of CO2 has increased from 280 to 400 ppm, which has contributed to the effects of global warming [11]. Farming communities have the appropriate knowledge of the environment in which they farm, to obtain the best yields. However, if the weather changes, agricultural practices must be adjusted to maintain productivity [13]. Due to, changing adaptive associations under global climate change will modify the outcome of microbial plant-soil interactions. Which will affect crop production, food, and feed supply and quality, like so negatively affecting plant physiology [14]. It has been reported that, with high or low temperatures, the growth of fungi and the production of their metabolites is inevitable. Atanda et al., observed that temperatures below 20 °C favored Penicillium, while temperatures above 20 °C increased the growth of Aspergillus species. They also report that legumes and cereals are the food products most likely to be contaminated by Aspergillus species, even during storage because there is no temperature control [15].
Aspergillus, Fusarium and Penicillium
Aspergillus flavus is one of the most frequently isolated species in agriculture and medicine, it is cosmopolitan and contaminates a wide range of crops in the world [23]. Aflatoxin contamination causes significant annual crop losses internationally. Contaminating food products include cereals, pistachios, tree nuts and peanuts, spices, and figs in hot climates, aflatoxin production occurs anywhere in the food supply chain, from preharvest to consumer (Wei et al., 2019; Zhang et al., 2022b). Most grain storage structures used by farmers do not provide the proper internal atmosphere, they do not give maximum protection against water, insects, and rodents; and they are not easy to clean. All these conditions promote fungal growth and aflatoxin production in stored grains and legumes [25]. Toxinogenic strains of Aspergillus flavus tend to produce higher levels of aflatoxin B1 (AFB1) and aflatoxin B2 (AFB2), while A. parasiticus produces more equal amounts of AFB1, AFB2, aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2) [26]. The phenotypic identification of this species is generally based on microscopic and macroscopic criteria, which consider the characteristics of conidiophores, phialides and spores, and colonial characteristics [27].
However, micro and macromorphological characteristics are not sufficient for correct identification in atypical Aspergillus isolates, which include those strains that present slow sporulation, or in the presence of cryptic or sister Aspergillus species. Furthermore, carrying out identification through these methods alone is time-consuming and requires considerable technical knowledge [28]. For this reason, there is a growing interest in polyphasic identification that includes not only phenotypic identification, but also genotypic identification, through molecular techniques. These molecular techniques are based on the partial or total sequencing of the genome of different genetic targets, including the internal transcribed spacer regions (ITS) of rDNA and genes that code for proteins such as calmodulin and β-tubulin (Nasri et al., 2015). Although Aspergillus fumigatus is the main causal agent of aspergillosis in humans and other animals, Aspergillus flavus is also of importance in this condition, which includes allergic, saprophytic colonizing and invasive aspergillosis [30]. The importance of this species is that it is the second main pathogen causing invasive and non-invasive aspergillosis [31]. Furthermore, A. flavus has been identified as one of the main causes of fungal keratitis, a fungal infection of the cornea [32]. Therefore, the correct identification of the species Aspergillus genus is important because these are the causal agent of a wide spectrum of clinical presentations, within these manifestations is bronchopulmonary aspergillosis, otomycosis, skin conditions, colonization of cavities and invasive aspergillosis (Nasri et al., 2015).
Fusarium is considered opportunistic due to its ability to grow at 37 °C. Some of its species are producers of toxins which can affect man and animals. About 100 species have been described, of which 12 are considered pathogenic for man, among these are F. solani, F. oxysporum and F. verticilloides. It is a genus of great economic importance because they act as phytopathogens [34,35]. The genus Penicillium is a cosmopolitan genus that can grow on various substrates such as grains, fruits, nuts; it is one of the main contaminants in the postharvest phase [36]. It is important in animal and human nutrition due to the deterioration it produces in grains, in addition to producing toxins [37]. Many of the species belonging to Penicillium are abundant in the soil, as they can compete for organic substrates. In addition to this, its proliferation in food is easy, representing a serious problem for their conservation, in addition to being a potentially mycotoxigenic fungus, some of its species are producers of the mycotoxin called ochratoxin present in various foods, and which is considered nephrotoxic, inmunotoxic, teratogenic, in addition, the IARC (International Agency for Research on Cancer) classifies it within class 2B for being a probable human carcinogen [38]. Some other Penicillium species are important fruit pathogens during the postharvest period, and others are beneficial and widely used in the pharmaceutical and food industries [5].
Mycotoxins
The first reported signs of mycotoxicosis due to the consumption of food contaminated by mycotoxins arose in the Middle Ages in Europe. There are around 400 mycotoxins with toxigenic potential, produced by around 100 fungi [39,40]. Mycotoxins are naturally generated by certain filamentous fungal species, are chemically stable and heat resistant, so they can persist during food processing (OMS 2018). Two routes of exposure to these metabolites have been identified, through inhalation (frequently spores) and through diet, which can generate health effects for both humans and livestock [41,42]. The main effects are inmunotoxic, teratogenic, nephrotoxic, and carcinogenic. The most studied mycotoxins due to their effects on human and animal health, in addition to being important from the agroeconomic point of view, commonly found in food are aflatoxins, zearalenone, fumonisins, trichothecenes, and ochratoxin A [43]. Mycotoxin contamination in the food chain is mainly conditioned by the diversity of fungal strains, the fungal vulnerability of the plant in the field, the microbial population, humidity, temperature, nutrients, and stress factors [44]. Fungal activity and toxin production elsewhere have been reported to be optimal at 25-37 °C [45]. In a study conducted by Zhao et al., they reported that 81.5% of feed ingredients were contaminated by aflatoxin B1, deoxynivalenol and zearalenone, while 95.7% of complete feeds were contaminated by these mycotoxins in various combinations [46]. In another study conducted by Ma et al they reported contamination by different mycotoxins in foods from different provinces of China with the following levels of contamination: AFB1 83.3%, ZEN 88.0% and DON 74.5% [47].
Aflatoxins
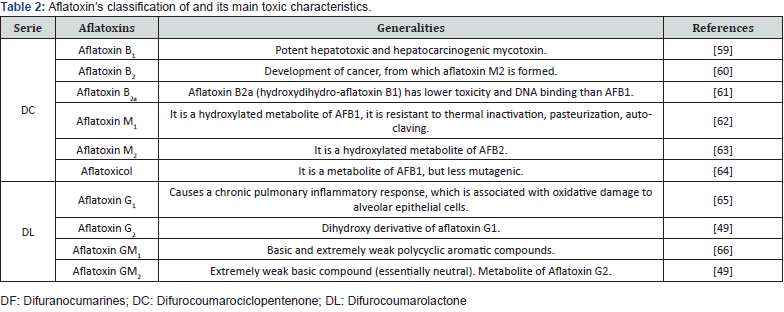
Aflatoxins consist of a group of approximately 20 related fungal metabolites, although the most studied for contaminating food are AFB1, AFB2, AFG1 and AFG2. Aspergillus flavus and A. parasiticus species are the main producers of aflatoxins and can grow on a wide variety of substrates. Their ability to produce aflatoxins depends on their individual metabolic system, essential for primary lipid metabolism, and specific enzymes (synthetases) capable of producing this secondary metabolite [48]. It should be noted that the synthesis of aflatoxins is not characteristic of the species, but of the strain [5]. These toxic substances are of low molecular weight, odorless, tasteless, and colorless, stable in food, it is also resistant, as it is not easily degraded by cooking processes [49-51]. High temperatures and humidity favor the growth of mycotoxin-producing fungi, so countries with these environmental conditions often experience increased contamination (Rushing and Selim, 2019). In addition to this, climate change has been modifying the variation in precipitation and temperature, this has meant that there are more regions prone to AFB1 contamination problems. Even that this contamination increases in crops where there were already problems due to this metabolite (Bbosa, G. S., Kitya, D., Lubega, A., Ogwal-Okeng, J., Anokbonggo, W. W., & Kyegombe, 2013, Rushing and Selim, 2019). Water content is a key determinant of aflatoxin development in food crops. To produce toxins by A. flavus, approximately 13% relative humidity and a water activity (aw) of 0.65 are required [15], however 0.77 of aw or more is optimal for growth and proliferation [54]. Aflatoxins are classified according to their chemical structure in the difurocomarocyclopentenone and difurocoumarolactone series, Table 2 shows the types of aflatoxins and their generalities.
Exposure to aflatoxins is of relevance in the health sector, in 2004 alone it was reported that approximately 4.5 billion people in developing countries were at risk of chronic and uncontrolled disease [15]. In a study carried out by Abdel et al. reported a maximum Aspergillus growth rate of 6.9 mm/day at 35 °C and a maximum aflatoxin production rate of 2278-3082 μg/g at 37 °C in maize [55]. Wajih ul Hassan et al. reported aflatoxin contamination in maize in Pakistan, in all the samples there was aflatoxin contamination with limits higher than 20 μg/kg, in addition there was a higher concentration of AFG1 in all the maize varieties analyzed [56]. Even though the globalized world has allowed countries to take advantage of the tools to reduce the levels of aflatoxins in food, it is evident that developed countries have less risk of eating food contaminated with these metabolites compared to developing countries, due to the policies and mismanagement of operations in agriculture (Waliyar et al., 2015). In a study conducted in regions of Ghana by Sugri et al. farmers were surveyed to assess their knowledge about aflatoxins, 78% of the respondents knew about aflatoxins, however, 68.1% did not perceive aflatoxins as a major food safety problem. In this same study, aflatoxin contamination in corn samples will be limited to a range of 0.011 to 308 mg/kg (Sugri et al., 2015).
Ochratoxin A
Among the ochratoxins that have been described are: β, α, A, B and C, however, it is reported that the most toxic is OTA (Ochratoxin A). OTA is one of the most important mycotoxins worldwide because it generates on human and animal health, within the effects it generates are nephrotoxic, mutagenic, teratogenic and inmunotoxic: this metabolite is generated by both Aspergillus and Penicillium species [67]. According to the IARC, it is in group 2B as a possibly carcinogenic substance [68]. The target organ of ochratoxin A is the kidneys, which is why it is associated with nephropathies [69]. It contaminates a wide variety of foods, including cereals, fruits, vegetables, spices, and animal products [70]. OTA is a white, odorless, crystalline solid compound, when absorbing ultraviolet light, it exhibits a strong fluorescence, which depends largely on the pH, the solubility in water is approximately 0.42 mg/L at 25 °C and exhibits moderate solubility in polar organic solvents such as chloroform, ethanol, and methanol [71]. Because OTA compromises food safety, it is necessary to monitor this metabolite in food, its detection is mainly based on chromatography and immunoassays, but when using these methods there are limiting factors such as costs, processing time and trained personnel (Sugri et al., 2015; Waliyar et al., 2015). In a study by Wajih et al. contamination in corn by OTA in Pakistan was determined, reporting that 71% of the samples presented contamination by this metabolite in a range of 2.14 to 214 μg/ kg [74]. Majeed et al. report contamination by ochratoxin A with a value of 5.29 μg/kg in corn samples [75]. In another study by Ibáñez et al. in cereal samples obtained from a Spanish market, ochratoxin A contamination was reported in 39% of the analyzed samples of wheat and rice, whose values were 0.37 μg/kg [76].
Fumonisins
The Fusarium genus is the most prevalent plant pathogen invading agricultural crops, and the mycotoxins produced by species of this genus are the most economically important [77]. Within the group of metabolites produced by this genus, the most important due to their toxicity in humans and animals are fumonisins (FB), zearalenone (ZEA) and trichothecenes [78]. There are about 15 fumonisins, but the most studied are fumonisins B1 (FB1), B2 (FB2) and B3 (FB3), of which the most toxic is FB1 and is classified within group 2B as a possibly carcinogenic substance in humans, by IARC [79, 80].
Control Strategies
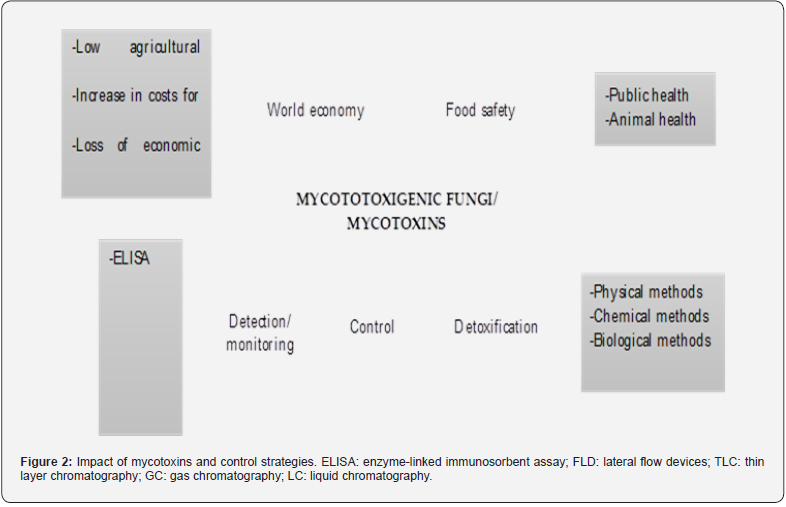
Since mycotoxins can cause toxicity in animals and humans, there is a growing interest in the control or elimination of mycotoxigenic fungi and/or their metabolites. One of the systems affected by these metabolites is the agri-food system, which must guarantee the supply of sufficient and quality food. However, among the substances that compromise food safety are mycotoxins, which represent a danger to food safety and to global health [81]. There are two strategic approaches aimed at the control of these fungal secondary metabolites. The first consists of the prevention of mycotoxins, which implies the inhibition of fungal growth, and the second consists of detoxification whose objective is to eliminate the fungal metabolite. Figure 2 shows the negative impact of mycotoxins on the world economy and food security. The latter being able to affect human and animal health; as well as strategies aimed at the control of mycotoxigenic fungi and their metabolites.
Detection
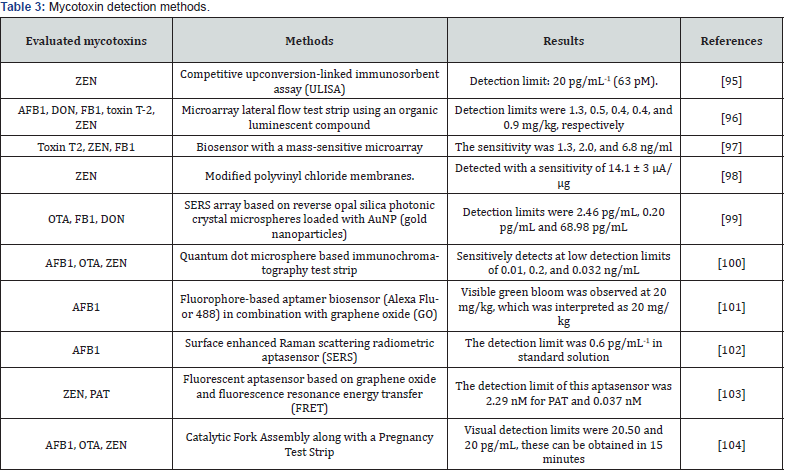
There are multiple techniques for the detection of mycotoxins, however, in a study conducted by Wei et al. the efficacy of surface plasmon resonance (SPR) was determined as a rapid detection method for mycotoxins in corn and wheat, the results showed that the minimum detection limits of ochratoxin A, AFB1, deoxynivalenol, and zearalenone were 1.27 ng/ml, 0.59 ng/ml, 3.26 ng/ml, and 7.07 ng/ml, respectively [82]. Transducers have also been used, where the main detection method is the use of optical, piezoelectric, and electrochemical spectroscopy. In addition, biological materials such as peptides, enzymes, antibodies, cells, and nucleic acids are important elements in detection in biosensor studies [83]. Chen et al, described an Escherichia coli-based biosensor to evaluate AFB1 and ZEN (zearalenone) in peanut and corn oil samples. In this study they report the decrease in the concentration of AFB1 in the range of 0.01-0.3 μg/mL and of ZEN in a range of 0.05-0.5 μg/mL. They conclude that the method establishes a new approach for the detection of mycotoxins [84].
The damage caused by mycotoxins in human and animal health requires the development of effective detection methods. Immunoassays are a sensitive and highly specific tool for the rapid detection of mycotoxins [85]. Zhang et al. developed a multiple surface enhanced Raman scattering (SERS)-based lateral flow immunosensor to determine six major mycotoxins in maize. The results showed a recovery of 78.9 to 106.2% and a high precision of the assay, in addition to being an assay that is completed in less than 20 minutes [86]. Yan et al. used a mimotope of deoxynivalenol which specifically binds to anti-DON antibody. In this study, an average recovery in corn and wheat samples of 90.4% to 118% and 101.3% to 111%, respectively, was obtained [85]. In another study conducted by Yang et al. developed an active signal electrochemiluminescence (ECL) biosensor for OTA determination, the results showed that ECL has a linear relationship with OTA in the range of 0.05 to 500 pg/mL with a correlation coefficient of 0.9957, with the limit of detection (LOD) being 0.02 pg/mL [87]. Therefore, the use of this biosensor for the detection of OTA in corn samples can give satisfactory results. Other studies carried out for the detection of mycotoxins which have proven to be effective and in some cases being able to detect multiple mycotoxins, are described in Table 3. Due to the importance of these metabolites in the agri-food chain, it is necessary to develop new methods for detecting mycotoxins in food and feed, which are rapid, low cost, as well as sensitive and selective [88], because these metabolites compromise food safety by putting human and livestock health at risk.
Detoxification
It has been reported that the use of chemical fertilizers as the main fungal control measure is no longer efficient because chemical substances can destroy the ecological environment of the soil, resulting in fungal resistance [89]. Due to the above, there is a greater interest in the regulation of detoxification and detoxification of mycotoxins in food, from a physical, chemical, biological and nutritional approach [90]. The use of natural substances as an alternative in the detoxification of mycotoxigenic fungi and their metabolites is of great importance in food production. For example, the use of essential oils as a control measure is efficient due to its low toxicity, high volatility and because it is biodegradable [91]. The biosynthesis of mycotoxins is modulated by oxidative stress that occurs during the secondary exchange of fungi, through reactive oxygen species [44]. Therefore, there is a growing interest in the use of natural antioxidant substances that inhibit the growth of mycotoxigenic fungi and their metabolites, due to their non-toxicity and because they are friendly to the environment [92].
In a study carry out by Silva et al., the antifungal activity against Aspergillus flavus and A. parasiticus of the essential oils of fennel, ginger, peppermint and thyme was evaluated, reporting the following effective concentrations: 50, 80, 50 and 50% respectively [93]. Kalagatur et al. evaluated the antifungal activity on strains of Aspergillus ochraceus and Penicillium verrucosum, of the essential oils of Cinnamomum zeylanicum and Cymbopogon martini, demonstrating a complete fungal inhibition of growth and OTA production at 1500 and 2500 μg/g in corn grains, respectively [44]. Gemeda et al. evaluated the antifungal activity of the essential oils of Cymbopogon martinii, Foeniculum vulgare and Trachyspermum ammi against Aspergillus strains, the results showed a better efficacy of T. ammi oil, showing mycelial inhibition absolute at 1 μl/mL, in turn completely inhibited spore germination at a concentration of 2 μl/mL. Furthermore, it totally inhibited the production of aflatoxins from A. niger and A. flavus at 0.5 and 0.75 μl/mL, respectively [94].
In a study conducted by Gómez-Maldonado et al. the antifungal activity was evaluated from extracts of manila seeds, in the study they report an inhibition of mycelial growth against the fungus Colletotrichum brevisporum of 100% after 9 days and spore germination of 0% after 20 hours, with manila seed extract at 3 g/L [105]. Andleeb et al. carried out a study in which they report an antifungal activity greater than 50% on strains of A. fumigatus and A. niger, by using extracts from flowers, berries and leaves of Argemone mexicana L. [106]. Rodriguez et al. carried out a study in which they report an 85% inhibition on the germination of F. oxysporum conidia on the fifth day of treatment, using phenolic extracts obtained from chiltepin fruits [107]. Trichoderma is a fungus widely studied in the world for its antifungal capabilities, as well as for promoting plant growth [108]. Trichoderma spp. it is cosmopolitan, it is characterized by the fact that it grows rapidly, in addition to having a metabolic diversity, even many of its species interact with animals, plants and other fungi [109]. In a study by He et al., showed that Trichoderma asperellum decreases the accumulation of DON (deoxynivatenol) and FB1 in the stem and cob of maize grown in soil [89]. Saravanakumar et al. determined that the cellulase genes Thph1 and Thph2 of Trichoderma harzianum, in addition to controlling foliar disease in corn, also act as biocontrol in stem rot caused by Fusarium [84].
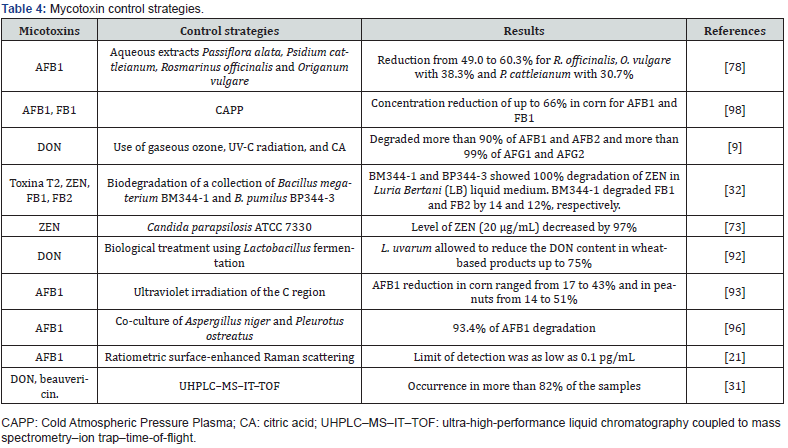
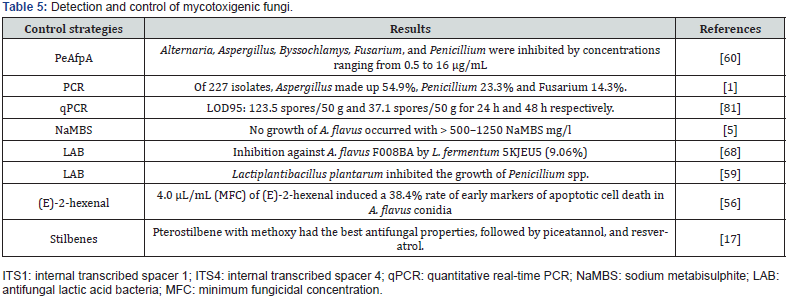
In another study by Li et al. evaluated the efficacy of Sporidiobolus pararoseus Y16 against grape rot caused by Aspergillus niger, showing that at different concentrations of S. pararoseus Y16 the decomposition of table grapes by A. niger was significantly inhibited [51]. For their part Kinyungu et al. evaluated biological control treatments from harvest to storage. Concluding that biocontrol before harvest does not replace the need for better postharvest practices, since the population of toxigenic A. flavus in the harvested grain increased and produced aflatoxins throughout the drying time when the humidity was high [48]. Yerkovich et al. report a reduction in the accumulation of deoxynivalenol of 60% in wheat crops, when Bacillus velezensis RC218 was applied, this in greenhouse tests. Although, as they mention, climate change must be considered, when applying combined strategies with fungicides, biocontrol, and cultivars to control the accumulation of mycotoxins [103]. Finally, Table 4 describes other studies carried out to evaluate the detoxification of the main mycotoxins that contaminate food. These methods have proven to be a useful alternative for the control of mycotoxins, which can also be friendly to the environment. Likewise, Table 5 mentions some of the methodologies used for the identification and control of mycotoxigenic fungi.
Conclusion
The impact of climate change is affecting the agri-food system, generating optimal conditions for the proliferation of mycotoxigenic fungi. Environmental factors such as temperature and relative humidity, as well as agricultural practices, contribute to the production of mycotoxins. The foregoing makes this a topic of relevance worldwide since the control of these fungi and their metabolites compromises food safety. One of the most visible effects is the decrease in productivity, and therefore in the supply of food for both human and animal consumption. Even the poor quality of food contaminated by these metabolites affects human and animal health. These are friendly to the environment since the use of chemical fertilizers as a control measure destroys the ecological environment of the soil and can generate resistance. Detection methods for mycotoxins in food and feed must be fast, cheap, sensitive, and selective. Detection proposals include ELISA, ULISA, MSMA, aptasensors, and biosensors. Among the control measures that can become effective alternatives for detoxification are the use of natural extracts, essential oils, ultraviolet irradiation, co-cultures, and biodegradation. All these methods are a useful alternative to guarantee safe food, of good quality and sufficient for consumption demands.
Acknowledgment
Author Sáez-Gomez gratefully acknowledges financial support for his PhD studies from Natl. Council for Science and Technology of Mexico (CONACYT).
References
- Aasa AO, Njobeh PB, Fru FF (2022) Incidence of filamentous fungi in some food commodities from Ivory Coast. J Agric Food Res 8: 100304.
- Abdel HA, Carter D, Magan N (2011) Discrimination between aflatoxigenic and non-aflatoxigenic Aspergillus section Flavi strains from Egyptian peanuts using molecular and analytical techniques. World Mycotoxin J, pp. 69-77.
- Adamchick J, Perez AM (2020) Choosing awareness over fear: Risk analysis and free trade support global food security. Glob Food Sec 26: 100445.
- Agrios G (2004) Plant Pathology. Academic P. Gainesville USA.
- Al-Jaza D, Medina A, Magan N (2022) Efficacy of sodium metabisulphite for control of Aspergillus flavus and aflatoxin B1 contamination in vitro and in chilli powder and whole red chillies. Food Control 135: 108786.
- Andleeb S, Alsalme A, Al-Zaqri N, Warad I, Alkahtani J, et al. (2020) In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. J King Saud University Sci 32(3): 2053-2058.
- Atanda SA Pessu PO, Agoda S, Isong IU, Adekalu OA, et al. (2011) Fungi and mycotoxins in stored foods. African J Microbiol Res 5: 4373-4382.
- Atongbiik AM, Opoku N, Amagloh FK (2017) Aflatoxin contamination in cereals and legumes to reconsider usage as complementary food ingredients for Ghanaian infants: A review. J Nutr Intermed Metab 10: 1-7.
- Babaee R, Karami-Osboo R, Mirabolfathy M (2022) Evaluation of the use of Ozone, UV-C and Citric acid in reducing aflatoxins in pistachio nut. J Food Compos Anal 106: 104276.
- Balajee SA, Houbraken J, Verweij PE, Hong SB, Yaghuchi T, et al. (2007) Aspergillus species identification in the clinical setting. Stud Mycol 59: 39-46.
- Bbosa GS, Kitya D, Lubega A, Ogwal-Okeng J, Anokbonggo WW, et al. (2013) Review of the biological and health effects of aflatoxins on body organs and body systems. In: Abyaneh MR (Ed.) Aflatoxins-recent advances and prospects. Intech Open, London.
- Begaa S, Messaoudi M (2019) Toxicological aspect of some selected medicinal plant samples collected from Djelfa, Algeria region. Biol Trace Elem Res 187(1): 301-306.
- Berthiller F, Sulyok M, Krska R, Schuhmacher R (2007) Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int J Food Microbiol 119: 33-37.
- Bogantes LP, Bogantes LD, Bogantes LS (2004) Aflatoxinas. Acta Med Costarric 46(4): 174-178.
- Bryden WL (2012) Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci Technol 173: 134-158.
- Bui-Klimke TR, Wu F (2016) Ochratoxin A and human health risk: A review of the evidence. Crit Rev Food Sci Nutr 1860-1869.
- Cai X, Qi J, Xu Z, Huang L, Li Y, Ren X, et al. (2022) Three stilbenes make difference to the antifungal effects on ochratoxin A and its precursor production of Aspergillus carbonarius. Food Microbiol 103: 103967.
- Chakraborty A, Singh J, Upadhyay H (2021) Fungal Diversity and Ecology: Major Challenges for Crop Production in Present Climate Scenario. In: Yadav AN (Ed.) Recent Trends in Mycological Research, Springer Nature, Geneva pp. 89-107.
- Chang CL, Leung M, Yang MH (2004) Alkyl and dialkylammonium tetrafluoroborate catalyzed cis-trans isomerization of 1,3,5-trimethyl-1,3,5-triphenylcyclotrisiloxane. Tetrahedron 60(41): 9205-9212.
- Charlermroj R, Phuengwas S, Makornwattana M, Sooksimuang T, Sahasithiwat S, et al. (2021) Development of a microarray lateral flow strip test using a luminescent organic compound for multiplex detection of five mycotoxins. Talanta 233: 122540.
- Chen P, Li C, Ma X, Wang Z, Zhang Y (2022) A surface-enhanced Raman scattering aptasensor for ratiometric detection of aflatoxin B1 based on graphene oxide-Au@Ag core-shell nanoparticles complex. Food Control 134: 108748.
- Chen Y, Yang Y, Wang Y, Peng Y, Nie J, et al. (2020) Development of an Escherichia coli-based electrochemical biosensor for mycotoxin toxicity detection. Bioelectrochem 133: 107453.
- Dautt-Castro M, Estrada-Rivera M, Olguin-Martínez I, Rocha-Medina MDC, Maria AIO, et al. (2020) TBRG-1 a Ras-like protein in Trichoderma virens involved in conidiation, development, secondary metabolism, mycoparasitism, and biocontrol unveils a new family of Ras-GTPases. Fungal Genet Biol 136: 103292.
- De la Torre-Hernández ME, Sánchez-Rangel D, Galeana-Sánchez E, Plasencia-de la Parra J (2014) Fumonisinas -Síntesis y función en la interacción Fusarium verticillioides-maí TIP 17(1): 77-91.
- Diao E, Ma K, Qian S, Zhang H, Xie P, et al. (2022) Removal of patulin by thiol-compounds: A review. Toxicon 205: 31-37.
- Dogliotti S, Giller KE, Van Ittersum MK (2014) Achieving global food security whilst reconciling demands on the environment: Report of the First International Conference on Global Food Security. Food Secur 6: 299-302.
- Dwivedy AK, Kumar M, Upadhyay N, Prakash B, Dubey NK (2016) Plant essential oils against food borne fungi and mycotoxins. Curr Opin Food Sci 11: 16-21.
- FAO (2022) Micotoxinas. Organ. las Nac. Unidas para la Aliment y la Agric.
- Gemeda N, Woldeamanuel Y, Asrat D, Debella A (2014) Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: A potential source of botanical food preservative. Asian Pac J Trop Biomed 4(Suppl 1): S373-S381.
- Gómez-Maldonado D, Lobato-Calleros C, Aguirre-Mandujano E, Leyva-Mir SG, Robles-Yerena L, et al. (2020) Antifungal activity of mango kernel polyphenols on mango fruit infected by anthracnose. LWT 126: 109337.
- González-Jartín JM, Ferreiroa V, Rodríguez-Cañás I, Alfonso A, Sainz MJ, et al. (2022) Occurrence of mycotoxins and mycotoxigenic fungi in silage from the north of Portugal at feed-out. Int J Food Microbiol 365: 109556.
- Hassan ZU, Al Thani R, Alsafran M, Migheli Q, Jaoua S (2021) Selection of Bacillus spp. with decontamination potential on multiple Fusarium mycotoxins. Food Control 127: 108119.
- He A, Sun J, Wang X, Zou L, Fu B, et al. (2019) Reprogrammed endophytic microbial community in maize stalk induced by Trichoderma asperellum biocontrol agent against Fusarium diseases and mycotoxin accumulation. Fungal Biol 123(6): 448-455.
- Hermosa R, Viterbo A, Chet I, Monte E (2012) Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158: 17-25.
- HMDB (2022) Showing metabocard for aflatoxin GM1 (HMDB0030477).
- Hong SB, Go SJ, Shin HD, Frisvad JC, Samson RA (2005) Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97(6): 1316-1329.
- Hua H, Xing F, Selvaraj JN, Wang Y, Zhao Y, et al. (2014) Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin A production. Plos One 9(9): e108285.
- Huang D, Cui LQ, Sajid A, Zainab F, Wu Q, et al. (2019) The epigenetic mechanisms in Fusarium mycotoxins induced toxicities. Food Chem Toxicol 123: 595-601.
- Huang D, Cui L, Guo P, Xue X, Wu Q, et al. (2017) Nitric oxide mediates apoptosis and mitochondrial dysfunction and plays a role in growth hormone deficiency by nivalenol in GH3 cells. Sci Rep 7(1): 17079.
- Huerta A, Dávila JE, Sánchez E, Heredia N, García S (2016) Occurrence of mycotoxins in alfalfa (Medicago sativa L.), sorghum. Agrociencia 50(7): 826-836.
- Ibáñez-Vea M, Martínez R, González-Peñas E, Lizarraga E, López de Cerain A (2011) Co-occurrence of aflatoxins, ochratoxin A and zearalenone in breakfast cereals from Spanish market. Food Control 22: 1949-1955.
- Janik E, Niemcewicz M, Ceremuga M, Stela M, Saluk BJ, et al. (2020) Molecular aspects of mycotoxins-A serious problem for human health. Int J Mol Sci 21(21): 8187.
- Jiao S, Liu J, Sun J, Chang Y, Wang S, et al. (2022) A highly sensitive and reproducible multiplex mycotoxin SERS array based on AuNPs-loaded inverse opal silica photonic crystal microsphere. Sensors Actuators B Chem 355: 131245.
- Kalagatur NK, Gurunathan S, Kamasani JR, Gunti L, Kadirvelu K, et al. (2020) Inhibitory effect of C Zeylanicum, C Longa, O Basilicum, Z Officinale, and C Martini essential oils on growth and ochratoxin A content of A. ochraceous and P. verrucosum in maize grains. Biotechnol Reports 27: e00490.
- Kang L, Guo N, Liu X, Wang X, Guo W, et al. (2020) High mobility group box-1 protects against Aflatoxin G1-induced pulmonary epithelial cell damage in the lung inflammatory environment. Toxicol Lett 331: 92-101.
- Karabulut S, Paytakov G, Leszczynski J (2014) Reduction of aflatoxin B1 to aflatoxicol: A comprehensive DFT study provides clues to its toxicity. J Sci Food Agric 94: 3134-3140.
- Karmanov AP, Kanarsky AV, Kocheva LS, Semenov EI, Belyy VA (2021) In vitro study of adsorption efficiency of natural lignins towards aflatoxin B2. React. Funct Polym 167: 105033.
- Kinyungu S, Isakeit T, Ojiambo PS, Woloshuk CP (2019) Spread of Aspergillus flavus and aflatoxin accumulation in postharvested maize treated with biocontrol products. J Stored Prod Res 84: 101519.
- Krishnan S, Manavathu EK, Chandrasekar PH (2009) Aspergillus flavus: an emerging non-fumigatus Aspergillus species of significance. Mycoses 52(3): 206-222.
- Lalah JO, Omwoma S, Orony DA (2019) Aflatoxin B1: Chemistry, environmental and diet sources and potential exposure in human in Kenya. Detect. Toxicol Eff.
- Li Q, Li C, Li P, Zhang H, Zhang X, et al. (2017) The biocontrol effect of Sporidiobolus pararoseus Y16 against postharvest diseases in table grapes caused by Aspergillus niger and the possible mechanisms involved. Biol Control 113: 18-25.
- Lillehoj EB, Ciegler A (1969) Biological activity of Aflatoxin B2
- Liu M, Zhao L, Gong G, Zhang L, Shi L, et al. (2022) Invited review: Remediation strategies for mycotoxin control in feed. J Anim Sci Biotechnol 13(1): 19.
- Lu YN, Shan Y, Huang H, Zhu L, Li B, et al. (2022) Quantum dot microsphere-based immunochromatography test strip enabled sensitive and quantitative on-site detections for multiple mycotoxins in grains. Food Chem 376: 131868.
- Ma R, Zhang L, Liu M, Su YT, Xie WM, et al. (2018) Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins (Basel) 10: 113.
- Ma W, Zhao L, Johnson ET, Xie Y, Zhang M (2022) Natural food flavour (E)-2-hexenal, a potential antifungal agent, induces mitochondria-mediated apoptosis in Aspergillus flavus conidia via a ROS-dependent pathway. Int J Food Microbiol 370: 109633.
- Magnan N, Hoffmann V, Opoku N, Gajate Garrido G, Kanyam DA (2021) Information, technology, and market rewards: Incentivizing aflatoxin control in Ghana. J Dev Econ 151: 102620.
- Majeed S, Iqbal M, Asi MR, Iqbal SZ (2013) Aflatoxins and ochratoxin A contamination in rice, corn and corn products from Punjab, Pakistan. J Cereal Sci 58(3): 446-450.
- Mareze J, Ramos-Pereira J, Santos JA, Beloti V, López-Díaz TM (2022) Identification and characterization of Lactobacilli isolated from an artisanal cheese with antifungal and antibacterial activity against cheese spoilage and mycotoxigenic Penicillium spp. Int Dairy J 130: 105367.
- Martínez-Culebras PV, Gandía M, Boronat A, Marcos JF, Manzanares P (2021) Differential susceptibility of mycotoxin-producing fungi to distinct antifungal proteins (AFPs). Food Microbiol 97: 103760.
- Martínez H, Hernández S, Reyes CGV (2013) El género Aspergillus y sus micotoxinas en maíz en México problemática y perspectivas. Rev Mex Fitopatol 31(2): 126-146.
- Martins HM, Martins ML, Bernardo FA (2000) Interaction of strains of non-toxigenic Aspergillus flavus with Aspergillus parasiticus on aflatoxin production. Brazilian J Vet Res Anim Sci 37(6).
- Mehrabi Z, Delzeit R, Ignaciuk A, Levers C, Braich G, et al. (2022) Research priorities for global food security under extreme events. One Earth 5(7): 756-766.
- Murphy PA, Hendrich S, Landgren C, Bryant CM (2006) Food mycotoxins: An update. J Food Sci 71(5): R51-R65.
- Nasri T, Hedayati MT, Abastabar M, Pasqualotto AC, Armaki MT, et al. (2015) PCR-RFLP on β-tubulin gene for rapid identification of the most clinically important species of Aspergillus. J Microbiol Methods 117: 144-147.
- National Center for Biotechnology Information (2022) PubChem Compound Summary for CID 186907, Aflatoxin B1.
- Nolan P, Auer S, Spehar A, Oplatowska SM, Campbell K (2021) Evaluation of mass sensitive micro-array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 222: 121521.
- Ogunremi OR, Freimüller LS, Mischler S, Miescher SS (2022) Antifungal activity of lactic acid bacteria isolated from kunu-zaki, a cereal-based Nigerian fermented beverage. Food Biosci 49: 101648.
- Okello DK, Kaaya AN, Bisikwa J, Were M, Oloka HK (2010) Management of aflatoxins in groundnuts: A manual for farmers, processors and traders in Uganda. Natl Agric Res Organ p. 1-38.
- Omotayo OP, Omotayo AO, Mwanza M, Babalola OO (2019) Prevalence of mycotoxins and their consequences on human health. Toxicol Res 35(1): 1-7.
- OMS (Organización Mundial de la Salud) (2018) Micotoxinas. Organ Mund la Salud.
- Ostry V, Malir F, Toman J, Grosse Y (2017) Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res 33(1): 65-73.
- Pan Y, Liu C, Yang J, Tang Y (2022) Conversion of zearalenone to β-zearalenol and zearalenone-14,16-diglucoside by Candida parapsilosis ATCC 7330. Food Control 131: 108429.
- Pankiew A, Chaisriratanakul W, Bunjongpru W, Srisuwan A, Jeamsaksiri W, et al. (2021) Modification of polyvinyl chloride membranes for mycotoxins detection. Sens Bio-Sensing Res 34: 100460.
- Paterson R, Lima N (2010) How will climate change affect mycotoxins in food? Food Res Int 43: 1902-1914.
- Peltomaa R, Farka Z, Mickert MJ, Brandmeier JC, Pastucha M, et al. (2020) Competitive upconversion-linked immunoassay using peptide mimetics for the detection of the mycotoxin zearalenone. Biosens Bioelectron 170: 112683.
- Pettersson H, Brown C, Hauk J, Hoth S, Meyer J, et al. (2011) Survey of T-2 and HT-2 toxins by LC-MS/MS in oats and oat products from European oat mills in 2005-2009. Food Addit Contam Part B Surveill 4: 110-115.
- Ponzilacqua B, Rottinghaus GE, Landers BR, Oliveira CAF (2019) Effects of medicinal herb and Brazilian traditional plant extracts on in vitro mycotoxin decontamination. Food Control 100: 24-27.
- Prencipe S, Siciliano I, Gatti C, Garibaldi A, Gullino ML, et al. (2018) Several species of Penicillium isolated from chestnut flour processing are pathogenic on fresh chestnuts and produce mycotoxins. Food Microbiol 76: 396-404.
- Rodríguez-Roa JH, Cárdenas M, Jiménez P (2013) Especies de Alternaria de La Sabana de Bogotá, Colombia Rev Fac Ciencias Básicas 9(2): 228.
- Roumani F, Azinheiro S, Rodrigues C, Barros-Velázquez J, Garrido-Maestu A, et al. (2022) Development of a real-time PCR assay with an internal amplification control for the detection of spoilage fungi in fruit preparations. Food Control 135: 108783.
- Rushing BR, Selim MI (2019) Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol 124: 81-100.
- Santana Oliveira I, Da Silva AG, De Andrade CAS, Lima Oliveira MD (2019) Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr Opin Food Sci 29: 64-79.
- Saravanakumar K, Dou K, Lu Z, Wang X, Li Y, et al. (2018) Enhanced biocontrol activity of cellulase from Trichoderma harzianum against Fusarium graminearum through activation of defense-related genes in maize. Physiol Mol Plant Pathol 103: 130-136.
- Schrenk D, Bodin L, Chipman JK, Del Mazo J, Grasl-Kraupp B, et al. (2020) Risk assessment of ochratoxin A in food. EFSA J 18(5): e06113.
- Setlem SK, Mondal B, Ramlal S (2022) A fluorescent aptasensor for the detection of Aflatoxin B1 by graphene oxide mediated quenching and release of fluorescence. J Microbiol Methods 193: 106414.
- Sharaf O (2016) Aflatoxin M1 levels in raw milk, pasteurised milk and infant formula. Ital J Food Saf 5(3): 5788.
- Silva FC, Chalfoun SM, Siqueira VM, Botelho DMS, Lima N, et al. (2012) Evaluation of antifungal activity of essential oils against potentially mycotoxigenic Aspergillus flavus and Aspergillus parasiticus. Rev Bras Farmacogn 22(5): 1002-1010.
- Sirma AJ, Ouko EO, Murithi G, Mburugu C, Mapenay I, et al. (2015) Prevalence of aflatoxin contamination in cereals from Nandi County, Kenya. Int J Agric Sci Vet Med 3: 55-63.
- Sugri I, Moses O, Asamoah L, Samuel BSJ, Stephen Nk, et al. (2015) Aflatoxin management in Northern Ghana: Current prevalence and priority. Aflatoxin management in Northern Ghana: Current prevalence and priority strategies in maize (Zea mays L.). J Stored Prod Postharvest Res 6: 48-55.
- Tilak R, Singh A, Prakash O, Maurya S, Chandra A, et al. (2010) Mycotic keratitis in India: a five-year retrospective study. Brief Original Article. J Infect Dev Ctries 4(3): 171-174.
- Trakselyte-Rupsiene K, Juodeikiene G, Hajnal EJ, Bartkevics V, Pugajeva I, et al. (2022) Challenges of Lactobacillus fermentation in combination with acoustic screening for deoxynivalenol and deoxynivalenol conjugates reduction in contaminated wheat - based products. Food Control 134: 108699.
- Udovicki B, Stankovic S, Tomic N, Djekic I, Smigic N, et al. (2022) Evaluation of ultraviolet irradiation effects on Aspergillus flavus and Aflatoxin B1 in maize and peanuts using innovative vibrating decontamination equipment. Food Control 134: 108691.
- Wajih ul Hassan S, Sadef Y, Hussain S, Rafique Asi M, Ashraf MY, et al. (2020) Unusual pattern of aflatoxins and ochratoxin in commercially grown maize varieties of Pakistan. Toxicon 182: 66-71.
- Waliyar F, Umeh VC, Traore A, Osiru M, Ntare BR, et al. (2015) Prevalence and distribution of aflatoxin contamination in groundnut (Arachis hypogaea L.) in Mali, West Africa. Crop Prot 70: 1-7.
- Wang L, Huang W, Shen Y, Zhao Y, Wu D, et al. (2022) Enhancing the degradation of Aflatoxin B1 by co-cultivation of two fungi strains with the improved production of detoxifying enzymes. Food Chem 371: 131092.
- Wei T, Ren P, Huang L, Ouyang Z, Wang Z, et al. (2019) Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem 300: 125176.
- Wielogorska E, Ahmed Y, Meneely J, Graham WG, Elliott CT, et al. (2019) A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem 301: 125281.
- Wu Z, Sun DW, Pu H, Wei Q, Lin X (2022) Ti3C2Tx MXenes loaded with Au nanoparticle dimers as a surface-enhanced Raman scattering aptasensor for AFB1 detection. Food Chem 372: 131293.
- Yan J, Shi Q, You K, Li Y, He Q (2019) Phage displayed mimotope peptide-based immunosensor for green and ultrasensitive detection of mycotoxin deoxynivalenol. J Pharm Biomed Anal 168: 94-101.
- Yang L, Zhang Y, Li R, Lin C, Guo L, Qiu B, et al. (2015) Electrochemiluminescence biosensor for ultrasensitive determination of ochratoxin A in corn samples based on aptamer and hyperbranched rolling circle amplification. Biosens Bioelectron 70: 268-274.
- Yazid SNE, Jinap S, Ismail SI, Magan N, Samsudin NIP (2020) Phytopathogenic organisms and mycotoxigenic fungi: Why do we control one and neglect the other? A biological control perspective in Malaysia. Compr Rev Food Sci Food Saf 19(2): 643-669.
- Yerkovich N, Cantoro R, Palazzini JM, Torres A, Chulze SN (2020) Fusarium head blight in Argentina: Pathogen aggressiveness, triazole tolerance and biocontrol-cultivar combined strategy to reduce disease and deoxynivalenol in wheat. Crop Prot 137: 105300.
- Zhang N, Li J, Liu B, Wang H, Zhang D, et al. (2022a) A facile “turn-on” fluorescent aptasensor for simultaneous detection of dual mycotoxins in traditional Chinese medicine based on graphene oxide and FRET. Toxicon 206: 42-50.
- Zhang S, Zhou S, Lyu B, Qiu N, Li J, et al. (2022b) Dietary exposure to fumonisins and ochratoxins in the Chinese general population during 2007-2020: Results from three consecutive total diet studies. Food Chem Toxicol 159: 112768.
- Zhang W, Tang S, Jin Y, Yang C, He L, et al. (2020) Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J Hazard Mater 393: 122348.
- Zhao L, Zhang L, Xu Z, Liu X, Chen L, Dai J, et al. (2021) Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018-2020. J Anim Sci Biotechnol 12(1): 74.
- Zhong ZT, Song LB, He YF, Zhang B, Chen W, et al. (2022) Detection of multiple mycotoxins based on catalytic hairpin assembly coupled with pregnancy test strip. Sensors Actuators B Chem 350: 130911.
- Zhu X, Yuan X, Zhang Y, Liu H, Wang J, Sun B (2022) The global concern of food security during the COVID-19 pandemic: Impacts and perspectives on food security. Food Chem 370: 130830.






























