The Effect of Drought on Phytochemical active Compounds Content in Chamomile and Yarrow
Banaz J Mahmood1, Hail Z Rihan1*, Mike Foulkes2 and Stephen Burchett S1
1Department of Science & Environment, University of Plymouth, UK
2Department of Science & Engineering, University of Plymouth, UK
Submission: October 12, 2017; Published: December 06, 2017
*Corresponding author: Hail ZR, University of Plymouth, B 501, Portland Square Building, Plymouth, UK, Email: hail.rihan@plymouth.ac.uk
How to cite this article: Mahmood BJ, Rihan HZ, Foulkes ME, Burchett S. The Effect of Drought on Phytochemical active Compounds Content in Chamomile and Yarrow. Agri Res & Tech: Open Access J. 2017; 12(4): 555855.DOI:10.19080/ARTOAJ.2017.12.555855
Abstract
The effect of drought stress conditions on the phytochemical compounds content of German chamomile (Matricaia chamomilla L.), common yarrow and summer berries yarrow (Achillea millefolium L.) was investigated. Treatments contained drought stress 70% field capacity (FC) pots and control (well-watered 100% FC) pots under controlled glasshouse conditions. Freeze dried and ground leaves were extracted at room temperature using Shaking extraction with methanol (99.9%) as an efficient solvent in order to determine the phytochemical compositions of phenols and flavonoids from dried chamomile and yarrow leaves. Samples were then analysed using High performance liquid chromatography with ultra-violet detection (HPLC-UV). Five times sample collections were applied over leaf production period for each plant species.
Results confirmed that these two plant species can produce a good mass production of phytochemical compounds content even when there is insufficient water in the soil. More phytochemically active compounds were identified in chamomile compared with yarrow. The main phenol and flavonoid compounds found were chlorogenic acid, caffeic acid, apig-7-glucoside, umbelliferon and luteolin. Total yields of chlorogenic acid, apig-7-glucoside and umbelliferon dominated over other compounds in chamomile, common yarrow and summer berries yarrow under both well-watered and drought stress conditions.
Keywords: Chamomile; Yarrow; Drought; Phenols; Flavonoids; Abiotic stress; HPLC-UV
Introduction
German chamomile and yarrow are two main medicinal plants belonging to the Asteracea family [1]. Both plants contain some active substances which are known as natural products, bioactive compounds or secondary metabolites which are affected by environmental conditions such as drought [2]. Drought stress or availability of water is considered to be an important abiotic factor that influences plant growth, development and biochemical processes such as the production of secondary metabolites and aromatic compounds [3-6]. In the controlling and management of agriculture production, drought stress is one of the most important environmental factors that determines plant growth worldwide. Medicinal plant phytochemical compounds content including total phenols, flavonoids and essential oil constituents such as limonene, terpinolene, myrcene, quercetin and sabinene are mainly influenced by drought stress [4,7,8]. In general, drought stress among cultivars caused a clear decrease in fresh weight, stem length, carotenoid and chlorophyll photosynthetic pigments, total soluble sugars, total flavonoids and total phenolic compounds [9]. Moreover, stress factors can damage plant mechanisms for example, an abnormal plant metabolism may occur and cause reduced growth and development and subsequently influence crop yield, or can lead to complete plant/crop death [10]. It has been reported that drought stress affects plant growth and the production of secondary products when grown under greenhouse conditions, for example plant vegetative growth has been reduced under stress [6].
To date chamomile and yarrow are cultivated around the world mainly for secondary products content. These natural compounds are affected by environmental stress factors such as drought [11]. Jeshni et al. [12] reported that irrigation, under 50% FC, could increase the content of medicinal components of chamomile plants. It has been found that drought stress levels not only effect the phytochemical compounds content of chamomile plants, but also significantly influence the fresh and dry flower yield [2]. Drought stress level (50% field capacity) was also found to be optimal for total phenol and flavonoid compound content of yarrow compared with 100%, 75% and 25% field capacity [13].
It is important to mention that several studies have reported the effect of drought stress on quality, yield and growth of field crops [14-16]. However only a few studies linking the effect of environmental stress on medicinal plants phytochemical compounds content have been investigated [17,18]. The current study therefore aimed to examine how chamomile and yarrow respond to the drought stress regime and to evaluate the impact of drought and well-watered regimes on the leaf phenol and flavonoid content in both German chamomile and yarrow plant species.
Materials and Methods
Growth conditions
Two varieties of yarrow seeds were performed, common yarrow were purchased from Richters Herbs (Source for Everything Herbal, 357 Durham Regional Hwy 47, Goodwood, ON L0C 1A0, Canada) and summer berries yarrow seeds were purchased from Chiltern seed (Wallingford, 0X10 6SL, England). German chamomile seeds were also purchased from Richters Herbs. Two water regimes, well-watered (WW) and drought stress (D) were applied. Seeds of all varieties were germinated in plastic seed trays (220x160mm, three trays per variety), with a loam based topsoil. The plants were then transferred into small plastic pots (88.9mm2) when they were 7-10cm tall (two plants per pot with forty pots per variety). Three months after germination, plantlets (20-25cm tall) were transplanted to 15 litre plastic pots (four plants per pot with 18 pots per variety); the pots were filled with the same amount of loam based topsoil. Plants were sprayed twice against whiteflies with bug clear ultra (Bayer Provado Ultimate Bug Killer). All plants were well-watered until they were two months old (leaf production growth stage). Then the two watering regimes were applied: well-watered (WW) and droughted (D). The moisture level in the well-watered pots (control) were allowed to drop to 70% of available water capacity, then pots were brought up to fully watered again (100%).
The moisture level in the droughted pots were allowed to drop to 50% available water capacity, and then brought up to 70% available water capacity again. The water content was measured by Theta probe (Delta-T Devices Ltd) for all treatments. Data was recorded at the soil surface of the pots daily until the end of experiment when plants started wilting and stopped growing. Leaves of all varieties were collected separately to identify the total phytochemical active compounds of phenols and flavonoids accumulated by plant materials under both the well-watered and drought growing regimes. Samples were generally collected five times every 10 days (five months after germination) over their leaf production period.
Phenols and flavonoids extraction
Extraction was carried out using 99.9% HPLC grade methanol. Chamomile and yarrow leaves were washed in distilled water and placed into a zip lock freezer storage bags. Prepared samples were then placed into the freezer (Indesit freezer) at -20 °C for one day and subsequently freeze dried in a freeze drier (Edwards Modulyo) at -50 °C under 10-1mbar vacuum for 2 days. After freeze drying, the leaves of the chamomile were ground (porcelain pestle and mortar), 2.0g of prepared ground chamomile leaves were weighed into 250mL conical flask. Then 60ml of methanol was added to the samples and placed on a shaker (KS 501 digital, IKA labortechnik, Germany) for 30 min (200rpm) at room temperature. The samples were then covered with parafilm and aluminium foil to avoid degradation of compounds and the loss of solvent, and then left in darkness for 5 days at room temperature to stand. After 5 days of dark, stored samples were adjusted to 100mL of methanol and filtered through filter paper (Whatman No.1) in a filter funnel [19] with rinsing. All three sample extract solutions were combined together, and then evaporated with a rotary evaporator (Buchi, Rotavapor R-3) at 40 °C and further dried using a Nitrogen blow down apparatus [20]. Samples were finally stored in the dark at 3 to 5 °C until HPLC analysis. The same process was adopted for yarrow leaves.
HPLC-UV analysis
All extracted samples were analysed using HPLC with UV detector. Leaves of both plants were prepared and extracted using methanol as a solvent. The extracted samples were then analysed using a Hewlett-Packard Ultimate 3000 chromatograph (Plymouth University, UK) with the Ultimate 3000 pump, injection loop volume (20μL) and a diode array detector (DAD) using 256nm wavelength. A reversed phase analytical columne with cartridge guard column (Thermo Scientific, BDS Hypersil C18, Dim 200x4.6mm, LOT: 9610, SN: 1184144U, particle size= 5μm) was used at 25 °C. Two Analytical gradients were created as a solvent: (A) 1% citric acid in ultrapure water (Elga) and (B) HPLC grade acetonitrile (90:10).
The extracted samples were prepared for analysis by making two different dilutions for each sample: in the first step 1ml methanol (99.9% HPLC grade) was added to the known mass of each final extracted sample and then returned to the fridge and stood for 24 hours. The second step was preparing 10 fold dilutions from the first step samples. 1.5ml of each prepared dilution was then placed into the HPLC vials separately and each sample was run for 20 minutes. The phenolic and flavonoid compounds were identified according to their retention times, peak area, with the injection volume 20956;L and flow rate 1ml min- 1. The detected samples were then compared with the standards.
Calibration curves
Standard calibration curves were prepared from known standards of chlorogenic acid, caffeic acid, apigenin-7-glucoside, umbelliferon and luteolin (Sigma Aldrich Ltd, Gillingham, Dorset, UK) and are the average of three repeat HPLC-UV runs. Five different dilutions were prepared for each standard (5.0, 10.0, 25.0, 50.0, and 100.0mgL-1), by using methanol 99.9% HPLC grade as solvent. Polynomial and linear regressions were carried out on the curves and subsequently used to develop a calibration to determine the concentration values in the extracts. All phenolic and flavonoid compounds found in the samples were identified from their retention times and those of the standards and were quantified using peak area.
Statistical analysiss
The experimental design of this study consisted of a randomized complete block design (RCBD) at Skarden Garden glasshouse/Plympouth University. All samples were prepared in triplicate. Data are presented as means±standard error (S.E.) and subjected to the two-way analysis of variance (ANOVA) using Minitab software (version 17) and comparisons of means were made using the least significant difference test (LSD) at 95% level of probability.
Results
The effect of water regimes on the phenolic and flavonoid compound levels in chamomile and yarrow leaves
All data obtained from samples were firstly compared with the standard peak areas and retention times, and then calculated by linear and polynomial equations of standard calibration curves to determine the final concentration (mgkg-1) of each phenol and flavonoid compound found in the original plant species. Five major phenolic and flavonoid compounds were identified: chlorogenic acid, caffeic acid, apigenin-7-glucoside, umbelliferon and luteolin.
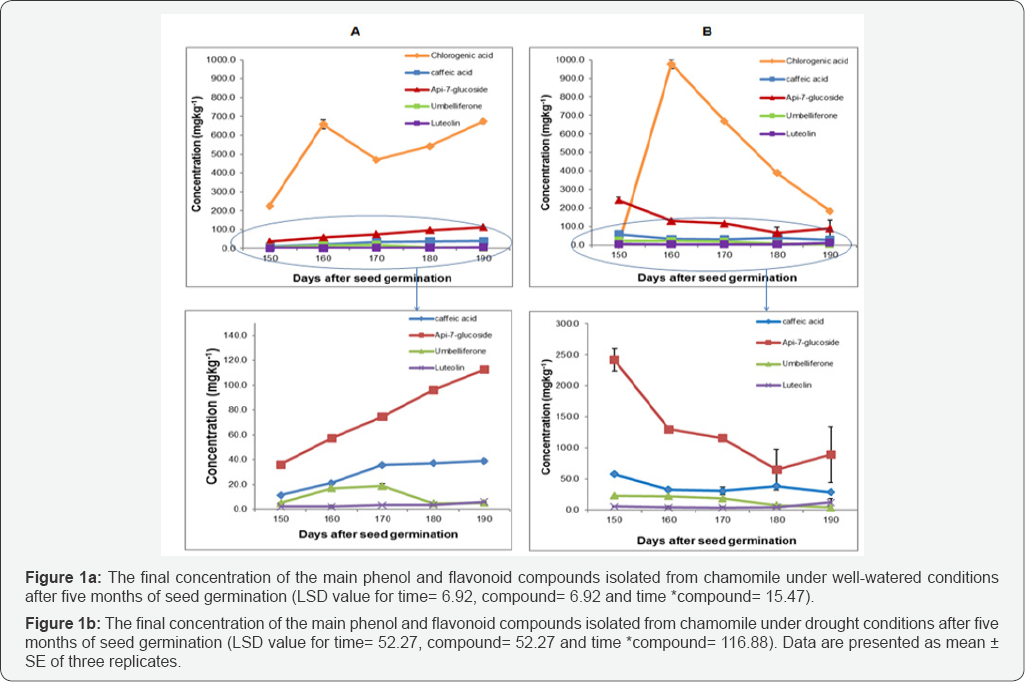
The levels of caffeic acid, apiginin-7-glucoside and luteolin were increased during the vegetative growth in chamomile, however chlorogenic acid production increased from 150 to 160 days after seed germination and declined from 160-170, it then increased again between 170 to 190 days after seed germination. Umbelliferon levels increased between 150 and 170 days after seed germination, and then reduced (Figure la). Analysis of variance shows a significant difference between all compounds under well-watered (WW) conditions (P≤0.001). Moreover, the interaction between time and chemical compounds content also produced a statistically significant (P≤0.001) effect on the phenolic and flavonoid content, where all compounds except chlorogenic acid and umbelliferon were progressively increased during a vegetative growth phase of the plants life-cycle.
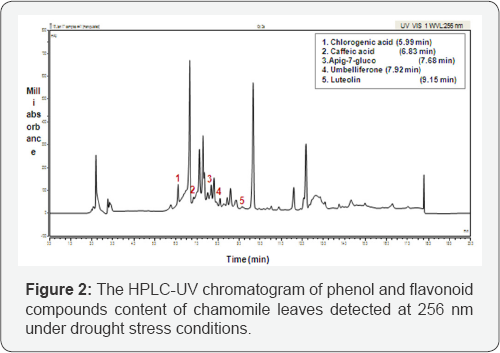
Under drought conditions chamomile plants or chamomile drought (Ch D), chlorogenic acid, umbelliferon and apig-7- glucoside were significantly (P≤0.001) decreased during the vegetative growth of chamomile, where chlorogenic acid was not detected after 150 days of seed germination. However some increasing levels were noticed in caffeic acid and luteolin between 170 to 190 days after seed germination (Figure 1 b). Results showed a significant decrease for most of the chamomile leaves phytochemical active compounds content under drought stress conditions (P≤0.001). The interaction between time and compounds content also had a significant effect on chlorogenic acid, umbelliferon and apig-7-glucoside (P≤ 0.001), where they were reduced over time. Chlorogenic acid was present at higher concentrations than the other compounds in both well-watered and drought conditions (514±44mgkg-1 and 444±99mgkg-1) respectively. Moreover, results showed that caffeic acid and luteolin were more drought resistant than other compounds especially in the later stage of growth. The HPLC-UV chromatogram of phenol and flavonoid compounds content of chamomile leaves under drought stress conditions is shown in Figure 2.
HPLC-UV results of common yarrow, type 1 (Y1) showed that four main compounds of both well-watered (WW) and drought (D) conditions were identified as follows; caffeic acid, apig-7-glucoside and luteolin were increased over time in (y1 WW) however, decreasing levels of umbelliferon were observed from 170 to 190 days after seed germination (Figure 3a). Statistical analysis showed significant differences between compounds during vegetative growth (P≤0.001). Furthermore, the interaction between time and compounds also showed a significant effect on phytochemically active compounds content at P≤0.001, where caffeic acid, apig-7-glucoside and luteolin gradually increased over time, while the increasing levels of umbelliferon were only obtained from 150-170 days after seed germination and then declined. No detection of chlorogenic acid was observed during this experiment.
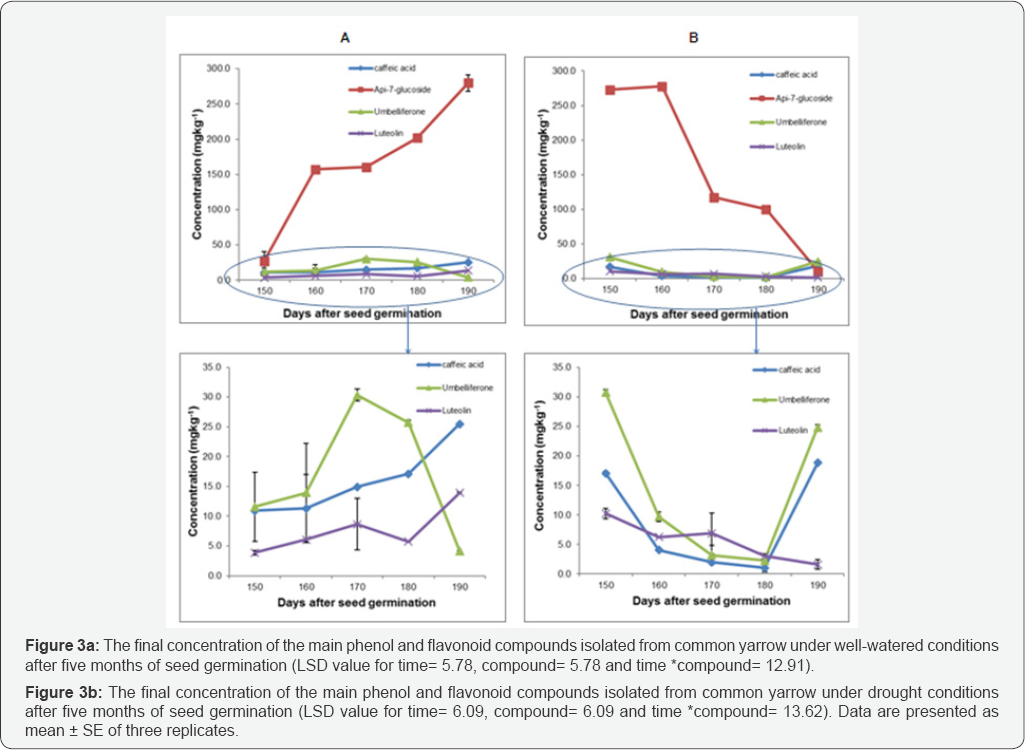
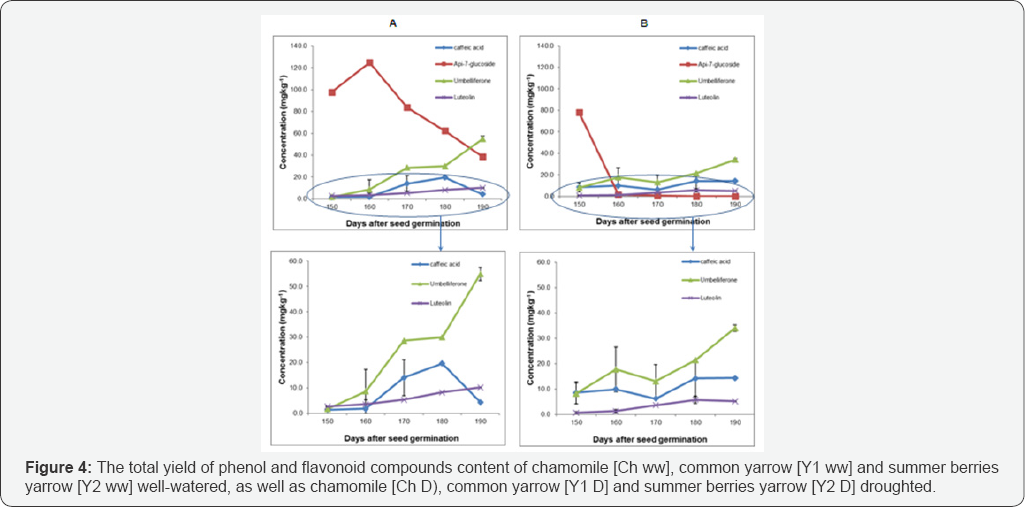
Luteolin and apig-7-glucoside detected in common yarrow were more affected under drought conditions compared with the other detected compounds. Caffeic acid and umbelliferon were also affected and decreased under drought stress until 180 days after seed germination, and then increased. Analysis of variance of common yarrow under drought stress showed that there was a significant effect on phytochemically active compounds content over time (P≤ 0.001), (Figure 3b). In addition, the interaction between time and compounds was also significant (P≤ 0.001) and showed that luteolin was the only compound that continually reduced, however some increasing levels occured for the other three compounds over the duration of vegetative growth. Apig-7-glucoside was the major phytochemically active compound in both growing conditions based on the total yield during vegetative growth. (165±22mgkg-1 and 156±28mgkg-1),respectively (Figure 4).
Similar to the common yarrow, four main phenolic and flavonoid compounds were also found in summer berries yarrow, type 2 (Y2). Also, chlorogenic acid was not detected however, umbelliferon and luteolin were start to increase over time. The concentration of apig-7-glucoside increased between 150 to 160 days after seed germination and then started to decline. However, caffeic acid increased at the beginning and started declining only from 180 to 190 days after seed germination (Figure 5a). The results showed that most of the phenol and flavonoid compounds found in Y2 well-watered were significantly increased (P≤0.001) during plant vegetative growth. Moreover, results of the interaction between time and compounds were also significant at p≤ 0.001 for umbelliferon and luteolin, where both of them progressively increased over time.
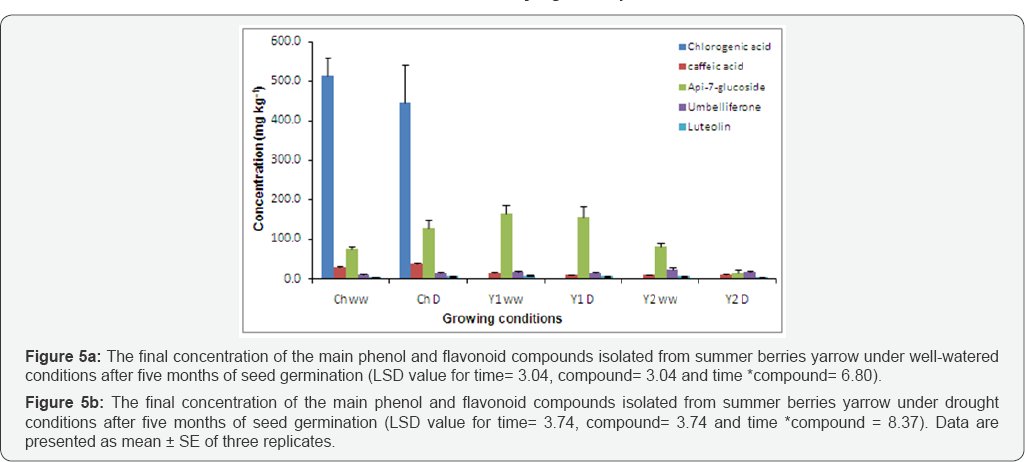
Among the phenolic and flavonoid compounds content of Y2 under drought stress conditions, apig-7-glucoside was the only flavonoid compounds that decreased over time (Figure 5b). Highly significant differences were found in summer berries phytochemically active compounds content under drought condition (P≤0.001). The interaction between time and compounds content also had a significant effect on the apig- 7-glucoside content (p≤0.001), where it gradually declined. Overall, apig-7-glucoside (81± 8mgkg-1) and umbelliferon (19±3mgkg-1) were the main compounds in both [Y2 WW] and [Y2 D], respectively (Figure 4).
Discussion
The total yield of some phenol and flavonoid compounds detected in chamomile plants such as caffeic acid, apig-7- glucoside, umbelliferon and luteolin were significantly increased and less affected by drought stress. However, most of the compounds found in yarrow varieties decreased under drought stress (Figure 4). This may be due to the fact that chamomile plants have small leaf structures which reduce water loss through transpiration or, it may be related to the deep root structures found in these plants [21]. Also, using and selecting different instrumental analysis such as HPLC and GC-MS for different plant parts is considered to be one of the main protocols to consider when trying to identify the maximum number of beneficial active compounds content especially for commercial medicinal plants [22-26].
Despite the successful methods used in this work, some difficulties for the detection of some compounds were observed. For example, chlorogenic acid was not observed in yarrow plants, which could be because of storage of the samples in a fridge or freezer for long periods of time or because of a lower level of limit of detection (chlorogenic acid= 0.46 mg L-1, caffeic acid= 1.49 mg L-1, apig-7-glucoside= 3.42mgL-1, umbelliferon= 0.11mgL-1, luteolin= 1.06mgL-1). Another problem can arise in detection, as very small concentrations of target compounds from extraction processes may lead to poor absorption by the detector.
Based on the current study, some compounds significantly increased over the time of vegetative growth under drought conditions. This is in agreement with several studies in the literature about the positive effect of abiotic stress on the accumulation of phenol compounds [27,28]. Regarding the development and increasing some active compounds under drought stress obtained in this current study, the results are also in agreement with those indicated by Salem et al. [29] who reported a significant increase of total phenolic acids content of Carthamus tinctorius (safflower) under water stress. Similarly, Kusvuran & Dasgan [30] showed that the levels of phenolic acid and flavonoid compounds detected in Phaseolus vulgaris L. (common bean) were increased under water stress conditions.
Moreover, it has been demonstrated that the content of flavonoid compounds such as kaempferol and quercetin of Arabidopsis thaliana L. (rockcress) were significantly increased after 14 days of water stress and drought stressed conditions compared with well-watered conditions [31]. Al Hassan et al.[32] revealed that under water stress the level of total phenol and flavonoid compounds content of Solanum lycopersicum (cherry tomato) leaves were increased. Also, Klunklin & Savage [33] demonstrated that the total phenolic compounds detected in tomatoes were significantly increased under drought stress compared with well-watered, in contrast total flavonoid compounds were decreased. Similarly, in the current study, it was clear that the total yield of caffeic acid detected in chamomile grown in the greenhouse was increased under drought stress. However, the content of flavonoid compounds such as apig- 7-glucoside and umbelliferon found in yarrow plants were significantly decreased under drought stress compared with well- watered conditions. This is possibly related to the mechanism of different enzymes activity as the most effective element under drought stress.
It has been confirmed previously that the total phenolic compounds content of Tridax procumbens (tridax daisy) leaves and flowers significantly increased under drought stress [7]. This result was supported in the current study where the total yield of caffeic acid detected in (Y2 D) increased under drought stress. However in (Y1 D) it decreased compared with well-watered conditions (Figure 4). The main reason for these differences may be linked to the variation of the mechanism of compounds biosynthesis of the two yarrow varieties; e.g. the reduction of the activity of some enzymes under drought stress, particularly those connected to the phenols and flavonoids biosynthesis [16].
Conversely, Król et al. [34] reported that all the levels of phenolic acid such as caffeic acid found in Vitis vinifera L. (grapevine) leaves were significantly decreased under drought stress conditions. Some differences were observed in yield components when plants were exposed to drought stress at different growth stages. Results from the present study of chamomile chlorogenic acid content under drought stress are also consistent with the results of Sofy et al. [35] who reported that the phenolic compounds content found in Hordeum vulgare (barley) shoots were decreased especially at the later stages of growth. This decrease could be either due to the reduction of plant hormones activity such as phenylalanine ammonia lyase during the biosynthesis of phenolic compounds or because of the osmotic stress. Furthermore, Moharramnejad et al. [36] showed that the accumulation of total phenolic compounds content of Zea mays L. (maize) was significantly decreased under drought stress.
Based on these investigations, the application of drought stress appears to produce a maximum yield of medicinal plant phenolic and flavonoid compounds content in chamomile and yarrow plant species. This could be important particularly in the area of marketing these materials. It is suggested that it is worth considering growing chamomile and yarrow plants in some dry areas [37]. This work still needs further investigation to improve and identify more phytochemical compounds whose content in plants can increase under drought stress conditions. This may be achieved through analysing these active compounds from different plant parts such as root, stem and seeds [38].
Conclusion
In summary, the identification of the main phenol and flavonoid compounds found in chamomile and yarrow leaves were analysed using HPLC-UV. These compounds were significantly affected by the applied water treatment and significantly changed under drought stress. For instance total yields of chlorogenic acid apig-7-glucoside and umbelliferon were found to be main compounds in chamomile and yarrow varieties, respectively under both well-watered and drought stress conditions. Results of this study showed that chamomile was highly drought resistant regarding the total yields of most compounds content compared with yarrow plants. In addition, the production of these compounds under drought stress is a good opportunity to produce some new commercial compounds able to cope with different environmental conditions.
Acknowledgement
The authors gratefully thank the technical support for sample analysis from the University of Plymouth. This study was also financially supported by Human Capacity Development Programme (HCDP) at the Ministry of Higher Education/ Kurdistan Regional Government.
References
- Hădărugăa NG, Hădărugăb DI, Tatuc C, Gruiac A, Costescua C, et al. (2009) Multivariate analysis (PCA) in Compositae biocompounds class. Journal of Agroalimentary Processes and Technologies 15(2): 201-210.
- Ahmadian A, Ghanbari A, Siahsar B, Haydari M, Ramroodi M, et al. (2011) Study of chamomiles yield and its components under drought stress and organic and inorganic fertilizers usage and their residue. Journal of Microbiology and Antimicrobials 3(2): 23-28.
- Farahani HA, Valadabadi SA, Daneshian J, Shiranirad AH, Khalvati MA (2009) Medicinal and aromatic plants farming under drought conditions. Journal of Horticulture and Forestry 1(6): 086-092.
- Saeidnejad AH, Kafi M, Khazaei HR, Pessarakli M (2013) Effects of drought stress on quantitative and qualitative yield and antioxidative activity of Bunium persicum. Turkish journal of Botany 37: 930-939.
- Salim EA, EL Hassan GM, Khalid HES (2014) Effect of spacing and seasonal variation on growth parameters, yield and oil content of mint plants. Journal of Forest Products & Industries 3: 71-74.
- Tátrai ZA, Sanoubar R, Pluhár Z, Mancarella S, Orsini F, et al. (2016) Morphological and Physiological Plant Responses to Drought Stress in Thymus citriodorus. International Journal of Agronomy p. 8.
- Gnanasekaran N, Kalavathy S (2017) Drought Stress Signal Promote the Synthesis of more Reduced Phenolic Compounds (Chloroform Insoluble Fraction) in Tridax procumbens. Free Radicals and Antioxidants 7(1): 128-136.
- Rajabbeigi E, Eichholz I, Beesk N, Ulrichs C, Kroh LW, et al. (2013) Interaction of drought stress and UV-B radiation-impact on biomass production and flavonoid metabolism in lettuce (Lactuca sativa L.). Journal of Applied Botany and Food Quality 86: 190-197.
- Cicevan R, AL Hassan M, Sestras AF, Prohens J, Vicente O, et al. (2016) Screening for drought tolerance in cultivars of the ornamental genus Tagetes (Asteraceae). Peerj 4: e2133.
- Fathi A, Tari DB (2016) Effect of Drought Stress and its Mechanism in Plants. International Journal of Life Sciences 10: 1-6.
- Saeedfar S, Negahban M, Mahmoodi SM (2015) The effect of drought stress on the essential oil content and some of the biochemical characteristics of anise hyssop (Agastache foeniculum [Pursh] Kuntze). Euro J Mole Biot 8(2): 103-114.
- Jeshni MG, Mousavinik M, Khammari I, Rahimi M (2015) The changes of yield and essential oil components of German Chamomile (Matricaria recutita L.) Under application of phosphorus and zinc fertilizers and drought stress conditions. Journal of the Saudi Society of Agricultural Sciences.
- Gharibi S, Tabatabaei B, Saeidi G, Goli S (2016) Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl Biochem Biotechnol 178(4): 796-809.
- Basu S, Ramegowda V, Kumar A, Pereira A (2016) Plant adaptation to drought stress. F1000Res.
- Blum A (2005) Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Crop and Pasture Science 56: 1159-1168.
- Farooq M, Hussain M, Wahid A, Siddique K (2012) Drought stress in plants: an overview. Plant responses to drought stress 1-33.
- Abdelmajeed NA, Danial EN, Ayad HS (2013) The effect of environmental stress on qualitative and quantitative essential oil of aromatic and medicinal plants. Archives Des Sciences 66: 100-120.
- Kirakosyan A, Kaufman P, Warber S, Zick S, Aaronson K, et al. (2004) Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiologia plantarum 121: 182-186.
- Nováková L, Vildová A, Mateus JP, Gonsalves T, Solich P (2010) Development and application of UHPLC-MS/MS method for the determination of phenolic compounds in Chamomile flowers and Chamomile tea extracts. Talanta 82(4): 1271-1280.
- Dias MI, Barros L, Dueñas M, Pereira E, Carvalho AM, et al. (2013) Chemical composition of wild and commercial Achillea millefolium L. And bioactivity of the methanolic extract, infusion and decoction. Food Chem 141(4): 4152-4160.
- Kouressy M, Dingkuhn M, Vaksmann M, Heinemann AB (2008) Adaptation to diverse semi-arid environments of sorghum genotypes having different plant type and sensitivity to photoperiod. Agricultural and forest meteorology 148: 357-371.
- Amber P, Akram A, Qureshi R, Akram Z (2012) HPLC analysis for secondary metabolites detection in Sclerotium rolfsii isolated from chickpea. Pak J Bot 44: 417-422.
- Higgs RE, Zahn JA, Gygi JD, Hilton MD (2001) Rapid method to estimate the presence of secondary metabolites in microbial extracts. Appl Environ Microbiol 67(1): 371-376.
- Hill CB, Roessner U (2013) Metabolic profiling of plants by GC-MS. The handbook of plant metabolomics, pp. 1-23.
- Khakimov B, Tseng LH, Godejohann M, Bak S, Engelsen SB (2016) Screening for triterpenoid saponins in plants using hyphenated analytical platforms. Molecules 21(12): 1614.
- Sato A, De Lima SS, Affonso VR, Esquibel MA, Lage CL (2006) Micro propagation of Chamomilla recutita (L.) Rauschert: A shock treatment model with growth regulators. Scientia horticulturae 109: 160-164.
- Hale BK, Herms DA, Hansen RC, Clausen TP, Arnold D (2005) Effects of drought stress and nutrient availability on dry matter allocation, phenolic glycosides, and rapid induced resistance of poplar to two lymantriid defoliators. J Chem Ecol 31(11): 2601-2620.
- Sharafzadeh S, Alizadeh O (2011) German and Roman Chamomile. Journal of applied pharmaceutical science 1(10): 1-5.
- Salem N, Msaada K, Dhifi W, Sriti J, Mejri H, et al. (2014) Effect of drought on safflower natural dyes and their biological activities. EXCLI j 13: 1-18.
- Kusvuran S, Dasgan HY (2017) Effects of drought stress on physiological and biochemical changes in Phaseolus vulgaris L. Legume Research: An International Journal p. 40.
- Mewis I, Khan MA, Glawischnig E, Schreiner M, Ulrichs C (2012) Water stress and aphid feeding differentially influence metabolite composition in Arabidopsis thaliana. PLoS One 7(11): e48661.
- AL Hassan M, Fuertes MM, Sánchez FJR, Vicente O, Boscaiu M (2015) Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not Bot Horti Agrobo 43(1): 1-11.
- Klunklin W, Savage G (2017) Effect on Quality Characteristics of Tomatoes Grown Under Well-Watered and Drought Stress Conditions. Foods 6(8): 56.
- Krül A, Amarowicz R, Weidner S (2014) Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) Under continuous of long-term drought stress. Acta Physiol Plant 36(6): 1491-1499.
- Sofy M, El-Monem MA, Sharaf AS, Osman M, Sofy A (2017) Physiological changes, antioxidant activity, lipid peroxidation and yield characters of salt stressed barely plant in response to treatment with Sargassum extract. International Journal of Advanced Research in Biological Sciences 4(2):90-109.
- Moharramnejad S, Sofalian O, Valizadeh M, Asgari A, Shiri M (2015) Proline, glycine betaine, total phenolics and pigment contents in response to osmotic stress in maize seedlings. Journal of bioscience & Biotechnology 4(3): 313-319.
- Farahani HA, Valadabadi SA, Daneshian J, Shiranirad AH, Khalvati MA (2009) Medicinal and aromatic plants farming under drought conditions. Journal of Horticulture and Forestry 1(6): 086-092.
- Rajabbeigi E, Eichholz I, Beesk N, Ulrichs C, Kroh LW, et al. (2013) Interaction of drought stress and UV-B radiation-impact on biomass production and flavonoid metabolism in lettuce (Lactuca sativa L.). Journal of Applied Botany and Food Quality 86: 190-197.






























