- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Exploring Microbial Electroactivity: From Skin Microbiota to Cable Bacteria in Microbial Fuel Cells
Yusra Zarlashat1*, Hassan Mushtaq2 and Syed Salman Shah3
1Department of Biochemistry, Government College University Faisalabad, Faisalabad, Pakistan
2Health Biotechnology Division, National Institute for Biotechnology and Genetic Engineering-C (NIBGE), Faisalabad, Pakistan
3Department of Biotechnology and Genetic Engineering, Hazara University, Mansehra, Pakistan
Submission:May 03, 2024; Published: May 16, 2024
*Corresponding author: Yusra Zarlashat, Department of Biochemistry, Government College University Faisalabad, Faisalabad, Pakistan, Email: yusrazarlashat@gcuf.edu.pk
How to cite this article: Yusra Zarlashat*, Hassan Mushtaq and Syed Salman Shah. Exploring Microbial Electroactivity: From Skin Microbiota to Cable Bacteria in Microbial Fuel Cells. Adv Biotech & Micro. 2024; 18(1): 555979 DOI:10.19080/AIBM.2024.18.555979
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Abstract
Bacteria with the remarkable ability to transport electrons both intra and extracellularly for renewable energy production and environmental remediation. Nowadays, some bacteria have applications in the field of environmental sciences as well as physics, involving the production of useful substances and electric current. Prokaryotes have the potential to use charged electrodes to donate and accept electrons. These bacteria use a mechanism namely extracellular electron transport (EET) for multiple purposes; small power sources, pollution remedy, water reclamation, and electrosynthesis. Researchers are currently working on bacteria having EET ability at a basic level and looking forward to remarkable applications in the future. Microbial fuel cells (MFCs) are recognized as the most potent candidate for future alternative energy production. Nanowires such as those produced by Geobacter sulfurreducens and Shewanella oneidensis, facilitate electron transfer over longer distances, enhancing the efficiency of bioelectricity generation. This review briefly explains the components of bacteria involved in the EET mechanisms for the production of electric current and the role of biofilm for electrogenic bacteria. It also highlights the different methods used to promote the EET mechanism and some unusual external electrons used recently in MFC.
Keywords: Electricity-Carrying Bacteria; Electrogenic Bacteria; Proton Motive Force, Extracellular Electron Transport; Biomass Concentration
Abbreviations: EET: Extracellular Electron Transport; MFCs: Microbial Fuel Cells; PMF: Proton Motive Force; DET: Directly Transferring Electron; EPS: Extracellular Polymeric Substances; EAB: Electroactive Bacteria
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Introduction
Bacteria capable of transporting electrons from the extracellular environment or through cell membranes are termed electricity-carrying bacteria [1]. Bacteria use both intra as well as extracellular systems linked with the acceptance or donation of electrons [2]. The electrons from organic or inorganic sources, are taken up by NAD brought to the cell wall, and donated to an electron acceptor [3]. Due to the accumulation of protons across the membranes, a proton gradient is generated known as the proton motive force leading to transport across the membrane, ATP production, and flagella movement [4]. Soluble protons are involved in this process which is pumped in and out of the membrane of bacterial cells leading to proton motive force (PMF). This is the reason bacteria are interacting for electricity production now [4].
Both yeast and bacteria were used to produce an electric current in an experiment by Potter in 1911 (Potter, 1911). Bioelectricity production from bacterial cells was first presented by him [5]. A great variety of bacteria is available in nature that is electrically active. Geobacter sulfurreducens and Shewanella oneidensis for microbial fuel cells (MFCs) first studied [6]. Both these strains of bacteria are studied in detail for being electrically active [7]. Pseudomonas and Clostridium bacteria are reported as exoelectrogens [8]. Rhodoferax ferrireducens are reported as having the potential to transfer electrons to an anode involving nano wires or c type cytochromes [9].
Electricity Production by using bacterial cells as a catalyst is done by the decomposition of biomass such as grass pieces, vegetables, food, fruit wastes, plant leaves, and mud [10]. Bioelectricity is produced by using microbial fuel cells and soil having industrial effluents i.e., brewery wastewater, colored wastewater, sludge, and ocean sediments [11-13]. Biofilm of mixed as well as pure bacterial cultures is used for electricity production via MFC [9].
Bacteria from human sources also can produce electricity, the largest organ of the human body “skin” is covered by many microbes that can transfer electrons efficiently to produce an electric current [14]. Enterococcus faecalis and Listeria monocytogenes both gram-positive bacteria can produce electric current as well as the mechanism for the transfer of electrons namely the EET pathway [15]. Both bacteria found in the gut of bacteria are now under study due to their ability for electric current production and their role in human health [16].
Electricity sources used by humans for many centuries are fossil fuels. As we know fossil fuels are renewable sources, but they require a lot of time for its formation and now worldwide these sources are depleting very fast due to the needs of the increasing population. Moreover, the production of electricity from these sources adds toxic gases and pollutants to the air to which scientists are looking for pollution-free sources [17]. Other renewable energy sources such as wind, geothermal, tidal, biomass, and solar are of great interest [18]. Still, now other sources of electricity production have not competed with conventional electricity production from fossil fuels but the hybrid system of solar energy with hydrogen fuel and solar energy with the wind will improve efficiency as well as electricity production [19]. These conversions of solar energy into bioelectricity and hydrogen are under study [17]. In this review, we will discuss history, electrochemically active bacteria, and mechanism of electron transfer, and potential applications of electricity-carrying bacteria.
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Electricity Carrying Bacteria
Both yeast and bacteria were used to produce an electric current in an experiment by Potter in 1911 (Potter, 1911). Many scientists have worked in the mid-20th century but at that time generation of current was not enough to be used for running any power machine. Two bacteria were reported at the same time in 1988 as having the ability to accept electrons by growing on solid metal oxides (manganese or iron) [20,21]. Shewanella facultative bacterium isolated from the Oneida Lake, N.Y was found capable of reducing manganese oxides and was tested in a laboratory where it reduces manganese by accepting its electrons [21]. Another delta proteobacteria namely Geobacter, oxygen-sensitive found in the Potomac River, N.Y was isolated and tested that it reduces iron oxides by accepting electrons [4].
Both bacteria are studied for three decades to explain the mechanism now known as extracellular electron transport (EET). This ability of these bacteria is different from all other microbial worlds [20]. All the energy-producing biosystems work on the same principle which is electron flow involving in the conservation of energy in soluble electron acceptor and donor in the biological membranes having minimum chances of losing an electron to the exterior of the cell [4]. In Shewanella species, there is a series of proteins having multiheme groups involving in the conduction of electrons [22,23]. This mechanism through these linked proteins involves the movement of electrons across the membrane and outer substrates (Figure 1). In different strains of Shewanella extracellular electrons, transport occurs by the different mechanism which includes compounds involved in endogenous electron shuttling, reduction by exogenous, direct reduction and reduction with the help of nanowires along the membranes in the form of cytochromes [24,25] (Figure 1).
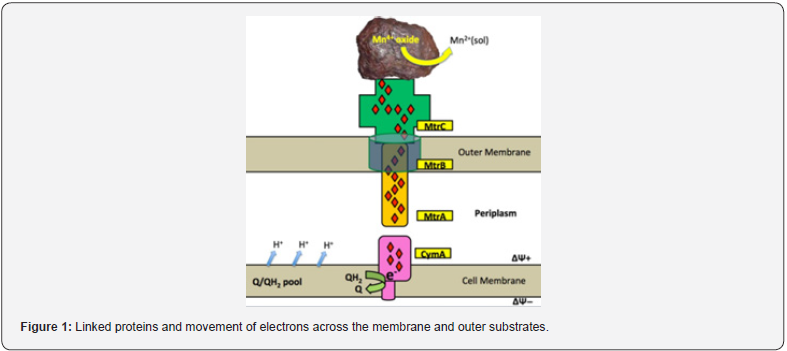
Geobacter species case it is found that there is physically utilization of c-type cytochromes with multiheme groups, but pili are involved in the process of conduction of electrons without any c-type cytochromes [26]. These bacteria have no c-type cytochromes but still, there is extracellular electron transport in them which means that there is another extracellular electron transport (EET) mechanism that needs to be discovered [27]. Both bacteria elaborated above are the first bacteria named “electric bacteria”. Before the experimental study of Dr. Byung‐Hong Kim [28] about EET, none of the electrically active bacteria was given attention. In his experiment S. oneidensis MR‐1 was studied which showed the production of electric current without any electron shuttle system. Many scientists in different laboratories of the world worked on this experiment and lead to the production of current [29,30]. Another experiment has reported that microbes can accept electrons from electrodes for their maintenance and growth. This process also involves the isolation of microbes from different environments with the help of electrodes and c-type multiheme cytochromes [31].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Electrochemically Active Bacteria
The natural environment is enriched by electrically active microbes. Sources of microbes include brewery wastewater, sludge, ocean sediments, dairy manure, and natural ecosystems. Microbial fuel cells can grow under anaerobic conditions, digestive sludge, rumen liquids, granular sludge, and domestic wastewater [13].
α-Proteobacteria
Acidiphilium cryptum from Rhodospirillales acetobacteraceae class is a gram-negative bacterium isolated from the drainage of mine water. These bacteria under acidic conditions are the first electrically active bacteria for microbial fuel cells [32]. Another gram-negative bacterium namely Rhodobacter sphaeroides from class Rhodobacteraceae, Rhodobacter uses different acids as a substrate to produce electric current [33]. Rhodopseudomonas belongs to α-proteobacteria is first electrically active from this class [13]. Gluconobacter oxydans a gram-negative bacterium reported in 2002, uses carbon dioxide as a substrate to produce electricity and it belongs to group Acetobacteraceae and Gluconobacter [34].
β-Proteobacteria
R. ferrireducens facultative, gram-negative bacterium belongs to class Comamonadaceae. It uses Fe (III) as an electron acceptor and fully oxides glucose to carbon dioxide at a temperature ranging from 25-30 ºC. From this class, it is the first bacteria that was reported as having the ability to oxides glucose completely to carbon dioxide and use its energy for electricity generation [13]. Comamonas denitrificans belong to denitrifying bacteria having the potential to yield electricity [13,35].
γ-Proteobacteria
Shewanella a facultative, anaerobic gram-negative bacterium belongs to Shewanell aceae class. It has been reported as a reference in MFC (microbial fuel cell). Visualization of electron transfer can be seen among the bacteria and electrodes. S. putrefactions IR-1 first electrically active bacterium reported as having the potential to accept electrons from electrodes [36]. Pseudomonas aeruginosa gram-negative, an aerobic facultative bacterium from class Pseudomonadaceae produces Pyocyanin as an electron acceptor not only for itself rather than for other strains during electricity production. It is the first bacterium that has an electron shuttle system [37]. Klebisella pneumonia is a gram-negative bacterium having the ability to oxidize different kinds of organic matter to produce electric current with the help of electrodes as an electron acceptor [38].
δ-Proteobacteria
G. sulfurreducens is an anaerobic gram-negative bacterium that uses Fe (III), Co (III)- EDTA, malic acid, and fumaric acid as an electron acceptor while hydrogen and acetic acid electron donors [39]. Sequencing of the whole genome of this bacterium revealed that it can be used as a reference bacterium to explain the mechanism by which they transfer electron from electrodes [13]. Geobacter uses iron as an electron acceptor and can reduce the radioactive pollutants from the environment such as benzene, short-chain fatty acids, ethanol, etc. That is why it is used as an eliminator of environmental pollutants [40]. Geopsychrobacter electrodiphilus a gram-negative bacterium has the potential to produce electric current by completely oxidizing citric acid, acetic acid, fumaric acid, and malic acid [41]. Desulfoblbus propionicus is also a gram-negative bacterium but with very low current production as compared to other bacteria [42].
ε-Proteobacteria
There is a production of 296mW/liter power by the two strains of genus Arcobacter that can grow in highly enriched acetate-fed MFC [43].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Mechanism of Electricity Production
Extracellular Electron Transfer
Mechanism of extracellular electron transfer (EET) can be explained by the following steps:
1) Directly transferring electron (DET) using nanowire or through direct contact.
2) Linked shuttle that may be endogenous or exogenous.
3) Extracellular polymeric substances (EPS) of biofilms [44] (Figure 2).
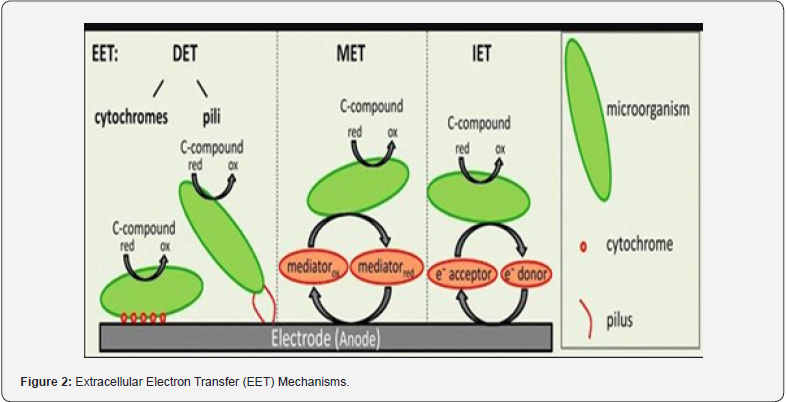
G. sulfurreducens and S. oneidensis have ability to transfer electron across the biological membranes with the help of c-type heme-containing cytochromes regarding DET. This cytochrome has multiheme with different redox potentials due to microorganism transfer electron across membranes [45]. In the case of MES mode flow of electrons in a cell is against the concentration gradient from low to high potential while opposite in the case of MFC. Moreover, DET is under experimental study due to hydrogenases and Rusticyanin protein [46,47]. S. oneidensis and G. sulfurreducens have extracellular appendages namely pili sometimes called nanowires that connect two bacteria due to which they can accept and donate an electron to solid surfaces at greater distances [48].
DET depends on the existence of biofilm to produce electricity and connection in the extracellular environment for the successful transfer of an electron between in and out of the cell [49]. In a biofilm, all the cells work collectively to transfer electrons and its thickness is directly proportional to the current production [48]. Electroactive bacteria (EAB) and formation of biofilm have great importance in the biochemical production of current. Soluble inert shuttle molecules in the MET are involved in the transport of electrons in and out from acceptor to donor with higher tendencies of electron transfer even at higher distances [2].
Mediators also help in the transfer of electrons across membranes. These mediators may be natural or artificial. S. oneidensis and P. aeruginosa secrete flavins, phenazines respectively which are natural mediators [2]. IET is based on the production of chemicals such as fumaric acid and hydrogen which will serve as an electron acceptor and donor in microbes. Microbes produce metabolic substances which are also involved in the transfer of an electron between microorganisms and the electrode. Irrespective of the mediators in MET there is an irreversible redox process due to electron transfer shuttling compounds. Electrically active bacteria are extended by the IET. Genetic tools are a great source that helps to study the cellular metabolism for the maintenance of efficiently working strains [2]. Genetic tools allow us to do desired modification with the help of which electron transfer across membranes is controlled [2].
Role of Nanostructured Material in EET
MFC and MES technologies were developed as a result of the discovery of bacterial bidirectional EET. However, several intrinsic challenges, such as low biofilm conductivity and weak bonding of bacteria to an electrode, and a lack of knowledge of the twoway EET mechanism, keep these technologies from becoming widely used. Biofilm ability is thought to be a key aspect of the EET process’s efficiency [50]. Unfortunately, biofilm conductivity alone is insufficient to efficiently transmit electrons to and from an electrode. To improve the bidirectional EET process, material scientists have recently begun to use recently manufactured nanostructured materials such as nanotubes of carbon, inorganic nanomaterials, semiconductors, conducting polymers, grapheme, and noble metal nanoparticles as bioanodes and biocathodes (Kalathil & Pant).
The use of nanostructured materials, in particular, has resulted in remarkable modifications in two ways EET process. Biofilm formation is a vital step in both MFCs and MES, a previous understanding of the basic mechanism required in bacterial adhesion to metal surfaces. The bacterial adhesion to diverse nanostructured surfaces has been studied in several ways, including theoretical and experimental studies. All of the advantages of employing such nanomaterials to improve electron transfer in BESs, as well as existing problems and future potential, are highlighted (Kalathil & Pant). In the ambient environment, thin-film devices have been built from protein wires extracted from G. sulfurreducens bacterium. The current density is approximately 17 microamperes per square centimeter. These devices provide approximately 0.5 volts on a 7-micron thick film [51].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
Biofilm Formation
A biofilm is a collection of bacteria encased in a complex, self-produced polymeric matrix that adheres to living or living surfaces [52]. Electroactive biofilms, on the other hand, are those that may respire final electrons released from metabolism on surfaces of the electrode. A single bacterial species can build a biofilm [53] or through multiple biofilms [54]. To generate power more efficiently in MFCs, electroactive biofilms are required. The amount of current production in MFCs is directly linked to biomass concentration in biofilm and the type of surface of the electrode. Electroactive biofilm prefers anode surfaces that are positively charged and hydrophilic [55]. The basic principle of microbial fuel cell creation also influences the performance of fuel cells [55].
Compare to bacteria that can form thin biofilms on the anode, bacteria that can create thick biofilms generate higher current densities e.g. G. sulfurreducens, develops a large biofilm with multiple layers (50 mm) is formed, and the gram-positive bacterium Thermincola ferriaceta also forms a thick biofilm (38 mm). On the other hand, the potent Thermincola and Clostridium ljungdahlii form a thin monolayer biofilm, resulting in low current density [27]. However, very thick biofilm deposition also restricts electron flow. As a result, thickness is advantageous for generating high current densities. As previously stated, electro-active biofilm is characterized by its ability to transmit electrons to electrode surfaces or to minimize the concentration of soluble as well as insoluble electron acceptors. These proteins have active redox potential (e.g., c-type cytochromes) [56].
The growth and activity of electroactive biofilms can be influenced by certain ions or minerals. Aside from having similar effects on different microbes, the same ions affect different microorganisms differently. The increased amount of Ca2 ions in the bio electrochemical system’s anolyte, for example, has proven lethal to exoelectrogens (mixed culture from anaerobic sludge) [57]. The study found that increasing the Ca2 concentration to 5 mM reduced the current output of the system by 72 percent when compared to the control system, which can be attributed to the buildup of non-active bacterial cells in the biofilms [57]. In contrast, adding CaCl2 (concentration of 1400 mM) to a mini MFC inoculated with S. oneidensis increases the density of current by about 80% in comparison to reference MFC which was primarily due to biological factors rather than ionic effects [58].
Furthermore, calcium ions encourage Shewanella xiamenensis for the production of EPS (extracellular polymeric substances). Furthermore, calcium ions encourage S. xiamenensis to produce EPS (extracellular polymeric substances). The structure of flagella and the cell membrane is influenced by the ions which stimulate the generation of EPS even more. At a 2 mM CaCl2 concentration, the EPS yield rose from 0.56 g/L to 1.74 g/L at a 20 mM CaCl2 concentration. The study went on to look at the influence of calcium ions on the production of current in an MFC and discovered that calcium ions had a beneficial impact on MFC performance, producing approximately 20% more current than the reference MFC [59]. What is in the environment that could be a rich source of exoelectrogens? Typically, anaerobic sediment, primary industrial effluent, sludge from industrial wastewater treatment plants, and even the soil contain exoelectrogens that can be extracted as pure or impure culture from the particular sources and used them in MFCs [60].
Effect of Certain Ions and Minerals in Biofilm Formation and Activity
The activity of electrically active biofilms is affected by the minerals as well as ions. It is reported that the same ions may have different effects on different strains of microbes. Higher the level of calcium ions in the anodic electrolyte have proven dangerous for the electrical activity of exoelectrogens. It is reported in an experimental study that only a 5mM concentration of calcium ions leads to a reduction of 72% current production as compared to a controlled system [57]. The addition of 1400mM Calcium chloride solution to the biofilm of S. oneidensis also increases the current production by 80% in comparison to controlled MFC [58]. The addition of calcium chloride is also involved in the increased synthesis of extracellular polymeric substances in S. xiamenensis, done by affecting the structure of flagella and membranes of the bacterial cell. With the increase of calcium chloride solution’s concentration production of extracellular polymeric substances also increases. When studied the effect of calcium on MFC to produce current it is revealed that it increases its production by 20% [59]. Sources for the utilization of MFC include industrial effluents, wastewater treatment, sludge, and soil [60].
Nanowires
Nanowires help in the transfer of electrons along long distances due to the thickness of biofilm [48]. When there is no oxygen or less amount of oxygen in the environment bacteria use it as an end point for electron acceptor [61]. Bacterial electron shuttle, unknown components, and proteins help in the transfer of electrons in making biofilm electrically active [52]. Due to the deficiency of pilA and omcZ in G. sulfurreducens bacteria, there is inhibition of biofilm formation as a result of which electric current also reduces [52]. PilA belongs to IV pili, has two segments; long PilA and short PilA [61]. It is reported that the long PilA segment is more important used to attach cells to graphite electrodes to form biofilms as compared to the short PilA [62]. However, short PilA is involved in the conductance of electrons across membranes via c-type cytochromes and OmcZ in the outer membrane [62].
It is also revealed from another study that pili do not involved in the transfer of electrons in the cells transpiring near to electrode, but it helps in the aggregation of cells that ultimately leads to the formation of biofilm [63]. It also promotes the formation of a thicker layer of biofilm formation by involving a series of a network of cytochromes [63]. When G. sulfurreducens grow in the form of biofilm certain genes are reported as compared to single cells [64]. Genetic studies have revealed that there is the involvement of genes that are coding pilus. It also revealed the role of these certain genes in the production of extracellular polymeric substances for biofilm formation and cyclopropane fatty acids [64]. Modification in the structure of biofilm occurs due to the presence of a sugar matrix that alters the receptor site and helps in the attachment of c-cyts [65].
It is reported that the morphology and structure of biofilm is associated with the growth phases of microbes. G. sulfurreducens in lag phase has a single layer of cells with less amount of c-Cyts due to which there is low production of current but when there is biofilm formation with three to four-fold more c-Cyts leading to high production of electric current [66]. Level of c-Cyts in G. sulfurreducens increases with the lag phase proceeding to log phase due to the increased concentration of c-Cyts [66]. Thicker the biofilm greater the transport of electrons and vice versa [67]. The morphology of biofilm greatly varies with the change of electrode in MFCs. G. sulfurreducens form layers of cells with pillars when grown on carbon cloth [64].
The structure of biofilm varies from gram-negative bacterium to gram-positive bacterium, which is based on the working principle of MFC, which may work as an open or closed-circuit system. It is reported that when microbial fuel cells behave as the closed system there is more attraction between bacteria and anode and vice versa in the case of an open system. Similarly, microbial fuel cells on the surface of bacteria are more easily available than the lower ones [1]. Biofilm formation by S. oneidensis MR-1 on the surface coated with minerals is experimentally treated with cyclic dinucleotide messenger. A phosphodiesterase namely pdeB has shown a negative effect on the formation of biofilm [68]. Deletion of this factor enhanced the production of biofilm formation under controlled conditions. When studied wild and mutant types of strains it is revealed from the results that the former leads to the formation of biofilm up to 10mm thick while 2nd one leads to two folds increase [68].
S. oneidensis MR-1 is very sensitive to the electron acceptors and becomes motile protein kinase in case of response to electron acceptors [69]. Pilin genes in Shewanella spp., are involved in the synthesis of a protein called Mannose-sensitive hemagglutinin which enhances its attachment and biofilm production ability [58]. As we know that biofilm formation in this species is based on c-Cyts which also secretes flavin useful for the acceptance of electrons. These help in the transfer of an electron from the inner surface to the outer side of the cell [69].
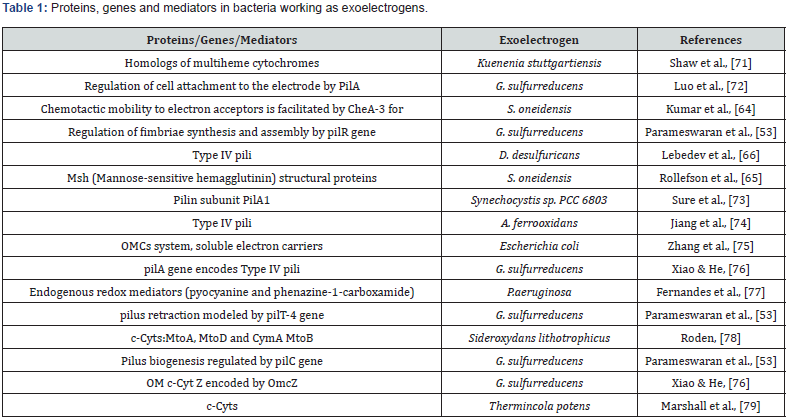
Desulfovibrio desulfuricans play an important role in the formation of biofilm in MFC which is termed as nanowires which leads to easy transfer of an electron to the anode. These nanowires are very tightly bound to the electrode leading to thicker electrically active biofilm formation by this bacterium [70]. First, it was found that the block that the exchange of water molecules on the nanowire–to-air interface due to the thickness of the upper interface of the nanowire film is put, the energy, while the removal of these density restores a continuous amount of power, and in the second case, an increase in the rate of exchange of water molecules is due to an increase in the relative humidity, respectively, and increases the amount of electrical energy, which is also reversible [51] (Table 1) [71-79].
Ion Channel
The investigation of microbial ion channels had also yielded valuable information related to neuronal signaling structures [80]. Electrical signaling is widely used in biological processes to communicate. Ion channels directly alter the action potential in neurons, which is one of the most well-known examples. For several years, research on microbial ion channels has supplied valuable information into the morphological basis of such neuronal signaling [81]. The prokaryotic K ion channel KcsA, in particular, generated the information related to structure for elasticity and selectivity of ions [80].
Microbes have severally significant classes of ion channels: Na channels, Cl channels, Ca-gated, K channels, and ionotropic glutamate receptors just like neurons [82]. Moreover, the native function of such ion channels in microbes is still unknown. Despite previous efforts to reveal ion channel role in microbes, in acid resistance reaction and regulation of fluid have been recognized, ion-specific channels do not seem so to be entirely associated with these biochemical functions. This is the reason that leads to doubts about another specific role of ion channels in prokaryotes [80].
Bio Surfactant
Surfactants belong to a group of very reactive compounds, they may be synthetic as well as natural leading to affect the efficiency of the MFC by different factors. Rhamnolipids and sophorolipids are the only two biological surfactants that are included [83]. It is reported that these bio-surfactants affects the attachment of biofilm, composition, structure as well as the survival of electrode due to interruption of electron transfer [84]. Brevibacillus 1 and Brevibacillus 2 are both species are reported as biosurfactants that can produce an electric current. Production of electric current by these biosurfactant bacteria ranges between 55 to 65 mW cm−2 [83].
Electron transfer and power output of MFC have been increased by improving the production of biosurfactants. Rhamnolipids synthesis in P. aeruginosa was increased by over expressing the gene rh1A and this leads to enhance the flow of electrons through the electron shuttle. It also enhanced the attachment of bacteria to the anode. This genetically modified strain gave us 2.5 times more production of electric current than that of the original strain [85]. Now after this scientists are working on exogenous surfactants which have proved more effective to produce electricity by MFC. Tween 80, EDTA, and polyethyleneimine are used as surfactants to improve the conduction of MFC and EET in bacteria [86,87]. As chemicals surfactants have side effects as well such as toxic to bacteria cells or leads to death of the bacterial cell [86,88]. That is why the addition of chemical surfactants to MFC needs to be improved to prevent bacterial death. As compared to these chemical surfactants the biosurfactants produced by bacteria are less toxic for MFC. Rhamnolipids and sophorolipid both are good biosurfactants that enhanced the performance of MFC and EET [88,89].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Electricity Production by Gut Bacteria
E. faecalis and L. monocytogenes both gram-positive bacteria can produce electric current as well as the mechanism for the transfer of electrons namely the EET pathway [15,90]. Both bacteria found in the gut of bacteria are now under study due to their ability for electric current production and their role in human health [16]. Series of experiments are conducted by scientists to explore the EET mechanism by other gram-positive bacteria [91].
Firmicutes and Bacteroidetes phyla both facultative anaerobes are a group of gram-positive and gram-negative bacteria found in the gut of a human. Due to their facultative nature, these bacteria grow in less oxygen but use high amount of nutrients leading to more and more EET processes [92,93]. Gut microbes are of great interest after the discovery of electron transfer pathways in them [94]. Five gut bacteria namely Lactobacillus rhamnosus, E. faecalis, Lactobacillus reuteri, Streptococcus agalactiae, and Staphylococcus aureus are reported as having the ability of EET pathway. E. faecalis, S. agalactiae, and S. aureus are reported as having the ability to transfer electrons and produce electric current as much as well-known S. oneidensis gram-negative bacteria. After this, experiments are conducted to evaluate the genes in S. aureus that are involved in electrogenicity [94].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Electricity Production by Skin Bacteria
The largest organ of the human body “skin” is covered by many microbes which can transfer electrons efficiently to produce electric current [14]. Staphylococcus capitis and Staphylococcus epidermidis found on the skin can produce electric current and that can be compared with the highly electrogenic gram-negative bacteria [95]. It is believed that bacteria produce electrons in the inner side of the membrane having an acceptor on the extracellular environment. Bacteria oxidize substances such as acetate to produce electrons which then transfer to other bacteria or metal ions i.e., manganese ion, ferric ion [96,97]. Above listed gram-positive gut bacteria when studied have revealed that they synthesize a special type of proteins that are involved in the process of EET leading to an increase in the bacterial growth rate [98,99].
Ferrozine assay is used to assess the electrogenic property of S. epidermidis skin bacteria. It is reported this bacterium is more electrogenic in the presence of glycerol fermentation and the addition of 5-methyl furfural leads to stop the process of fermentation because of which electricity production also diminishes [100]. S. epidermidis and Staphylococcus hominis when exposed to only 2% glycerol for a period of 20 min, an increase of 3 mV is noticed [100].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Electricity Production by Cable Bacteria
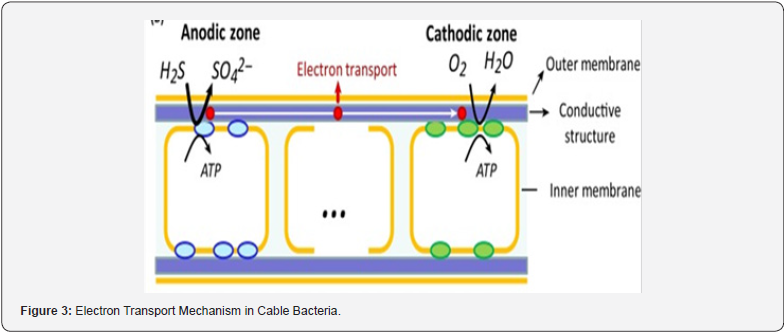
Newly discovered cable bacteria are reported as having the ability to produce electric current and can run it along their whole filamentous body [101]. They produce electric current by oxidizing sulfide in the deep layers of sediments and reducing oxygen at the surface of sediment-water [102,103]. Cable bacteria have been discovered at the anaerobic interface in a variety of aquatic sediment environments, including water bodies, as of their discovery [104,105], freshwater [106], and aquifer [107] ground water. Cable bacteria have a significant impact on the elemental cycling of sulfur, iron, methane, and phosphorus in such environments [108-111].
Furthermore, cable bacteria were discovered connected to the anode of an anoxic planktonic microbial fuel cell. [112] or in connection with oxygen-rich areas around plant roots and insect tubes in water bodies [111,113]. Cable microorganisms are members of the Desulfobulbaceae family, which also includes planktonic sulfate-reducing bacteria and sulfur- disproportioning bacteria [114]. The filament of the cable microbe is linear and usually consists of hundreds of cells. Though its cells are differentiated by an inflexible septum, they communicate a cytoplasmic space which includes a system of conductive fibers that operate along the longitudinal direction of the filament. [103,115,116] and are linked among both cells by a cartwheelshaped framework inside the septum [117,118]. The cell division process in cable microorganisms seems to be very similar to the Gram-negative reference microbe E. coli [114] (Figure 3).
Long-Distance Electron Transport in Cable Bacteria
Microscopy demonstrates that cable microbes polymerize to form fibers composed of elongated chains of cells extending up to 30-70 mm in length and containing more than 104 cells. These long microbial fibers are naturally slightly bent in the outer surface of water bodies, under which they form dense filament systems [101].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Microbial Fuel Cell
The microbial fuel cell (MFC) is now the most popularly used bacterial EET method, in which bacteria produce electricity by using electrons extracted from the EET [119,120]. MFCs use microbes as an enzyme to metabolize biological molecules such as non-carbon materials like sulfur compounds and plant material including, fruit wastes, food wastes, grass pieces, plant leaves, edible wastes, and muds to generate electricity [121]. As an electron donor for energy production, numerous simple to different substrates has been used. With varying efficiencies, these include ribose and, galactose, acetate, whey, sucrose, xylose, molasses, cellulose, and glucose [122,123]. A few researchers demonstrated that hydrogen can be produced efficiently in MFC, and is used in the mechanism for electricity supply and wastewater treatment. Hydrogen can be used to reduce carbon emissions because it is compatible with both burning and electrochemical processes for electricity production. There are various methodologies for hydrogen production, including water electrolysis and bacterial production [121].
The electricity produced from waste materials, energy production in MFCs can be boosted in several ways. The types of electrodes, electrode dimensions, proton exchange membranes, and other factors all have an impact on electricity production. An appropriate trial on ammonium-treated carbon electrodes was conducted to enhance output power. In this experiment, the anode treated with ammonium is dependent on two distinct factors that influence energy production, like power plant startup, high microbial bond strength, and improved of electron transfer ability to the exterior by microbes. Because of the ammonium treatment, electron transfer has been enhanced [124].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Electricity Production by Using MFC
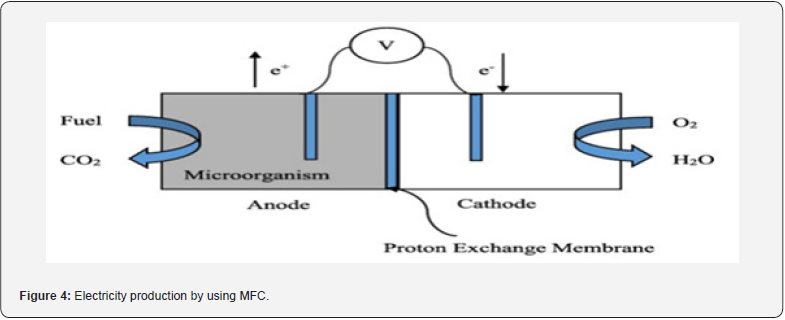
Components of MFC are the anode, cathode, external wire, and membrane sensitive to protons. Anaerobic a well as aerobic conditions are set at the anode and cathode, respectively. The communicator can be used or not for the working of MFC. Bacteria are also added to aid in the process of oxidation for microbial fuel cells [125]. Suitable substances are given to the bacteria at the anode which anaerobically metabolizes them and releases an electron. These electrons are then transferred to the cathode through an external wire to generate electricity. Electrically active bacteria break down organic substances and covert chemical energy released from bonds into electrical energy. MFCs are of different designs that may be stacked MFC, single or twochambered MFC [126]. There is a special type of membrane called proton exchange membrane which separates two electrodes and helps in the movement of an electron across cathode for the production of electricity [127] (Figure 4).
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Types of MFCs
Single Chamber MFC
Anode and cathode in MFC with a single chamber may or may not be separated by a membrane. A separating membrane is occasionally linked with a cathode [128]. Electrons produced by the oxidation of carbon compounds at an anode are transferred to the cathode via an external circuit [129]. The presence of cathode is more important in this type of MFC due to its role in oxidation at pH 7 [130]. There is low liquid volume in the case of air cathodes used in this type of MFC while the use of smallsized air cathodes has few drawbacks for output power due to the nature of inoculum, electrode spacing, and nature of PEM [10].
Two-Chamber MFC
Mostly used microbial fuel cells are in the form of this type. In this type, there is the aerated cathode and anaerobic anode. A salt bridge or proton exchange membrane is used to connect these two electrodes. At the anode, formation of biofilm under anaerobic conditions is facilitated by microbes and it is aerobic in the cathode chamber [131]. Positive ions move from the cathode towards the anode leading to a decrease in pH at anode whole increase at the cathode which tends to decrease the electrical potential at the electrode [132]. The performance of MFC is controlled by many parameters like pH, flow rate, nature of electrodes, and external flow [133,134].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
MFC and Electricity Carrying Bacteria
R. sphaeroides could grow in aerobic as well as the anaerobic environment. These bacteria when grown in the presence of light under anaerobic conditions they form photosynthetic apparatus in the cytoplasm of the cell to produce electric current [17]. Rhodobacter capsulate [135], Rubrivivax gelatinosus [136], Rhodopseudomonas faecalis [137], Rhodopseudomonas palustris are photosynthetic microbes commonly involved in the production of hydrogen [138,139]. R. sphaeroides is reported as having electrical activity greatest of all [17]. Klebsiella sp. is a facultative anaerobe that can generate electric current by consuming substrates food wastes, glucose, and sucrose [140- 142]. It also can degrade RB19 for the production of electric current. Klebsiella sp. C is involved in the synthesis of mediators that help in the production of electric current [143].
P. aeruginosa is used as a catalyst in the double chamber MFC that uses substrates fructose, sucrose, and glucose to produce an electric current. It uses pentoses and hexoses via anodic respiration to produce power. Bacteria have high affinity in the case of glucose than sucrose and fructose (Ali et al.,). Mesophilic Aeromonads grow in water bodies and are facultative anaerobes. Aeromonas hydrophila bacteria are involved in the breakdown of chitin due to the presence of enzymes Endo-chitinase and b-Nacetylglucosaminidase. These enzymes lead to more degradation of chitin because of which more power is generated [128] (Table 2).
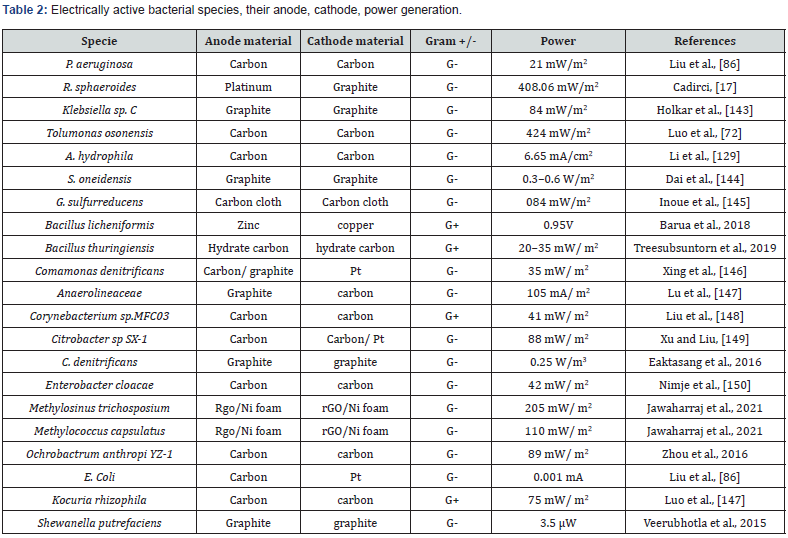
S. oneidensis is reported as having electrical activity and is involved in the production of electric current [144-150]. Bacillus and Klebsiella both strains can produce electric current [151]. Organic carbon content in the soil is also one of the major factors which influence the production of electric current [11].
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
Conclusion
Shewanella oneidensis and Geobacter sulfurreducens demonstrate pioneering electricity-carrying bacteria, showing complex electron transfer mechanisms involving multiheme proteins and unique extracellular electron transport capabilities, respectively. The diverse range of bacteria, from gut microbes like E. faecalis and L. monocytogenes to skin bacteria like Staphylococcus capitis and Staphylococcus epidermidis, as well as newly discovered cable bacteria, highlight the potential of microbial electrogenesis across various environments [152- 160]. Electricity-carrying bacteria including Staphylococcus capitis, Staphylococcus epidermidis, and cable bacteria, exhibit diverse mechanisms for bioelectricity production, ranging from skin microbiota to deep sediment environments. Their potential in MFCs highlights a promising avenue for sustainable energy generation from various substrates, underscoring the importance of harnessing microbial electrogenic properties for future energy needs. Electro microbiology is generally an emerging field of biology and microbiology, with a wider range of new developments and ever-growing discoveries. There are many other potential applications currently under study [161-167]. However, we need new methods that can meet our sustainable system requirements. The field of electro microbiology can provide us with some useful and exciting tools for finding a sustainable future. The production of bioelectricity by conversion of organic waste into useful energy through well-organized wastewater treatment is an effective way, which can be used as an alternative energy source to substitute non-renewable energy.
- Review Article
- Abstract
- Introduction
- Electricity Carrying Bacteria
- Electrochemically Active Bacteria
- Mechanism of Electricity Production
- Biofilm, Nanowire, Ion channel and Bio Surfactant Formation Leads to Increased Current
- Electricity Production by Gut Bacteria
- Electricity Production by Skin Bacteria
- Electricity Production by Cable Bacteria
- Microbial Fuel Cell
- Electricity Production by Using MFC
- Types of MFCs
- MFC and Electricity Carrying Bacteria
- Conclusion
- Author Contribution
References
- Read ST, Dutta P, Bond PL, Keller J, Rabaey K (2010) Initial development and structure of biofilms on microbial fuel cell anodes. BMC Microbiol 10: 98.
- Sydow A, Krieg T, Mayer F, Schrader J, Holtmann D (2014) Electroactive bacteria-molecular mechanisms and genetic tools. Appl Microbiol Biotechnol 98(20): 8481-8495.
- Mukhaifi EA, Abduljaleel SA (2020) Electric bacteria: a review. J Adv Lab Res Bio 11(1): 7-15.
- Nealson KH (2017) Bioelectricity (electro microbiology) and sustainability. Microbial Biotechnol 10(5): 1114-1119.
- Bullen RA, Arnot TC, Lakeman JB, Walsh FC (2006) Biofuel cells and their development. Biosensors Bioelectronics 21(11): 2015-2045.
- Dolch K, Danzer J, Kabbeck T, Bierer B, Erben J, et al. (2014) Characterization of microbial current production as a function of microbe-electrode-interaction. Bioresource Technol 157: 284-292.
- Bücking C, Schicklberger M, Gescher J (2013) The biochemistry of dissimilatory ferric iron and manganese reduction in Shewanella oneidensis. In Microbial Metal Respiration. Springer, Berlin, Heidelberg, pp. 49-82.
- Li M, Zhou M, Tian X, Tan C, McDaniel CT, et al. (2018) Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol Adv 36(4): 1316-1327.
- Ali N, Anam M, Yousaf S, Maleeha S, Bangash Z (2017) Characterization of the electric current generation potential of the pseudomonas aeruginosa using glucose, fructose, and sucrose in double chamber microbial fuel cell. Iran J Biotechnol 15(4): 216.
- Cai T, Meng L, Chen G, Xi Y, Jiang N, et al. (2020) Application of advanced anodes in microbial fuel cells for power generation: A review. Chemosphere 248: 125985.
- Jiang YB, Zhong WH, Han C, Deng H (2016) Characterization of electricity generated by soil in microbial fuel cells and the isolation of soil source exoelectrogenic bacteria. Front Microbiol 7: 1776.
- Samsudeen N, Radhakrishnan TK, Matheswaran M (2015) Bioelectricity production from microbial fuel cell using mixed bacterial culture isolated from distillery wastewater. Bioresource Technol 195: 242-247.
- Zhang YC, Jiang ZH, Ying LIU (2015) Application of electrochemically active bacteria as anodic biocatalyst in microbial fuel cells. Chinese J Anal Chem 43(1): 155-163.
- Grice EA, Segre JA (2011) The skin microbiome. Nature reviews microbiology 9(4): 244-253.
- Light SH, Su L, Rivera-Lugo R, Cornejo JA, Louie A, et al. (2018) A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562(7725): 140-144.
- Du Toit A (2018) Exporting electrons. Nature Rev Microbiol 16(11): 657-657.
- Cadirci BH (2018) An electricity production study by Rhodobacter sphaeroides. Int J Hydrogen Energy 43(38): 18001-18006.
- Panwar NL, Kaushik SC, Kothari S (2011) Role of renewable energy sources in environmental protection: A review. Renew Sustain Energy Rev 15(3): 1513-1524.
- Khare V, Nema S, Baredar P (2016) Solar-wind hybrid renewable energy system: A review. Renew Sustain Energy Rev 58: 23-33.
- Lovley DR, Phillips EJ (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54(6): 1472-1480.
- Myers CR, Nealson KH (1988) Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240(4857): 1319-1321.
- Clarke TA, Edwards MJ, Gates AJ, Hall A, White GF, et al. (2011) Structure of a bacterial cell surface decaheme electron conduit. Proc Natl Acad Sci 108(23): 9384-9389.
- Richardson DJ, Butt JN, Fredrickson JK, Zachara JM, Shi L, et al. (2012) The ‘porin-cytochrome’model for microbe‐to‐mineral electron transfer. Molecular Microbiol 85(2): 201-212.
- El-Naggar MY, Finkel SE (2013) Live wires. Scientist 27(5): 38-43.
- Pirbadian S, Barchinger SE, Leung KM, Byun HS, Jangir Y, et al. (2014) Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc Natl Acad Sci 111(35): 12883-12888.
- Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, et al. (2011) Tunable metallic-like conductivity in microbial nanowire networks. Nature Nanotechnol 6(9): 573-579.
- Wrighton KC, Thrash JC, Melnyk RA, Bigi JP, Byrne-BKG, et al. (2011) Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl Environ Microbiol 77(21): 7633-7639.
- Kim BH, Kim HJ, Hyun MS, Park DH (1999) Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J Microbiol Biotechnol 9(2): 127-131.
- Lovley DR (2006) Bug juice: harvesting electricity with microorganisms. Nature Rev Microbiol 4(7): 497-508.
- Rabaey K, Rodríguez J, Blackall LL, Keller J, Gross P, et al. (2007) Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J 1(1): 9-18.
- Beckwith CR, Edwards MJ, Lawes M, Shi L, Butt JN, et al. (2015) Characterization of MtoD from Sideroxydans lithotrophicus: a cytochrome c electron shuttle used in lithoautotrophic growth. Front Microbiol 6: 332.
- Borole AP, O’Neill H, Tsouris C, Cesar S (2008) A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum. Biotechnol Lett 30(8): 1367-1372.
- Gomelsky M, Kaplan S (1996) The Rhodobacter sphaeroides 2.4.1 rho gene: expression and genetic analysis of structure and function. J Bacteriol 178(7): 1946-1954.
- Walker AL, Walker CW (2006) Biological fuel cell and an application as a reserve power source. J Power Sources 160(1): 123-129.
- Gumaelius L, Magnusson G, Pettersson B, Dalhammar G (2001) Comamonas denitrificans sp. nov., an efficient denitrifying bacterium isolated from activated sludge. Int J Syst Evolutionary Microbiol 51(3): 999-1006.
- Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, et al. (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci 105(10): 3968-3973.
- Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70(9): 5373-5382.
- Deng L, Li F, Zhou S, Huang D, Ni J (2010) A study of electron-shuttle mechanism in Klebsiella pneumoniae based-microbial fuel cells. Chin Sci Bullet 55(1): 99-104.
- Liu Y, Kim H, Franklin R, Bond DR (2010) Gold line array electrodes increase substrate affinity and current density of electricity-producing G. sulfurreducens biofilms. Energy & Environ Sci 3(11): 1782-1788.
- Call DF, Logan BE (2011) A method for high throughput bio electrochemical research based on small scale microbial electrolysis cells. Biosensors Bioelectron 26(11): 4526-4531.
- Holmes DE, Nicoll JS, Bond DR, Lovley DR (2004) Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl Environ Microbiol 70(10): 6023-6030.
- Niessen J, Schröder U, Harnisch F, Scholz F (2005) Gaining electricity from in situ oxidation of hydrogen produced by fermentative cellulose degradation. Lett Appl Microbiol 41(3): 286-290.
- Fedorovich V, Knighton MC, Pagaling E, Ward FB, Free A, et al. (2009) Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell. Appl Environ Microbiol 75(23): 7326-7334.
- Choi O, Sang BI (2016) Extracellular electron transfer from cathode to microbes: application for biofuel production. Biotechnol Biofuels 9(1): 1-14.
- Firer-Sherwood M, Pulcu GS, Elliott SJ (2008) Electrochemical interrogations of the Mtr cytochromes from Shewanella: opening a potential window. J Biol Inorganic Chem 13(6): 849-854.
- Liu W, Lin J, Pang X, Cui S, Mi S, et al. (2011) Overexpression of rusticyanin in Acidithiobacillus ferrooxidans ATCC19859 increased Fe (II) oxidation activity. Current Microbiol 62(1): 320-324.
- Rosenbaum M, Aulenta F, Villano M, Angenent LT (2011) Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresource Technol 102(1): 324-333.
- Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, et al. (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72(11): 7345-7348.
- Rabaey K, Girguis P, Nielsen LK (2011) Metabolic and practical considerations on microbial electrosynthesis. Curr Opin Biotechnol 22(3): 371-377.
- Malvankar NS, Lau J, Nevin KP, Franks AE, Tuominen MT, et al. (2012) Electrical conductivity in a mixed-species biofilm. Appl Environ Microbiol 78(16): 5967-5971.
- Liu X, Gao H, Ward JE, Liu X, Yin B, et al. (2020) Power generation from ambient humidity using protein nanowires. Nature 578(7796): 550-554.
- Malvankar NS, Lovley DR (2012) Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. Chem Sus Chem 5(6): 1039-1046.
- Parameswaran P, Bry T, Popat SC, Lusk BG, Rittmann BE, et al. (2013) Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ Sci Technol 47(9): 4934-4940.
- Snider RM, Strycharz-GSM, Tsoi SD, Erickson JS, Tender LM (2012) Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc Natl Acad Sci 109(38): 15467-15472.
- Guo K, Freguia S, Dennis PG, Chen X, Donose BC, et al. (2013) Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems. Environ Sci Technol 47(13): 7563-7570.
- Brutinel ED, Gralnick JA (2012) Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl Microbiol Biotechnol 93(1): 41-48.
- Liu T, Cai X, Ding G, Rao L, Yuan Y, et al. (2015) Calcium-dependent electroactive biofilm structure and electricity generation in bioelectrochemical systems. J Power Sources 294: 516-521.
- Fitzgerald LA, Petersen ER, Ray RI, Little BJ, Cooper CJ, et al. (2012) Shewanella oneidensis MR-1 Msh pilin proteins are involved in extracellular electron transfer in microbial fuel cells. Process Biochem 47(1): 170-174.
- Chen T, Zhou Y, Ng IS, Yang CS, Wang HY (2015) Formation and characterization of extracellular polymeric substance from Shewanella xiamenensis BC01 under calcium stimulation. J Taiwan Institute Chem Engineer 57: 175-181.
- Chabert N, Ali OA, Achouak W (2015) All ecosystems potentially host electrogenic bacteria. Bioelectrochem 106: 88-96.
- Richter K, Schicklberger M, Gescher J (2012) Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl Environ Microbiol 78(4): 913-921.
- Richter LV, Sandler SJ, Weis RM (2012) Two isoforms of Geobacter sulfurreducens PilA have distinct roles in pilus biogenesis, cytochrome localization, extracellular electron transfer, and biofilm formation. J Bacteriol 194(10): 2551-2563.
- Bonanni PS, Schrott GD, Busalmen JP (2012) A long way to the electrode: how do Geobacter cells transport their electrons? Biochem Soc Transac 40(6): 1274-1279.
- Kumar R, Singh L, Zularisam AW (2016) Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew Sustain Energy Rev 56: 1322-1336.
- Rollefson JB, Stephen CS, Tien M, Bond DR (2011) Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J Bacterial 193(5): 1023-1033.
- Lebedev N, Strycharz-Glaven SM, Tender LM (2014) High resolution AFM and single-cell resonance Raman spectroscopy of Geobacter sulfurreducens biofilms early in growth. Front Energy Res 2: 34.
- Jana PS, Katuri K, Kavanagh P, Kumar A, Leech D (2014) Charge transport in films of Geobacter sulfurreducens on graphite electrodes as a function of film thickness. Physical Chem Chemical Phys 16(19): 9039-9046.
- Chao L, Rakshe S, Leff M, Spormann AM (2013) PdeB, a cyclic di-GMP-specific phosphodiesterase that regulates Shewanella oneidensis MR-1 motility and biofilm formation. J Bacteriol 195(17): 3827-3833.
- Harris HW, El-Naggar MY, Bretschger O, Ward MJ, Romine MF, et al. (2010) Electrokinesis is a microbial behavior that requires extracellular electron transport. Proc Natl Acad Sci 107(1): 326-331.
- Eaktasang N, Kang CS, Ryu SJ, Suma Y, Kim HS (2013) Enhanced current production by electroactive biofilm of sulfate-reducing bacteria in the microbial fuel cell. Environ Engineer Res 18(4): 277-281.
- Shaw DR, Ali M, Katuri KP, Gralnick JA, Reimann J, et al. (2020) Extracellular electron transfer-dependent anaerobic oxidation of ammonium by anammox bacteria. Nature Commun 11(1): 1-12.
- Luo J, Yang J, He H, Jin T, Zhou L, et al. (2013) A new electrochemically active bacterium phylogenetically related to Tolumonas osonensis and power performance in MFCs. Bioresource Technol 139: 141-148.
- Sure S, Torriero AA, Gaur A, Li LH, Chen Y, et al. (2015) Inquisition of Microcystis aeruginosa and Synechocystis nanowires: characterization and modelling. Antonie Van Leeuwenhoek 108(5): 1213-1225.
- Jiang X, Hu J, Petersen ER, Fitzgerald LA, Jackan CS, et al. (2013) Probing single-to multi-cell level charge transport in Geobacter sulfurreducens DL-1. Nature Communications 4(1): 1-6.
- Zhang T, Cui C, Chen S, Yang H, Shen P (2008) The direct electrocatalysis of Escherichia coli through electro activated excretion in microbial fuel cell. Electrochem Commun 10(2): 293-297.
- Xiao L, He Z (2014) Applications and perspectives of phototrophic microorganisms for electricity generation from organic compounds in microbial fuel cells. Renew Sustainable Energy Rev 37: 550-559.
- Fernandes AFT, da Silva MBP, Martins VV, Miranda CES, Stehling EG (2014) Isolation and characterization of a Pseudomonas aeruginosa from a virgin Brazilian Amazon region with potential to degrade atrazine. Environ Sci Pollution Res 21(24): 13974-13978.
- Roden EE (2012) Microbial iron-redox cycling in subsurface environments. Biochem Society Transac 40(6): 1249-1256.
- Marshall CW, May HD (2009) Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica. Energy & Environ Sci 2(6): 699-705.
- Prindle A, Liu J, Asally M, Garcia-OJ, Süel GM (2015) Ion channels enable electrical communication in bacterial communities. Nature 527(7576): 59-63.
- MacKinnon R (2004) Nobel Lecture. Potassium channels and the atomic basis of selective ion conduction. Biosci Rep 24(2): 75-100.
- Ren D, Navarro B, Xu H, Yue L, Shi Q, et al. (2001) A prokaryotic voltage-gated sodium channel. Science 294(5550): 2372-2375.
- Naik S, Jujjavarapu SE (2021) Enhanced bioelectricity generation by novel biosurfactant producing bacteria in microbial fuel cells. Environ Technol & Innov 23: 101665.
- Markande AR, Patel D, Varjani S (2021) A Review on biosurfactants: properties, applications and current developments. Bioresource Technol 330: 124963.
- Zheng T, Xu YS, Yong XY, Li B, Yin D, et al. (2015) Endogenously enhanced biosurfactant production promotes electricity generation from microbial fuel cells. Bioresource Technol 197: 416-421.
- Liu L, Tsyganova O, Lee DJ, Su A, Chang JS, et al. (2012) Anodic biofilm in single-chamber microbial fuel cells cultivated under different temperatures. Int J Hydrogen Energy 37(20): 15792-15800.
- Wen Q, Kong F, Ma F, Ren Y, Pan Z (2011) Improved performance of air-cathode microbial fuel cell through additional Tween 80. J Power Sources 196(3): 899-904.
- Shen HB, Yong XY, Chen YL, Liao ZH, Si RW, et al. (2014) Enhanced bioelectricity generation by improving pyocyanin production and membrane permeability through sophorolipid addition in Pseudomonas aeruginosa-inoculated microbial fuel cells. Bioresource Technol 167: 490-494.
- Wen Q, Kong F, Ren Y, Cao D, Wang G, et al. (2010) Improved performance of microbial fuel cell through addition of rhamnolipid. Electrochem Commun 12(12): 1710-1713.
- Pankratova G, Leech D, Gorton L, Hederstedt L (2018) Extracellular electron transfer by the Gram-positive bacterium Enterococcus faecalis. Biochem 57(30): 4597-4603.
- Schwab L, Rago L, Koch C, Harnisch F (2019) Identification of Clostridium cochlearium as an electroactive microorganism from the mouse gut microbiome. Bioelectrochem 130: 107334.
- Singh RK, Chang HW, Yan DI, Lee KM, Ucmak D, et al. (2017) Influence of diet on the gut microbiome and implications for human health. J Translational Med 15(1): 1-17.
- Cahoon LA, Freitag NE (2018) The electrifying energy of gut microbes. Nature 562(7725): 43-44.
- Tahernia M, Plotkin KE, Mohammadifar M, Gao Y, Oefelein MR, et al. (2020) Characterization of Electrogenic Gut Bacteria. ACS omega 5(45): 29439-29446.
- Mohammadifar M, Tahernia M, Yang JH, Koh A, Choi S (2020) Biopower-on-Skin: Electricity generation from sweat-eating bacteria for self-powered E-Skins. Nano Energy 75: 104994.
- Byrne JM, Klueglein N, Pearce C, Rosso KM, Appel E, et al. (2015) Redox cycling of Fe (II) and Fe (III) in magnetite by Fe-metabolizing bacteria. Science 347(6229): 1473-1476.
- Shi L, Dong H, Reguera G, Beyenal H, Lu A, et al. (2016) Extracellular electron transfer mechanisms between microorganisms and minerals. Nature Rev Microbiol 14(10): 651-662.
- Kim MY, Kim C, Ainala SK, Bae H, Jeon BH, et al. (2019) Metabolic shift of Klebsiella pneumoniae L17 by electrode-based electron transfer using glycerol in a microbial fuel cell. Bioelectrochem 125: 1-7.
- Wang W, Du Y, Yang S, Du X, Li M, et al. (2019) Bacterial extracellular electron transfer occurs in mammalian gut. Anal Chem 91(19): 12138-12141.
- Balasubramaniam A, Adi P, Do Thi TM, Yang JH, Labibah AS, et al. (2020) Skin bacteria mediate glycerol fermentation to produce electricity and resist UV-B. Microorganisms 8(7): 1092.
- Meysman FJ (2018) Cable bacteria take a new breath using long-distance electricity. Trend Microbiol 26(5): 411-422.
- Nielsen LP, Risgaard-PN, Fossing H, Christensen PB, Sayama M (2010) Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 463(7284): 1071-1074.
- Pfeffer C, Larsen S, Song J, Dong M, Besenbacher F, et al. (2012) Filamentous bacteria transport electrons over centimeter distances. Nature 491(7423): 218-221.
- Malkin SY, Rao AM, Seitaj D, Vasquez-CD, Zetsche EM, et al. (2014) Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor. ISME J 8(9): 1843-1854.
- Burdorf LD, Tramper A, Seitaj D, Meire L, Hidalgo-Martinez S, et al. (2017) Long-distance electron transport occurs globally in marine sediments. Biogeosci 14(3): 683-701.
- Risgaard-PN, Kristiansen M, Frederiksen RB, Dittmer AL, Bjerg JT, et al. (2015) Cable bacteria in freshwater sediments. Appl Environ Microbiol 81(17): 6003-6011.
- Müller H, Bosch J, Griebler C, Damgaard LR, Nielsen LP, et al. (2016) Long-distance electron transfer by cable bacteria in aquifer sediments. ISME J 10(8): 2010-2019.
- Risgaard-PN, Revil A, Meister P, Nielsen LP (2012) Sulfur, iron-, and calcium cycling associated with natural electric currents running through marine sediment. Geochimica et Cosmochimica Acta 92: 1-13.
- Seitaj D, Schauer R, Sulu-Gambari F, Hidalgo-MS, Malkin SY, et al. (2015) Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins. Proc Natl Acad Sci 112(43): 13278-13283.
- Sulu-GF, Seitaj D, Meysman FJ, Schauer R, Polerecky L, et al. (2016) Cable bacteria control iron–phosphorus dynamics in sediments of a coastal hypoxic basin. Environ Sci Technol 50(3): 1227-1233.
- Scholz VV, Meckenstock RU, Nielsen LP, Risgaard-PN (2020) Cable bacteria reduce /methane emissions from rice-vegetated soils. Nature Communications 11(1): 1-5.
- Reimers CE, Li C, Graw MF, Schrader PS, Wolf M (2017) The identification of cable bacteria attached to the anode of a benthic microbial fuel cell: evidence of long-distance extracellular electron transport to electrodes. Front Micrbiol 8: 2055.
- Aller RC, Aller JY, Zhu Q, Heilbrun C, Klingensmith I, et al. (2019) Worm tubes as conduits for the electrogenic microbial grid in marine sediments. Sci Adv 5(7): eaaw3651.
- Geerlings NM, Geelhoed JS, Vasquez-CD, Kienhuis MV, Hidalgo-MS, et al. (2021) Cell cycle, filament growth and synchronized cell division in multicellular cable bacteria. Front Microbiol 12: 620807.
- Jiang Z, Zhang S, Klausen LH, Song J, Li Q, et al. (2018) In vitro single-cell dissection revealing the interior structure of cable bacteria. Proc Natl Acad Sci 115(34): 8517-8522.
- Meysman FJ, Cornelissen R, Trashin S, Bonné R, Martinez SH, et al. (2019) A highly conductive fiber network enables centimetre-scale electron transport in multicellular cable bacteria. Nature Commun 10(1): 1-8.
- Cornelissen R, Bøggild A, Thiruvallur ER, Koning RI, Kremer A, et al. (2018) The cell envelope structure of cable bacteria. Front Microbiol 9: 3044.
- Eachambadi TR, Bonné R, Cornelissen R, Hidalgo‐MS, Vangronsveld J, et al. (2020) An Ordered and Fail‐Safe Electrical Network in Cable Bacteria. Adv Biosystems 4(7): e2000006.
- Gao Y, Mohammadifar M, Choi S (2019) From Microbial Fuel Cells to Biobatteries: Moving toward On‐Demand Micropower Generation for Small‐Scale Single‐Use Applications. Adv Materials Technol 4(7): 1900079.
- Logan BE, Rossi R, Saikaly PE (2019) Electroactive microorganisms in bio electrochemical systems. Nature Rev Microbiol 17(5): 307-319.
- Saripan AF, Reungsang A (2013) Biohydrogen production by Thermoanaerobacterium thermosaccharolyticum KKU-ED1: Culture conditions optimization using mixed xylose/arabinose as substrate. Electronic J Biotechnol 16(1): 1-1.
- Nimje VR, Chen CY, Chen HR, Chen CC, Huang YM, et al. (2012) Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresource Technol 104: 315-323.
- Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Bioresource Technol 131: 564-571.
- Logan BE, Shaoan C (2007) Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun 9(3): 492-496.
- Saeed HM, Husseini GA, Yousef S, Saif J, Al-Asheh S, et al. (2015) Microbial desalination cell technology: a review and a case study. Desalination 359: 1-13.
- Tharali AD, Sain N, Osborne WJ (2016) Microbial fuel cells in bioelectricity production. Front Life Sci 9(4): 252-266.
- Mandal SK, Das N (2021) Application of microbial fuel cells for bioremediation of environmental pollutants: an overview. J Microbiol Biotechnol Food Sci 2021: 437-444.
- Rismani-YH, Carver SM, Christy AD, Tuovinen OH (2008) Cathodic limitations in microbial fuel cells: an overview. J Power Sour 180(2): 683-694.
- Li M, Zhou S, Xu M (2017) Graphene oxide supported magnesium oxide as an efficient cathode catalyst for power generation and wastewater treatment in single chamber microbial fuel cells. Chem Eng J 328: 106-116.
- Cheng S, Liu H, Logan BE (2006) Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Communications 8(3): 489-494.
- Watanabe K (2008) Recent developments in microbial fuel cell technologies for sustainable bioenergy. J Biosci Bioeng 106(6): 528-536.
- Naidoo QL, Naidoo S, Petrik L, Nechaev A, Ndungu P (2012) The influence of carbon based supports and the role of synthesis procedures on the formation of platinum and platinum-ruthenium clusters and nanoparticles for the development of highly active fuel cell catalysts. Int J Hydrogen Energy 37(12): 9459-9469.
- Almatouq A, Babatunde AO (2018) Identifying optimized conditions for concurrent electricity production and phosphorus recovery in a mediator-less dual chamber microbial fuel cell. Appl Energy 230: 122-134.
- Penteado ED, Fernandez-MCM, Zaiat M, Gonzalez ER, Rodrigo MA (2017) Influence of carbon electrode material on energy recovery from winery wastewater using a dual-chamber microbial fuel cell. Environ Technol 38(11): 1333-1341.
- Obeid J, Magnin JP, Flaus JM, Adrot O, Willison JC, et al. (2009) Modelling of hydrogen production in batch cultures of the photosynthetic bacterium Rhodobacter capsulatus. Int J Hydrogen Energy 34(1): 180-185.
- Li RY, Fang HH (2008) Hydrogen production characteristics of photoheterotrophic Rubrivivax gelatinosus L31. Int J Hydrogen Energy 33(3): 974-980.
- Ren NQ, Liu BF, Ding J, Guo WQ, Cao GL, et al. (2008) The effect of butyrate concentration on photo-hydrogen production from acetate by Rhodopseudomonas faecalis RLD-53. Int J Hydrogen Energy 33(21): 5981-5985.
- Oh YK, Seol EH, Kim MS, Park S (2004) Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rhodopseudomonas palustris P4. Int J Hydro Energy 29(11): 1115-1121.
- Chen CY, Lu WB, Wu JF, Chang JS (2007) Enhancing phototrophic hydrogen production of Rhodopseudomonas palustris via statistical experimental design. Int J Hydrogen Energy 32(8): 940-949.
- Jia J, Tang Y, Liu B, Wu D, Ren N, et al. (2013) Electricity generation from food wastes and microbial community structure in microbial fuel cells. Bioresource Technol 144: 94-99.
- Li X, Liu L, Liu T, Yuan T, Zhang W, et al. (2013) Electron transfer capacity dependence of quinone-mediated Fe (III) reduction and current generation by Klebsiella pneumoniae L17. Chemosphere 92(2): 218-224.
- Liu T, Li X, Zhang W, Hu M, Li F (2014) Fe (III) oxides accelerate microbial nitrate reduction and electricity generation by Klebsiella pneumoniae L17. J Colloid Interface Sci 423: 25-32.
- Holkar CR, Arora H, Halder D, Pinjari DV (2018) Biodegradation of reactive blue 19 with simultaneous electricity generation by the newly isolated electrogenic Klebsiella sp. C NCIM 5546 bacterium in a microbial fuel cell. Int Biodeteriorat Biodegrad 133: 194-201.
- Dai HN, Nguyen TAD, LE LPM, Van Tran M, Lan TH, et al. (2021) Power generation of Shewanella oneidensis MR-1 microbial fuel cells in bamboo fermentation effluent. Int J Hydrogen Energy 46(31): 16612-16621.
- Inoue K, Leang C, Franks AE, Woodard TL, Nevin KP, et al. (2011) Specific localization of the c‐type cytochrome OmcZ at the anode surface in current‐producing biofilms of Geobacter sulfurreducens. Environ Microbiol Reports 3(2): 211-217.
- Xing D, Cheng S, Logan BE, Regan JM (2010) Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction. Appl Microbiol Biotechnol 85(5): 1575-1587.
- Luo J, Li M, Zhou M, Hu Y (2015) Characterization of a novel strain phylogenetically related to Kocuria rhizophila and its chemical modification to improve performance of microbial fuel cells. Biosensors Bioelectronics 69: 113-120.
- Liu M, Yuan Y, Zhang LX, Zhuang L, Zhou SG, et al. (2010) Bioelectricity generation by a Gram-positive Corynebacterium sp. strain MFC03 under alkaline condition in microbial fuel cells. Bioresource Technol 101(6): 1807-1811.
- Xu S, Liu H (2011) New exoelectrogen Citrobacter sp. SX‐1 isolated from a microbial fuel cell. J Appl Microbiol 111(5): 1108-1115.
- Nimje VR, Chen CY, Chen CC, Tsai JY, Chen HR, et al. (2011) Microbial fuel cell of Enterobacter cloacae: Effect of anodic pH microenvironment on current, power density, internal resistance and electrochemical losses. Int J Hydrogen Energy 36(17): 11093-11101.
- Jamlus NIIM, Masri MN, Wee SK, Shoparwe NF (2021) Electricity Generation by Locally Isolated Electroactive Bacteria in Microbial Fuel Cell. In IOP Conference Series: Earth Environ Sci 765(1): 012115.
- Do MH, Ngo HH, Guo WS, Liu Y, Chang SW, et al. (2018) Challenges in the application of microbial fuel cells to wastewater treatment and energy production: a mini review. Sci Total Environ 639: 910-920.
- Do MH, Ngo HH, Guo WS, Liu Y, Chang SW, et al. (2020) Microbial fuel cell-based biosensor for online monitoring wastewater quality: A critical review. Sci Total Environ 712: 135612.
- Eroğlu İ, Tabanoğlu A, Gündüz U, Eroğlu E, Yücel M (2008) Hydrogen production by Rhodobacter sphaeroides OU 001 in a flat plate solar bioreactor. Int J Hydrogen Energy 33(2): 531-541.
- Feng Y, Barr W, Harper WF (2013) Neural network processing of microbial fuel cell signals for the identification of chemicals present in water. J Environ Manag 120: 84-92.
- Gul H, Raza W, Lee J, Azam M, Ashraf M, et al. (2021) Progress in microbial fuel cell technology for wastewater treatment and energy harvesting. Chemosphere 281: 130828.
- Jasim A, Ullah MW, Shi Z, Lin X, Yang G (2017) Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydrate Polymers 163: 62-69.
- Kalathil S, Pant D (2016) Nanotechnology to rescue bacterial bidirectional extracellular electron transfer in bioelectrochemical systems. RSC Adv 6(36): 30582-30597.
- Kitafa BA, Al-saned AJO (2021) A Review on Microbial Fuel Cells. Eng Technol J 39(1A): 1-8.
- Kumar R, Singh L, Zularisam AW, Hai FI (2018) Microbial fuel cell is emerging as a versatile technology: a review on its possible applications, challenges and strategies to improve the performances. Int J Energy Res 42(2): 369-394.
- Larsen S, Song J, Dong M, Besenbacher F, Meyer RL, et al. (2012) Filamentous bacteria transport electrons over centimeter distances. Nature 491(7423): 218-221.
- Li SW, He H, Zeng RJ, Sheng GP (2017) Chitin degradation and electricity generation by Aeromonas hydrophila in microbial fuel cells. Chemosphere 168: 293-299.
- Parkash A (2016) Microbial fuel cells: a source of bioenergy. J Microb Biochem Technol 8(3): 247-255.
- Puig S, Serra M, Coma M, Cabré M, Balaguer MD, et al. (2011) Microbial fuel cell application in landfill leachate treatment. J Hazardous Mater 185(2-3): 763-767.
- Rabaey K, Angenent L, Schroder U, Keller J (2009) Bio electrochemical systems. IWA publishing, London, England, United Kingdom.
- Ramadan BS (2017) Challenges and opportunities of microbial fuel cells (MFCs) technology development in Indonesia. In MATEC web of conferences EDP Sci 101: 02018.
- Sharma R, Munns K, Alexander T, Entz T, Mirzaagha P, et al. (2008) Diversity and distribution of commensal fecal Escherichia coli bacteria in beef cattle administered selected subtherapeutic antimicrobials in a feedlot setting. Appl Environ Microbiol 74(20): 6178-6186.






























