Antibiofilm Activity of Ethanolic Extracts from Nypa fruticans and Pleurotus ostreatus against Produced Water Biofilm
Stanley HO1* Okhuahesogie E1 and Ugboma C J2
1 Department of Microbiology, University of Port Harcourt, Nigeria
2 Department of Microbiology, Rivers State University, Nigeria
Submission: January 23, 2018; Published: May 01, 2018
*Corresponding author: Stanley HO, Department of Microbiology, Faculty of Sciences, University of Port Harcourt, PMB 5323, Choba, 500004 Port Harcourt, Rivers State, Nigeria,.
How to cite this article: Stanley HO, Okhuahesogie E, Ugboma C J. Antibiofilm Activity of Ethanolic Extracts from Nypa fruticans and Pleurotus ostreatus against Produced Water Biofilm. Adv Biotech & Micro. 2018; 9(1): 555755. DOI: 10.19080/AIBM.2018.09.555755
Abstract
The antibiofilm activity of the ethanolic extracts from Nypa fruticans and Pleurotus ostreatus were tested against biofilm attached on Polyvinylchloride (PVC) slides, using produced water as aqueous medium.10g of dry powder from dried Nypa fruticans and Pleurotus ostreatus was extracted with 50% ethanol (100ml) using a shaker. 5ml of ethanolic extract was introduced into each test conical flask and biofilm was allowed to stand for two (2) hours and the PVC slides are removed for biofilm examination. Biofilm mass assessment was carried out by direct comparison of mass of samples (before extract application) and test samples (after extract application). Biofilm protein constituent was assessed using the Modified Lowry method while the polysaccharide constituent was assessed using the phenol-sulphuric acid method. Agar well diffusion assay was utilized in assessing the susceptibility of biofilm microorganisms. Staphylococcus spp was highly susceptible to extract from Nypa fruticans with zone of inhibition of 16mm. Polysaccharide content was highly denatured by Nypa fruticans extract with a reduction in polysaccharide content of 60% when compared with polysaccharide constituent before application of extracts. The use of both extracts showed more effectiveness in reducing 65% of the biofilm mass. Analysis of variance revealed that the difference between mass of biofilms on PVC slides before application of extract and mass of biofilm on PVC slides after application is significant (P=0.05).¬ Ethanolic extracts from Nypa fruticans and Pleurotus ostreatus could be considered in the control of biofilms and the utilization of more than a single extract will be more potent.
Keywords: Denaturation; Inhibition; Microorganisms; Polyvinyl-chloride; Mass
Introduction
Microbes are generally known to produce extracellular polysaccharide matrix and this is sometimes responsible for their resistance to treatment. The formation of these biofilms confers the well cementing of the microbes onto biotic and abiotic surfaces they therefore create a microenvironment needed for their survival [1]. When biofilms are left uncontrolled they could cause problems wherever they develop like glasses, metals and plastics as well as distortion of industrial processes, products and systems. Increased growth of biofilms has led to a very high distortion in flow pipes, decomposition of wood as well as plugging of flow pipes and valves. Reduction of cooling effect on heat exchangers may arise from this immense growth [2].
Phytochemical screening of Nypa fruticans showed that polyphenols are present in the husk and while the seed had traces of alkaloids [3]. Different medicinal plants contain phenols and flavonoids with antioxidant activity and they were also able to inhibit the growth of Escherichia coli and Staphylococcus aureus [4]. Pleurotus ostreatus was cytotoxic against Gram negative and Gram positive bacteria with an average antimicrobial property. This indicates their potential for use in microbial control [5]. This study was aimed at determining the biocidal activity of phenols extracted from Nypa fruticans and an edible mushroom (Pleurotus ostreatus). To extract their ethanolic content and also to investigate the susceptibility of biofilms based on their age. This study was carried out in Port Harcourt, Nigeria in January 2015.
Methodology
Produced water sample was collected aseptically from Obirikom Egbema oil field in Ogba/Egbema/Ndoni Local Government Area of Rivers State. Whole bunch unripe fruit of Nypa fruticans was collected from Eagle Island in Port Harcourt city local government area of Rivers state. Commercially cultivated edible mushroom oyster mushroom (Pleurotus ostreatus) was gotten from a mushroom farm in Port Harcourt city Local government area of Rivers State.
Isolation of test organisms
Isolation of test organisms was carried out according to basic microbiological techniques using nutrient agar which was prepared according to manufacturer’s instruction. Identification of isolates was done using biochemical test in line with Bergey’s manual of determinative bacteriology [6].
Alcoholic extraction of phenol
10g from dried endosperm of Nypa fruticans and dried Pleurotus ostreatus were extracted with 50% ethanol (100ml) using a shaker. A constant speed at 30 °C for 1hour was maintained in the shaker. Filtration of the extract was done using Whatman No. 4 filter paper [7,8].
Preparation of polyvinyl chloride (PVC) slides
Locally obtained Polyvinyl chloride (PVC) material was cut into slides. The size of the slides were 5cmx 1.4cmx0.1cm (Length, width and thickness respectively). The weights of the slides were measured before beginning the experiment. A hole was created on each PVC slide and a nylon thread was passed through the hole so as to aid in suspending the slides inside the conical flasks. A total of twenty-eight (28) polyvinyl chloride slides were cut for use in the experiment [9].
Determination of antimicrobial activity of extracts
Suspensions of the tested microorganisms were spread onto the surface of Nutrient agar plates and wells of 5 mm diameter were cut from the agar. The wells were then filled with 50μl of different concentrations (25%, 50%, 75% and 100%) of extract from both mushroom and Nypa palm. The plates were incubated for 24 hours at 37 °C. The inhibition zone diameter around the wells where measured so as to determine the sensitivity of tested microorganisms to extracts [10].
Experimental setup
A total of twenty-eight (28) conical flasks were utilized. These conical flasks served as batch reactors, each batch reactor contained 100ml of produced water sample and one prepared PVC slide for adhesion of biofilms. Chocolate agar was used as enrichment medium and 10ml was added to each batch reactor. The batch reactors were incubated at room temperature for 5 days. This is to enable adhesion of sessile microorganisms to PVC slides. The PVC slides were however inspected after 5 days for the formation of biofilms. Four (4) out of the 28 batch reactors were utilized as control test and they contained 100ml of produced water sample, 10ml of chocolate medium and a PVC slide for biofilm formation. The remaining twenty-four (24) test reactors were utilized in the experiment to test the anti-biofilm activity of extracted phenol. Eight (8) reactors were used for extracted phenol from Nypa fruticans, another eight (8) reactors was used for extracted phenol from Pleurotus ostreatus and the final eight (8) reactors were used for extracts of both Nypa fruticans and Pleurotus ostreatus. The slides are left inside the reactor and monitored for biofilm growth after 5 day. 5ml of Nypa phenol was introduced into two designated reactors (R1a-N and R1b-N), 5ml of Pleurotus phenol was applied to two designated reactors (R1a-P and R1b-P) and 5ml of Nypa+Pleurotus (2.5ml each) phenol was applied to two designated reactors (R1a- NP and R1b-NP). The ethanolic extracts were left to act with biofilms formed on all the PVC slides and they were left for a contact time of two hours. After the contact time of two hours, the PVC slides were retrieved from the various reactors including one slide from one control reactor. The slides with biofilms were conditioned for biofilm weighing, polysaccharide as well as protein constituent analysis. The results revealed the effect of the ethanolic extracts on the 5 days old biofilm. After 10 days, the process was repeated and also for 15days and 20 days and 25 days. The results were recorded [11].
Biofilm mass assessment
Direct technique: The PVC slides have been weighed earlier in the experiment and also identified before being placed in the reactors at the start of the experiment. After the contact time of two hours, the slides were retrieved and washed with 0.9% NaCl solution. This is done to remove phenol detached biofilm. The slides are then air dried so as to obtain a constant mass. The total mass for the slides alongside the remaining attached biofilm was gotten by measuring with a weighing balance [11].
Indirect technique: The indirect methods measure specific biofilm constituents such as protein and polysaccharide. One of such methods is the spectrophotometric analysis of biofilm constituents [11].
Protein concentration determination using modified lowry method: A sterile spatula was used to scrape biofilms from PVC slides into tubes and a 1M phosphate buffer solution was made. Biofilms were disrupted by agitating the solution vigorously in a vortex for 3 minutes after which the suspension is centrifuged for 10 minutes. A spectrophotometer was used to measure the absorbances of test samples as well as control. Using half-log dilution of Bovine Serum Albumin (BSA) as protein standard, the unknown sample concentration was measured by tabulating the corrected absorbance (Δ A750nm) of all unknown samples against that of the known concentration. Standard curves were generated by plotting Δ A750nm against the serially diluted concentrations of Bovine Serum Albumin.
Glucose concentration determination using phenol sulphuric acid method
A spectrophotometer was used to measure the sample absorbance. A half-log dilution of glucose standard was made so as to cover the expected concentrations of the unknown. The concentrations of the unknown sample were measured by tabulating the corrected absorbance (Δ A630nm) of all unknown samples against that of the known concentration. Standard curves were generated by plotting Δ A630nm against the serially diluted concentrations of Glucose standard [11].
Statistical analysis
Analysis of variance and post hoc test (multiple comparisons): The One-Way ANOVA was employed in determining if there is significance in the difference that exists between test samples and control sample while the Post Hoc Test confirms where the differences occurred between groups
Results
This study compared the biocidal activity of ethanolic extracts from Nypa palm and Oyster mushroom against biofilms formed from produced water and the biofilm microorganisms. Table 1 below shows the zones of inhibition caused by the ethanolic extract from Nypa fruticans at various concentrations while Table 2 shows the zones of inhibition caused by ethanolic extract from Pleurotus ostreatus also at various concentrations. All readings were done in duplicates and the mean values recorded as seen in Table 1 & 2.
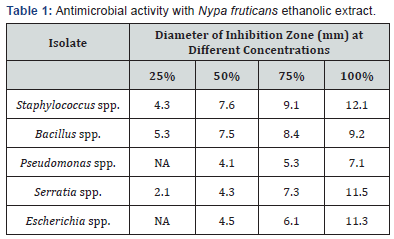
*Key: NA=No Activity
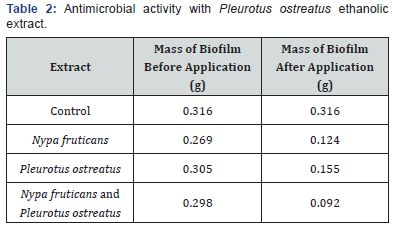
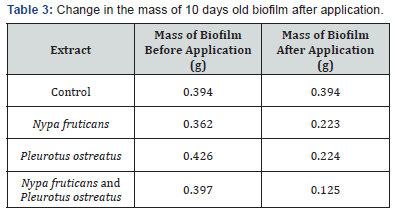
The effects of ethanolic extracts against the biofilm formed on the slides are shown in Table 3-6 below. There were significant reductions in the mass of biofilms formed on the slides before application of extracts and the mass of biofilms on the slides after the 2 hours contact time with the ethanolic extracts. Ethanolic extracts had significant effects on the polysaccharide concentration as well as the protein concentration of biofilms as measured with a spectrophotometer. Ethanolic extract from N. fruticans reduced about 94% of polysaccharide at day 10 while P. ostreatus reduced about 82% of protein concentration at day 10 (Table 7 & 8).
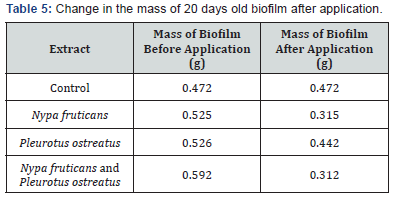
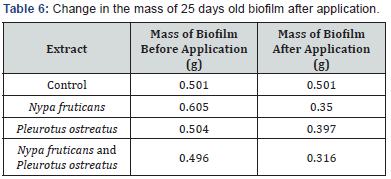
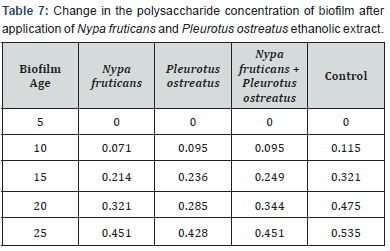
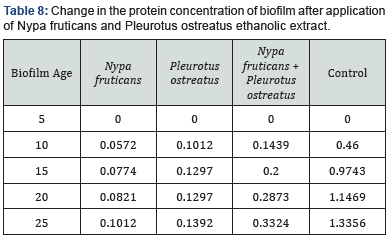
Discussion
This study compared the ethanolic extract from both P. ostreatus and N. fruticans and they both had antimicrobial effects on the isolates from produced water. Staphylococcus spp had the largest zone of inhibition to ethanolic extract from N. fruticans with a 12.1mm diameter while for P. ostreatus the zone of inhibition diameter was 9.3mm. This is in agreement with Benedec et al. [12] suggestion that polyphenols from plants may be used for their bioactive properties. The reduction in biofilm protein and polysaccharide constituent as well as protein constituent of biofilm is in line with the report of Igwe et al. [11] where monochloramine caused a 60.6% reduction in polysaccharide content and 62.9% reduction in protein constituent [13].
Conclusion
This study has showed that the development of biofilms on materials of industrial use associated with produced water could be controlled with the use of non-toxic bioactive plant extracts. The use of natural biocide in the removal of biofilms in PVC pipes should be considered as an option and more research should be conducted to know the mode of action of these ethanolic extracts against biofilms.
References
- Donlan R (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9): 881-890.
- Al-Haj H (2012) Fouling in heat exchangers. MATLAB-A fundamental tool for scientific computing and engineering applications.
- Osabor V, Egbung G, Okafor P (2008) Chemical profile of Nypa fruiticans from cross river Estuary, South Eastern Nigeria. Pakistan Journal of Nutrition 7(1): 146-150.
- Kaur S (2014) Study of Total Ethanolic and Flavonoid Content, Antioxidant Activity and Antimicrobial Properties of Medicinal Plants. Journal of Microbiology & Experimentation 1(1): 00005.
- Sala UG, Sarwar H, Monirul I, Asaduzzaman M, Jahan bulbul I, et al. (2015) Evaluation of antimicrobial, antioxidant and cytotoxic property of Pleurotus ostreatus mushroom. International Research Journal of Biological Science 4(1): 29-33.
- Bergey DH, Holt JG (1994) Bergeys manual of determinative bacteriology. The Williams and Wilkins Company, Baltimore, USA.
- Prasad N, Yang B, Kong K, Khoo H, Sun J, et al. (2013) Phytochemicals and antioxidant capacity from Nypa fruticans wurmb. Fruit. Evidence- Based Complementary and Alternative Medicine 5(9): 1-9.
- Gan CH, Nurul Amira B, Asmah R (2013) Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis). International Food Research Journal 20(3): 1095-1102.
- Onojake MC, Abanum UI (2012) Evaluation and management of produced water from selected oil fields in Niger Delta, Nigeria. Archives of Applied Science Research 4(1): 39-47.
- Parihar S, Virani K, Pithawala E, Shukla M, Lahiri S, et al. (2015) Pytochemical screening, total ethanolic content, antibacterial and antioxidant activity of wild edible mushroom Pleurotus ostreatus. International Research Journal of Pharmacy 6(1): 65-69.
- Igwe I, Puyate Y (2013) Comparison of the effects of Monochloramine and Glutaraldehyde (Biocides) against biofilm microorganisms in produced water. International Journal of Engineering Trends and Technology 5(1): 54-61.
- Benedec D, Vlase L, Oniga I, Mot A, Damian G, et al. (2013) Polyethanolic composition, antioxidant and antibacterial activities for two romanian subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 18(8): 8725-8739.
- Wandati T, Kenji G, Onguso J (2013) Phytochemicals in edible wild mushrooms from selected areas in Kenya. Journal of Food Research 2(3): 231-332.






























