Pre Clinical In vitro Investigation of the Cytotoxic Effect of Ficus species of Phytochemicals on Hepatoma Hep G2 Cells using MTT and Neutral Red Toxicity Assays
Fokunang Charles1*, Tembe-Fokunang Estella1, Ngameni Bathelemy2, Salwa Barkwan3, Hoare Gary3, Ngadjui Bonaventure2 and Paul Tomkins3
1 Department of Pharmacotoxicology and Pharmacokinetics, University of Yaoundé1, Cameroon
2 Department of Pharmacognosy and Medicinal Chemistry, University of Yaoundé1, Cameroon
3 Centre for Biopolymer and Bio- molecular Research, Athlone Institute of Technology, Republic of Ireland
Submission: December 25, 2017; Published: March 26, 2018
*Corresponding author: Fokunang Charles, Pharmacotoxicology and Pharmacokinetics laboratory, Preclinical Animal Toxicology Unit, Faculty of Medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon. P.O. Box 33032, Yaoundé, Cameroon.
How to cite this article: Fokunang C, Tembe-F E, Ngameni B, Salwa B, Hoare G, Ngadjui B, Paul T. Pre Clinical In vitro Investigation of the Cytotoxic Effect of Ficus species of Phytochemicals on Hepatoma Hep G2 Cells using MTT and Neutral Red Toxicity Assays. Adv Biotech & Micro. 2018; 8(5): 555748. DOI: 10.19080/AIBM.2018.08.555748
Abstract
Background: The use of plants in traditional medicine has been well documented, and hundreds of plant species, and their extracts, are used in developing countries to treat numerous diseases despite the fact that only a small number are approved for therapeutic use by the FDA. However, the cytotoxic effects of these plants have not been studied in detail, nor have their molecular structures been identified.
Aim:This study was to investigate the cytotoxic effects of these medicinal plants using both MTT and Neutral red assays.
Method: The stem, leaf, bark and trunk extracts from each plant species were used to determine the cytotoxic effects of the plant species, Hep G2 cells were exposed to extracts taken from plant species at concentrations of 0.1, 1, 10 and 100μg/ml and the cytotoxic effects determined using both MTT and Neutral Red assays.
Results: Using both MTT and Neutral Red assays, the cytotoxicity of each plant species was determined. It was found that extracts of F. ovata incubated on cells resulted in no cytotoxic effect, but for cells treated with all other extracts of the plant species, cytotoxic effects were observed in at least one concentration tested.
Conclusion: The results obtained for cytotoxicity assays indicated that F. ovata may be suitable for use as medicinal agent as the extract tested did not infer cytotoxicity. However, the other plant species tested during this study may not be suitable for use in medicine because of their potential cytotoxic effects.
Introduction
Natural products have been used since millennia for the treatment of human diseases and as a result, a large proportion of current drugs in modern medicine have been developed from natural molecules. The search for new biologically active natural products continues to be an intense field of research [1,2]. It has been reported that the high natural biodiversity represents a broad range of diverse chemical structures with potentially new molecules having promising biological activities for many therapeutic areas [3]. Such new natural molecules can potentially serve as chemical entities for the design and the synthesis of novel drugs [4]. Plants have historically proven their value as a rich source of molecules with therapeutic potential and many major current drugs are natural products-derived compounds [2,5]. The natural products firstly commercialized for therapeutic use are morphine, isolated from Papaver somniferum [3, 4] and aspirin [6], based on the natural product salicin from Salix alba [7].
For thousands of years, plants have been recognised to play a therapeutic role in curing diseases and infections, and currently, there is a wide range of herbal remedies available in health food shops and other outlets, which many people are using to treat numerous problems, despite the fact that most herbal and plant remedies have not been approved for use by the FDA [2,7]. It is now realised that the therapeutic effects of plant products are mainly due to the phytochemicals found in these plants, as many are known to have a pharmacokinetic or pharmacodynamic interaction with certain drugs [8]. Because of these potential benefits of using plants in medicine, many species are now being analysed to identify the phytochemicals found in them.
Despite this, however, the safety of using plants in medicine is continuously being reviewed, and much work is being done to determine not only the efficacious effects of plants, but also their potential harmful effects [6,8]. Although it has been purported by some herbal remediests that the use of plants as herbal medicine is safe due to the fact that it is a “natural” substance, there have been a number of publications that indicate otherwise [4,9]. Some herbal medicines are known to have resulted in severe sideeffects after ingestion, which may be due to the toxic properties of the herbs or plants used, while the interactions of the plants or herbal medicine with other drugs being used by the patient can also lead to adverse effects [6,10]. For example, a number of severe effects, including heart attack, stroke and even death have been reported following the use of products containing Ma huang (ephedrine) and kola nut due to the interaction of the caffeine in the kola nut and the ephedrine [11]. Numerous members of the Ficus species have been documented to be used for both food and medicine, and their use has been most widely documented in the Middle East, where many members of this species are known to grow.
Materials and Methods
Plant material
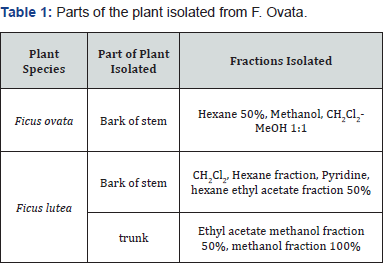
A number of species from the Ficus genus, along with species from the Dorstenia and Triumfetta genus were tested during this study. All plant species were collected from the jungle in Cameroon. The species studied are outlined in the Table 1 & 2, in addition to the parts of the plant that were isolated and the fractions tested.
Cell Culture
In this study, a human hepatocellular carcinoma cell line, Hep G2, was used to test the toxicity of each plant species. One sample from each plant species was incubated on HepG2 cells at concentrations of 0.1μg/ml, 1μg/ml, 10μg/ml and 100μg/ ml to determine if this fraction inferred any cytotoxic effects to the cells. Results were compared to control samples. The plant species tested, parts of the plant tested and extract solvent are outlined in Table 2.

Subculture of cells
Medium was removed from the dish and a solution of 0.25% trypsin/1mM EDTA was added. Cells were incubated at 37 oC/5% CO2 for 10 minutes, and an equal volume of medium was added to deactivate the trypsin. The culture was transferred to a 50ml conical tube and centrifuged at 2000rpm for 5 minutes. Cells were re-suspended in medium and seeded into T-175 flasks. Cultures were replenished with medium every 3-4 days and passaged again when 90-95 % confluent.
Preparation of cells from a monolayer culture for counting
Cells were first viewed to assess their morphology and their degree of confluence. Following this, medium was removed from the flask, and 3mls phosphate buffered saline (PBS) added to the flask, which was gently shook to wash the cells. The PBS was removed, and 6mls trypsin-EDTA added to the flask, which was again gently shook to soak the cells. The flask was then placed in an incubator (37 oC, 5% CO2) for 3-4 minutes, and upon removing the flask the cells were observed under the microscope to assess their degree of detachment. When cells were fully detached from the flask, 6mls fresh medium was added to the cells, mixed well and the cell suspension was then removed and placed in a universal. The cells were pelleted by centrifugation at 2000rpm for 5 minutes. The supernatant was then removed, 2mls fresh medium was added to the universal and the pellet was re-suspended in this fresh medium.
To count the cells, the haemocytometer and cover-slip were washed in 70% ethanol and wiped dry. The slide and cover-slip were moistened and placed in contact so that both grids on the slide were covered. 0.2mls was taken from the cell suspension prepared in the universal, and mixed with 0.3mls PBS and 0.5mls 0.4% trypan blue (viability dye). This mixture was allowed to stand for 5 minutes. Following this, a small volume of the dyed cells was taken and added to both channels of the haemocytometer slide using a micropipette. All colorless cells were counted using the 5 large squares of the haemocytometer, and this number was then placed into the following formula to determine cell density.
cellcount= 104 x No. cells counted x dilution factor/ No squares counted
Toxicity assays
Methyl Tetrazolium Assay (MTT): The MTT assay was used to determine the proliferation rate of cells treated with plant extracts at different concentrations taken from a number of different species. In this assay, metabolically active cells convert yellow tetrazolium salt MTT to purple formazan crystals, and the number of viable cells in the population is proportional to the amount of formazan produced. Cells were seeded in a 96-well plate at the required density, and incubated for 24 hrs (37 oC, 5% CO2) to allow the cells to adhere. 10μl plant extract chemical was added to the cells at concentrations of 0.1, 1, 10 and 100μg/ ml. Untreated cells (control) received medium only. The plate was then incubated for 24 hrs (37 oC, 5% CO2). The chemical and medium were both removed from the cells, which were then washed with 100μl PBS. This was removed from the cells, and 100μl fresh medium and 10μl of MTT to each well and incubated for 3 hours. After 3hrs, 100μl DMSO was added, and the plate was gently shaken to solubilise the dye. The absorbance was then read at 540nm using a Biotek Multiwell Plate Reader. A set of negative controls (untreated cultures) was used in each experiment, and all experiments were run in triplicate.
Neutral red assay
Cells were seeded in a multi-well plate at the required density, and the plate was incubated for 24 hrs (37oC, 5% CO2) to allow the cells to adhere. 10μl plant extract chemical was added to the cells at concentrations of 0.1, 1, 10 and 100μg/ml. As a control, medium was added to the cells (with no plant extract). The plate was then incubated for 24 hrs (37oC, 5% CO2). The medium and plant extract was then removed from the cells, and replaced with 100μl neutral red medium. The plate was then incubated (37oC, 5% CO2) to allow uptake of dye into the cells. The neutral red medium was then removed from all wells, which were washed with PBS that was then removed. 100μl neutral red extract was added to the cells, and incubated for 15 minutes at room temperature. The absorbance was then read at 540nm using a Biotek Multiwell Plate Reader.
Statistical analysis
Results obtained for both MTT and Neutral Red assays were analysed statistically using student T-tests. Absorbance levels for control samples were compared to the absorbance levels obtained for each concentration of extract used to determine if there were any significant differences between absorbance levels. In addition, results obtained for the concentrations of the extracts used were compared to each other to determine if there were any significant differences between the different concentrations.
Results
Cytotoxicity assay results
Hep G2 cells were treated with extracts taken from each of the species tested to determine if they resulted in any cytotoxic effects to this cell line. Concentrations of 0.1, 1, 10 and 100μg/ml of each plant species were prepared and dissolved in methanol, and incubated on the cells for 24hrs. The toxicity of each species was determined carrying out both MTT and Neutral Red assays. For the control, cells were incubated with medium only for 24hrs.
Student T-tests were performed on the results obtained to determine if any significant differences could be detected between control samples, and those treated with plant extract, and to determine if there were any significant differences between the various concentrations of plant extract used in the study.
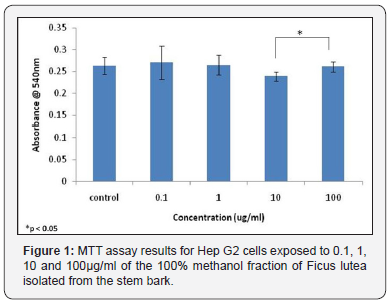
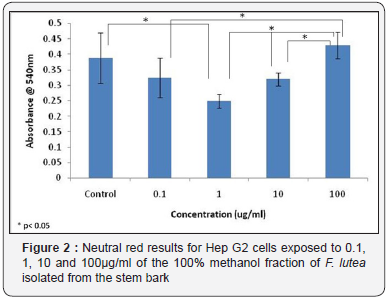
or F. lutea, HepG2 cells were treated with 0.1, 1, 10 and 100 μg/ml of the 100 % methanol fraction of the stem bark. Significance tests showed for the MTT assay, no significant differences were observed between control samples treated with medium only, and samples treated with varying concentrations of the plant extract (Figure 1). However, results of the MTT assay for F. lutea did show a significant difference in absorbance levels between cells treated with 10μg/ml of extract and those treated with 100μg/ml of extract (Figure 1). The neutral red assay results for F. lutea showed a significant difference in absorbance levels between control samples, and those treated with 1μg/ml of sample (Figure 2). In addition, significant differences were observed between samples treated with 0.1μg/ml of extract 100μg/ml of extract, while significant differences were also detected between samples treated with 1μg/ml of extract and 10μg/ml of extract, and those treated with 1μg/ml of extract and 100μg/ml of extract (Figure 2).
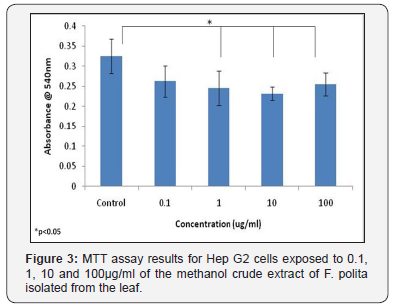
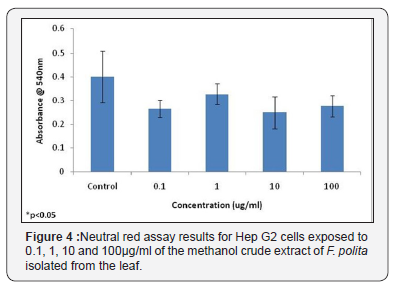
Neutral red assay results for Hep G2 cells exposed to 0.1, 1, 10 and 100μg/ml of the 100% methanol fraction of F. lutea isolated from the stem bark The methanol crude extract fraction of the leaf of F. polita was also tested using HepG2 cells. While no significant differences were observed in the neutral red assay between control samples and those treated with the different concentrations of plant extract, MTT assay results did show a significant difference between control samples and those treated with 1, 10 and 100μg/ml of plant extract (Figure 3). No significant differences were observed between the different concentrations of extract tested in either the MTT assay or neutral red assay (Figure 3 & 4).
HepG2 cells were treated with various concentrations of the 100% ethyl acetate fraction of the stem bark of F. trichopoda, and both neutral red and MTT assays used to determine any cytotoxic effects on the cells. Results obtained for the MTT assay indicated that a significant difference in absorbance levels was found between control samples, and those treated with 10μg/ml of plant extract, but not samples treated with 100μg/ml of plant extract (Figure 5). In addition, it was also determined that there was a significant difference in absorbance levels in cells treated with 10μg/ml of the extract and those treated with 100μg/ml of extract (Figure 5).

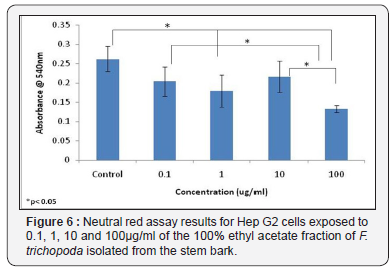
Results obtained for the neutral red assay showed a significant difference in absorbance levels between control samples and those treated with both 1μg/ml of extract 100μg/ ml of extract (Figure 6). In addition, significant differences were observed in results obtained in the neutral red assay for cells treated with 0.1μg/ml and 100μg/ml of plant extract, and for cells treated with plant extract concentrations of 10μg/ml and 100μg/ml (Figure 6).
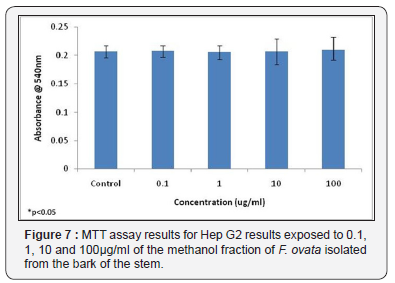
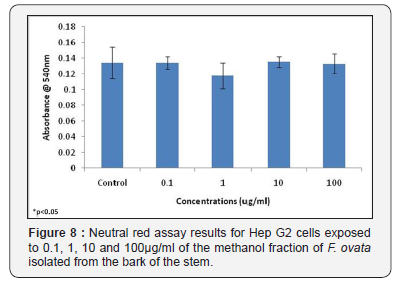
The cytotoxic effects of the methanol fraction of the bark of the stem of F. ovata was also examined using HepG2 cells, and the results obtained for both the MTT assay and neutral red assay showed that, for this plant species, no significant difference in absorbance levels between control samples and those treated with varying concentrations of plant extract was detected (Figure 7 & 8). In addition, no significant differences were observed in either assay used between any of the different concentrations of extract tested (Figure 7 & 8).
Discussion
In order to determine the cytotoxic effects of each of the species, a number of concentrations were tested on HepG2 cells, and their cytotoxic effects determined using both MTT and Neutral Red assays. It was found that for F. ovata, no cytotoxic effects on HepG2 cells were observed for any concentration of the species used. The highest concentration of the extract tested for each species was 100μg/ml, and much higher concentrations may need to be tested on cells in order to observe any cytotoxic effects. For cells treated with extracts of F. lutea, the only significant difference observed in cytotoxic effects in the MTT assay was between cells treated with 10μg/ml and 100μg/ml of extract. However, Neutral Red assay results for this species in relation with other studies indicated that higher concentrations of this species may contribute to significant cytotoxicity [12,13]. It was found that significant differences in cytotoxicity were observed in cell samples treated with 100μg/ml of plant extract when compared to those treated with 0.1, 1 and 10μg/ ml, indicating that the bark of the stem of F. lutea may not be suitable for medicinal purposes at higher concentrations.
Leaf extracts of F. polita may also result in some cytotoxic effects. MTT assay results for this species indicated that significant differences in cytotoxic effects were observed between control samples, and those treated with 1, 10 and 100μg/ml of plant extract. However, neutral red assay results did not show significant differences in cytotoxicity between control samples and the different concentrations of extract tested, which may indicate that the neutral red assay was not as sensitive for detecting cytotoxic effects as the MTT assay for this species.
Earlier studies with other Ficus species showed that these plants have rather a great significance for their traditional use in the treatment of other pathologies than cancer [14-16]. Studies showed in some cases that Ficus thonningii and F. platyhylla showed very weak cytotoxicity with IC50 values 1500.0lg/ml on NBMH mammalian cell lines [16,17]. The ethanolic extracts from F. bryopteris did not display significant cytotoxic activity against the rat skeletal myoblast cell line (L-6 cells) with IC50 values >90, 32.6, and >90 lg/mL, for extracting solvents, toluene, ethyl acetate, and butanol, respectively [5,18].
Some cytotoxic effects were observed on HepG2 cells when treated with extracts of F. trichopoda at varying concentrations. Results of the Neutral Red assay indicated that at concentrations of both 1μg/ml and 100μg/ml of extract, cytotoxic effects were observed on the cells when compared to control samples. However, no cytotoxic effects were observed between control samples, and those treated with 10μg/ml of extract, indicating that this experiment may need to be repeated again to ensure accurate results. In addition, Neutral Red assay results also indicated that at a concentration of 100μg/ml of extract, significant differences in cytotoxic effects were observed on the cells when compared to samples treated with both 0.1μg/ ml and 10μg/ml of extract. These results indicate that high concentrations of the ethyl acetate fraction of the stem bark of F. trichopoda may cause cytotoxic effects, and so its use as a medicinal plant should be limited this in contrast with other studies that showed that cytotoxicity of Ficus sp to be dose dependent [7,19].
MTT assay results also showed a similar trend in results for cells treated with extracts of F. trichopoda. These results indicated that cytotoxicity was observed when HepG2 cells were treated with 10μg/ml of plant extract when compared to control samples. However, since no significant difference was observed between control samples and those treated with 100μg/ml of extract, this experiment should be repeated to ensure the accuracy of the results. MTT assay results also indicate that significant differences in cytotoxicity were observed in cells treated with 10μg/ml of plant extract, and those treated with 100μg/ml of extract. Taken together, both the MTT and neutral red assay results indicate that some cytotoxic effects may be observed when higher concentrations of extracts of F. trichopoda are used, and so if used as a medicinal plant, low concentrations of extract should be used.
Other studies have demonstrated that the crude extracts of the wood of F. elastica aeriel roots and S. vogelii leaves presented low antiplasmodial and very important antitrypanosomal activities associated with a low cytotoxicity [3,20]. The comparison between the cytotoxicity effects suggests that the decreased viability of parasites may not be caused by a general cytotoxicity of the extracts [21,22]. These results indicate that the selected medicinal plants should be explored more actively in order to isolate the main compounds responsible for the pharmacological action [22,23]. It is important to mention that to the best of our knowledge, this study represents the first report on cytotoxic, evaluation for extract of Ficus ovata. The obtained results support to some extent the safe traditional uses of these plants for the treatment of some poverty related diseases in folk medicine. Isolation, purification, and structure elucidation of constituents from these plants are important to support discovery of new chemical entities for biological activities..
Acknowledgement
The authors are thankful to the Centre for Biopolymer and Bio- molecular Research, Athlone Institute of Technology, County Westmeath, and Republic of Ireland for providing the reagents and laboratory facilities for the study. The natural product chemistry, Faculty of Science of the University of Yaoundé 1 for the extraction and phytochemical screening of the plant materials.
Sources of Funding
This work was funded by the Centre for Biopolymer and Biomolecular Research, Athlone Institute of Technology, County Westmeath, Republic of Ireland, and the research mobilization grant from the Ministry of Higher Education, Cameroon.
Policy Issues
All statements and opinions are the responsibility of the authors. All authors have participated in the research, and have reviewed and agree with the content of the article.
References
- Rout SD, Panda T, Mishra N (2009) Ethno-medicinal plants used to cure different diseases by tribal’s of Mayurbhanj district of North Orissa. Ethno-Med 3(1): 27-32.
- Sirisha S, Sangeeta N, Chetty L (2010) Antioxidant properties of Ficus species-a review. Int J Pharm Tech Res 2(4): 2174-2182.
- Sivakumar K, Sivakumar N, Vijayabaskaran P, Pret al. (2008) Antioxidant activities of Triumfetta rhomboidea against dalton’s ascites lymphoma bearing swiss albino mice. Research J Med Sci 2(4): 203-208.
- Uttara M (2008) Evaluation of antioxidant activity of aqueous extract bark of Ficus glomerata. Research J Pharma Technol 1(4): 537-538.
- Kantor R Roth (2007) Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health. Med 13: 30-35.
- Devmurari G, Ghodasara I, Jivani L (2010) Antibacterial activity and Phytochemical study of ethanolic extract of Triumfetta Rhomboidea Jacq. International Journal of Pharm Tech Research 2 (2): 1182-1186.
- Gbeassor K, Koumaglo A, Agbo A, Souza de C, Agbo K, et al. (1990) In vitro anti-malarial activity of six medicinal plants. Phytother Res 4(3): 115-117.
- Kubmarawa A, Ajoku P, Enwerem O, Okorie DA (2016) Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria. Journal Biotechnol 6(14): 1690-1696.
- Kuete V, Kamga H, Sandjo Z, Ngameni B, Poumale Herve MP, et al. (2011) Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl. (Moraceae). BMC Complement Altern Med 11(6): 61-68.
- Lee R (2000) Research and future trends in the pharmaceutical development of medicinal herbs from Chinese medicine. Public Health Nutr 3(4A): 515-522.
- Ao L, Elozaawely P, Xuan G, Tran D, Shinchiki T, et al. (2008) Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L.fil. extract. Food Control 19(10): 940-948.
- Ayisi N, Nyadedzor P (2003) Comparative in vitro effects of AZT and extracts of Ocimum gratissimum, Ficus polita, Clausena anisata, Alchornea cordifolia, and Elaeophorbia drupifera against HIV-1 and HIV-2 infections. Antiviral Res 58(1): 25-33.
- Mevy B, Rabier D, Ruzzier M, Dherbornez M, Ruzzier M, et al. (2006) Composition and antimicrobial activities of the essential oils of Triumfetta rhomboidea Jacq. Flavour and fragrance journal 21(1): 80- 83.
- Rodriguez-fragoso R, Reyes-esparza P, Burchiel H, Herrera-Ruiz D, Torres E (2008) Risks and benefits of commonly used herbal medicines in Mexico. Toxicol Appl Pharmacol 227(1): 125-135.
- Dias DA, Urban S, Roessner U (2012) A historical overview of natural products in drug discovery. Metabolites 2(2): 303-336.
- Falade MO, Akinboye DO, Gbotosho GO (2014) In vitro and In vivo antimalarial activity of Ficus thonningii Blume (Moraceae) and Lophira alata Banks (Ochnaceae), identified from the ethnomedicine of the Nigerian middle belt. J Parasitol Res doi: 10.1155/2014/972853
- Galati EM, Monforte MT, Tripodo MM, d’Aquino A, Mondello MR, et al. (2001) Antiulcer activity of Opuntia Ficus indica (L.) Mill. (Cactaceae): ultrastructural study. J Ethnopharmacol 76: 1-9.
- Jio fack T, Fokunang C, Guedje N, Kemeuze V, Fongnzossie E, et al. (2010) Ethnobotanical uses of medicinal plants of two ethnoecological regions of Cameroon. Int J Med Med Sci 2(3): 60-79.
- Mbosso EJ, Teinkela X, Siwe N (2017) Biological activities of plant extracts from Ficus elastica and Selaginella vogelli: An antimalarial, antitrypanosomal and cytotoxity evaluation. Saudi J Biol Sci 25(1): 2017.
- Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3): 629-661.
- Rao CV, Verma AR, Vijayakumar M, Rastogi S (2008) Gastroprotective effect of standardized extract Ficus glomerata fruit on experimental gastric ulcers in rats. J Ethnopharmacol 115(2): 323-326.
- Pistelli L, Chiellini EE, Moselli I (2000) Flavonoids from Ficus pumila. Biochem Syst Ecol 28(3): 287-289.
- Truong NH, Seung HK, Jin KK (2012) Chemical constituents of the Ficus elastica leaves and their antioxidant activities. Bull Kor Chem Soc 33: 3461-3464.






























