The Significance of Pseudomonas aeruginosa Infection and Biofilms to the Cystic Fibrosis Patients: The Need for the Development of New Therapies
Justin Asbury and Jalal A Jazayeri*
School of Biomedical Sciences, Charles Sturt University, Boorooma Street, Wagga Wagg, NSW 2678, Australia
Submission: January 23, 2018; Published: February 02, 2018
*Corresponding author: Jalal A Jazayeri, School of Biomedical Sciences, Charles Sturt University, Boorooma Street, Wagga Wagg, NSW 2678.
How to cite this article: Justin A, Jalal J. The Significance of Pseudomonas aeruginosa Infection and Biofilms to the Cystic Fibrosis Patients: The Need for the Development of New Therapies. Adv Biotech & Micro. 2018; 8(4): 555742. DOI: 10.19080/AIBM.2018.08.555742
Abstract
Pseudomonas aeruginosa (PA) is an opportunistic pathogen, responsible for severe, chronic lung infections in immune compromised persons. Cystic fibrosis (CF) patients are among the most heavily affected people group, with 80% or more becoming chronically infected by early adulthood. One of the most important features of PA infection is the formation of biofilms. Biofilms are heavily resistant to antimicrobial therapy and are the foundation of chronic lung infections evident in CF patients. The urgent need for new therapeutic options against PA is highlighted by increasing antibiotic resistance and the inability of all current treatments to eliminate PA biofilms. Such new treatments may include: quorum sensing inhibitors, antimicrobial peptides and bacteriophage therapy. In particular, this review will examine the pathogenic mechanisms of PA, the ability of PA to form biofilms and explore potential future therapies. The protection offered to PA bacteria by biofilm formation is most likely multi-factorial in nature. Therefore, it is probable that no one treatment will prove to be fully effective against PA infection and combination therapy is the most likely way forward.
Keywords: Pseudomonas aeruginosa; Cystic fibrosis; Biofilms
Introduction
Pseudomonas aeruginosa (PA) is a motile, Gram-negative, bacillus bacterium of the Pseudomonadaceae family [1]. PA is found extensively in nature and is known to inhabit most natural and man-made environments [2]. PA is robust, with many features enabling continued survival under both favourable and hostile conditions [3]. One such feature is PA’s minimal nutritional requirements and the ability to utilise a diverse range of foods for energy. PA is classed as a facultative anaerobic organism [4]. As such, the self-production of ATP to meet metabolic demand can be met and sustained in the presence or complete absence of oxygen. PA also has the ability to convert to a mucoid or biofilm forming phenotype, resulting in the hyper-production of secreted alginate. This situation enables PA to establish a biofilm mode of growth, offering additional protection from environmental factors, enhancing survival [5,6].
PA is a known opportunistic pathogen, causing chronic infection and severe disease in humans and animals. PA regularly infects immune compromised patients or patients with pre-existing respiratory diseases, such as CF [2]. Four main problems exist regarding the PA infection in CF patients. Firstly, PA is contained in respiratory aerosols and is readily transmissible between immune compromised and hospitalised patients [2]. Secondly, the very high incidence of PA infection among immune-compromised patients increases the probability of inter-patient transmission [3]. Thirdly, PA is known to cause severe and extensive chronic infection, resulting in irreversible damage to respiratory tissues and a steady decline in lung function over time [3]. Fourth, PA contained within biofilms is highly resistant to the functions of the host immune system and all current antimicrobial agents. Once an infection is established it usually persistes for the entire life of a patient and cannot be fully eradicated by current antimicrobial agents [5]. This final point particularly emphasises the urgency in developing new strategies and treatments in the fight against PA infection.
PA and cystic fibrosis patients
Pathogenesis and pathophysiology of cystic fibrosis
Cystic fibrosis (CF) is an autosomal recessive genetic condition, caused by a mutation on human chromosome 7. Specifically, this mutation occurs within the gene encoding the cystic fibrosis trans-membrane conductance regulator (CFTR) [7,8]. The CFTR is a trans-membrane protein responsible for the transport of thiocyanate and chloride across the cellular membrane. A three nucleotide deletion within the CFTR gene is the predominant causative mutation found in CF patients and results in the creation of a defective protein. Specifically this mutation results in the removal of a phenylanalyne residue at the 508th position of the translated CFTR protein. Subsequently, a defective CFTR (or no protein at all) is inserted into the cell wall, resulting in impaired chloride movement across the cell membrane [7]. The lack of chloride transport within the respiratory system has an osmotic effect, reducing the volume of the paraciliary fluid in the lower respiratory tract [9]. Consequently, respiratory mucous is abnormally thick and sticky. This abnormally viscous respiratory mucous hampers mucociliary clearance and subsequent obstruction of the respiratory passages occurs [9]. There are numerous theories that attempt to explain why CF patients are more susceptible to PA infection. One theory suggests reduced mucocilary clearance contributes to the susceptibility of the CF lung to bacterial infection. In this case, bacterial attachment to the respiratory epithelium is more likely and the initial phases of infection are favoured [5]. Another theory suggests the elevated salt content of airway mucous inhibits functions of the innate immune system, rendering the subject more prone to infection by inhaled pathogens [10].
Cystic fibrosis patients are predisposed to PA infection
The patho-physiological processes occurring within the lungs of a CF patient, predispose that individual to repeated respiratory infection [11]. A concerning statistic is that around 80% of CF patients will have established chronic PA lung infection by early adult life [12]. Some studies suggest cross infection between CF patients in hospitals and respiratory clinics contributes heavily to this high incidence [5]. PA infections include periods of slow disease progression, combined with intermittent episodes of acute exacerbation [7]. Often PA infections produce few or no notable symptoms in the early chronic phase [6]. During acute exacerbations, a notable decline in lung function occurs that is not always fully reversible [7]. Even when CF is well managed by medications, a slow decline in lung function still occurs over time due to the irreversible damage caused by the PA infection. 80-95% of all CF patients ultimately succumb to respiratory failure caused by the damaging effects of PA infection and the associated inflammation [13].
The Biofilm Mode of Growth
One of the most important patho-physiological features occurring in CF patients is the ability of PA to form bacterial biofilms. Almost all bacteria have the ability to form biofilms as a means of survival in adverse conditions [5,14]. Biofilms are a self-secreted mix of polysaccharides, proteins and extracellular DNA fragments. These components form an organised matrix that attaches to living and non-living surfaces, encasing the bacterium [15,16]. Oddly, the pathophysiological processes within the lung of a CF patient appear to provide conditions favourable for the initiation and development of biofilms. Impaired mucocilary clearance and the anaerobic environment found within the thick, dehydrated respiratory mucous provide ideal conditions for biofilm formation [6]. PA that colonise the epithelium of the CF airway alters its own gene expression to switch to a mucoid (or biofilm producing) phenotype [13,17]. When a biofilm is formed, the encased bacteria are shielded from the immunological responses of the host [3,18]. Similarly, the structure and function of the biofilm offers the encased bacteria increased protection from antimicrobial agents [15]. Once established, a biofilm infection usually persists unless the affected tissue is surgically removed [19]. The adaptive response of biofilm formation is one explanation demonstrating how chronic PA infections endure for decades or even the entire life of a CF patient [15]. Likewise, the biofilm formation can suggest why no current antimicrobial agents are successful in completely eradicating PA infections from the lungs [5,15].
Formation of biofilms
The initiation and formation of a biofilm into a mature structure is a successive series of events. The exact details and number of steps involved in biofilm formation are still somewhat debated. However, most authors agree at least three phases occur. These phases are: attachment of the bacterium to a living or nonliving surface; production of the extracellular polymer matrix and; the liberation of bacterial cells from the mature biofilm structure (Figure 1) [5]. Extremely fast and efficient processes enable PA to establish a mature biofilm within 5-7 days of initial infection [5]. This efficiency probably explains why PA is the most notable gram-negative bacterium causing chronic human disease. Given that CF and other immune-compromised patients are already at greater risk of the initial stages of PA infection; the speed of PA biofilm production further highlights the urgency in developing more adequate preventative and eliminative treatment strategies.
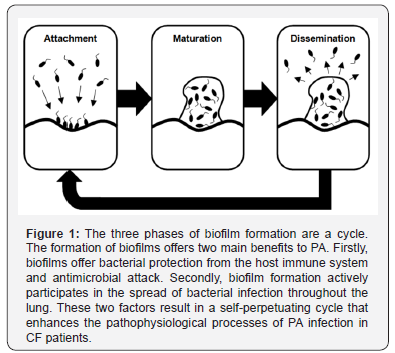
The attachment phase
The attachment phase is seen as the critical step initiating the formation of a bacterial biofilm [5]. The attachment phase is considered to occur as a sequential two-step process by some authors [5]. The initial phase includes a reversible attachment governed primarily by non-covalent forces. PA is a motile bacterium; therefore cell surface flagella and pili manoeuvre this bacterium against the surface and aid the establishment of the initial attachment. The subsequent attachment phase establishes an irreversible connection between the surface and bacterium. Within the human body, attachment of PA can occur on a range of living and non-living surfaces. These surfaces include: the airway epithelium, endo-tracheal tubes, intravenous catheters, heart valves (artificial and native) and coronary stents [5]. Research has indicated the cellular features of flagella and pili to be crucial in the attachment phase. The significance of flagella and pili contributing to the initial phases of PA infection is further highlighted by mutant strains of PA that lack flagella. These strains appear to be unable to attach to the endothelial surface of the lung; therefore the initial phase of infection and subsequent biofilm formation cannot occur [7,20].
The maturation phase
Following the attachment phase, PA experiences a change in gene expression resulting in an altered mucoid (alginate overproducing) or biofilm producing phenotype [15]. This mucoid phenotype is responsible for the formation of the biofilm structure. Micro-colonies are formed between numerous surface-attached bacteria and the polymer biofilm matrix is constructed by a secretion process [5]. One highly important feature governing the formation and maturation of the biofilm structure is a cell-to-cell communication process known as quorum sensing (QS) [21]. QS between gram-negative bacteria is achieved by the release of signal inducing N-acyl-L-homoserine lactone (AHL) molecules [22]. These signalling molecules are released in very low concentrations into the extracellular fluid where they bind surface receptors of nearby cells. Activation of quorum receptors regulates gene expression by enhancing or suppressing the transcription of certain genes in the target cell [5]. However, AHL is only released in minute quantities by each cell. As cell population increases, so does the concentration of AHL within the biofilm matrix or extracellular fluid. Once a threshold population is reached (or quorum), the signalling molecules can be effective in their regulatory processes [5,22]. In this way, the whole colony of bacteria within the biofilm can function with entirely coordinated gene expression. The maturation process of the biofilm structure is strongly governed by QS [12]. Likewise, the release of virulence factors is governed in the same way [17,23].
The dissemination phase
Dissemination is the final stage of biofilm development. A fully mature biofilm acts as a sanctuary, enabling sustained bacterial survival in hostile environments. Biofilms that form in the lungs of CF patients protect the bacteria from immunological clearance and antimicrobial attack [3]. This protection allows continued clonal propagation to occur, increasing the total population of bacterial cells within the biofilm [24]. Dissemination allows the release of mature bacterial cells from the biofilm into the surrounding environment. A process of localised dissolution occurs, degrading the biofilm structure, liberating bacterial cells [5]. Once outside of the biofilm, the released cells are able to spread to local and distant regions of lung tissue and the process of re-infection and new biofilm formation can begin once more. Clearly, the issue of biofilm formation is not just one of bacterial protection as biofilms are also an active participant in the spread of bacterial infection. Biofilms also actively participate in the spread of bacterial infection (Figure 2). For CF patients this is indeed a concern, as the diseased lung is prone to both the initial infection of PA and is favourable to biofilm formation [6,16,17]. In this way, biofilms contribute to the decline in lung function and disease progression consistently noted within CF patients.
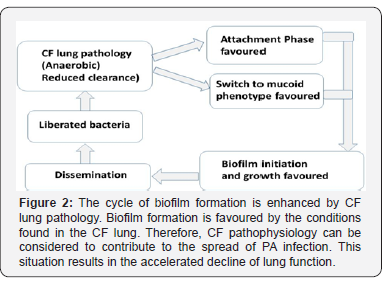
Biofilms offer Increased Protection for PA
Biofilms offer increased bacterial resistance
Biofilms are infamous for conveying increased protection to bacteria from the host immune system and antimicrobial agents. Exactly how this increased protection is offered is a hotly disputed topic. The exact pharmacokinetics and pharmacodynamics of antimicrobials within biofilms are yet to be fully ascertained [6,12,25-27]. Three situations demonstrate that biofilms are indeed responsible for the increased protection of PA in chronic infection. Firstly, the bacteria contained within biofilms are found to have a 100-1000 fold increase in the minimum inhibitory concentrations of antimicrobials when compared to planktonic bacterium [13]. Secondly, bacteria that are liberated from the biofilm revert to having the same resistance as other planktonic bacterium [19]. Thirdly, bacteria that are known to have no resistance to antimicrobials still experience increased protection in the biofilm mode of growth [19]. To complicate matters further, there is a general consensus among researchers that the protective mechanisms of biofilms are likely to be multifactorial [15].
Structure of biofilms and increased bacterial resistance
Several key features of the biofilm structure have been suggested as mechanisms of increased bacterial resistance. The physical construction of the biofilm has been hypothesised by many to be a primary determinant of biofilm resistance. Such theories state the polymer matrix acts as a physical barrier, separating the encased bacterium from the external environment [5,9]. For an antimicrobial agent to be effective, direct physical contact must be made with the target bacterium. In this way the exposed planktonic bacteria are more susceptible to antimicrobial attack, while bacteria within the biofilm remain shielded and protected [9]. In a similar theory, the biofilm matrix is thought to retard diffusion of antimicrobial agents that manage to penetrate within the structure [9]. However, both of these theories are not without conjecture. Long-term exposure to an antimicrobial agent would still result in significant antibiotic concentrations entering and remaining within the biofilm structure, despite any theoretical retardation of diffusion [9]. Other studies have also suggested the rate of diffusion is only lowered by a factor of 2-3 and this is not sufficient to explain the vast level of protection offered by the biofilm [19]. Clearly, additional factors must contribute to the protective and sustaining attributes of biofilms.
Internal variation of biofilms
Biofilms form organised colonies and have extensive internal variation [9]. Bacteria near the surface of the biofilm consume most of the available oxygen and nutrients. This situation results in gradients of oxygen and nutrient supply throughout the biofilm [18]. Basal areas of the biofilm tend to contain the lowest concentrations, forming niches of bacterial cells starved of oxygen and nutrient supply. Bacteria within these niches are sustained in an almost dormant state. The cells are still viable; however are slow growing and have an extremely low metabolic rate [5]. Most antimicrobial agents mount an attack against metabolically active cells and require cell growth to produce their deleterious effects [15]. Survival of these dormant cells in the presence of antimicrobial agents suggests at least one mechanism of biofilm tolerance may be mediated by these near dormant bacterial cells. Likewise, the lack of oxygen is known to lower the effectiveness of some antimicrobial agents and may also contribute to bacterial protection [28,29].
Persister cells
A sub-population of bacterial cells known as persister variants (PV) are also said to exist and may help explain the inability of antibiotics to fully eradicate biofilm infections. PV comprise a very low proportion of the entire bacterial population and possess a phenotype that is highly protective and resistant to antimicrobial agents [28]. The exact mechanism explaining how PV offer protection is not known; however several theories exist. One theory proposes that bacteria within a biofilm are readily eliminated by antimicrobial agents while the PV are able to resist the onslaught and live on [19]. A second theory suggests the mode of antimicrobial action is to induce programmed cell death of the target bacteria. PV in this theory are thought to have defective apoptotic mechanisms; therefore cannot be eliminated by antibiotics that use this mode of action [19]. An apparent cooperation is thought to exist between the freely growing bacteria and slow growing PV cells. When antimicrobial or host immune system attack successfully eliminates planktonic and freely growing biofilm bacteria, the PV live on to re-establish the population. When bacterial activity is not impeded, the PV will lie dormant while the freely growing bacteria proliferate and spread infection [30]. PV are thought to exist in far greater proportions within biofilm colonies than their planktonic relatives [19]. The PV theories may help explain why only planktonic bacteria can be effectively eliminated by antimicrobials, while bacterial colonies within biofilms are only contained.
Current Treatments for CF Patients
Current treatments fail to eradicate PA infection
All current CF treatments fail to destroy the biofilm mode of growth of PA bacterium. A range of antibiotic treatments are the most commonly used agents against PA infection within CF patients [5,31]. However, all present antibiotics are ineffective against bacterial colonies contained within biofilms; therefore fail to fully eradicate PA within the lungs. Biofilms allow continued survival of PA, even when bacterial numbers have been drastically reduced. As some bacterial cells always remain, the infection persists and bacterial numbers are restored when antimicrobial treatment ceases [9,19]. Current treatments are merely designed to delay the progression of chronic PA infection and are aimed at treating the symptoms of CF by reducing the bacterial load [12].The overall goals of current treatments are: to improve quality of life; maintain lung function for as long as possible and; increase the life expectancy of CF patients. These goals are primarily achieved by slowing the progressive decline in lung function caused by PA inhabitation – not full elimination of the responsible pathogen.
Prevention is the most effective treatment against PA
The relative ineffectiveness of all current treatments against biofilms makes prevention of PA infection paramount for the health and survival of CF patients [5,32]. One of the most basic prevention methods can be achieved by isolating noninfected patients from those with known PA colonisation [5]. However, the success of isolation is limited and CF patients are frequently in contact with other immune compromised patients in respiratory clinics, hospitals and day-to-day life. The extremely widespread occurrence of PA in nature means even total isolation from infected patients is unlikely to prove fully effective in the long term [5]. Early aggressive antimicrobial treatment with high-dose antibiotics is considered to be one successful method preventing the onset of chronic PA infection [5]. PA bacteria that are planktonic or in the early stages of biofilm formation are still highly susceptible to antimicrobial attack [5,9]. Courses of high-dose antibiotics are able to eradicate PA from the lungs before the mature biofilms are able to form and chronic infection is established [5]. However, the biggest issue for aggressive antibiotic therapy is reliable positive detection of the very early stages of PA infection [22]. Often a direct microscopic examination is required as the agar culture of early biofilm fragments is difficult [22]. Because there is a lack of objective testing to positively identify early PA infection, often antimicrobial therapy is commenced only on suspicion of infection. This method is open to the subjectivity of the diagnosing physician [1,22]. Likewise, patient adherence to treatments plays a role in the effectiveness of early eradication methods [22]. The already intense, time consuming treatment regimen for CF patients makes correct and thorough adherence to any additional prescribed therapies a major challenge.
Combination therapy
The most common treatment for CF associated lung infections are the antibiotics tobramycin and ciprofloxacin. Both tobramycin and ciprofloxacin are highly effective against planktonic PA bacteria [3,5,33]. These agents are used to decrease the microbial load of chronic infection or form part of early aggressive strategies to eradicate PA before mature biofilms can be established [5]. Tobramycin is nebulised and inhaled by the CF patient on a daily or twice-daily basis. Inhalation allows the drug to be administered in higher concentrations within the respiratory passages than would be possible by the oral/ systemic route [33]. Tobramycin treatment is often prescribed on an intermittent basis, with patients taking regular courses of the antibiotic every other month [2]. Ciprofloxacin is an oral antibiotic and prescribed in conjunction with tobramycin. Ciprofloxacin is also highly active against planktonic PA and may also offer some anti-inflammatory benefit within the respiratory system of CF patients [12]. The concurrent prescription of an oral and inhaled drug has the benefit of a more thorough delivery of antimicrobial treatment within the respiratory system. Oral antibiotics are transported to the target site within the patient’s blood supply, resulting in the majority of the drug being delivered to the lower respiratory passages. Inhaled antibiotics are administered directly into the upper reaches of the respiratory system and are mostly deposited within the conductive airways and sputum, with little reaching the respiratory zone [12,33].
Problems that must be addressed for successful treatment of PA
There are several disadvantages to current treatments that must be overcome if successful PA eradication is to occur in CF patients. Most current treatments are antimicrobial in nature. However, the persistence of biofilms, despite antibiotic treatment, presents a significant problem. All antimicrobial agents are developed and chosen for use upon their action against planktonic bacteria and herewith lays a problem [9]. The antimicrobial agents chosen are able to effectively lower the microbial load within the lungs by destroying planktonic bacteria; however these same agents are relatively ineffective against bacteria contained within biofilms. . Another significant issue is PAs highly adaptive and tolerant nature. PA alone has demonstrated a significant rise in antibiotic resistance over the last decade [12,24]. Rising resistance levels threaten the continued use of antibiotic treatments against PA in the longterm. Therefore, it is imperative that research is continued to identify the resistive mechanisms at work within biofilms and to find new non-antibiotic solutions to counter these processes.
Possible Future Treatments
Quorum sensing inhibitors (QSI)
As previously stated, quorum sensing is a sophisticated cellto- cell communication system that allows highly coordinated gene expression thorough an entire bacterial colony. Quorum sensing is integral to the evolution of bacterial colonies into mature biofilm structures and the secretion of virulence factors [23]. Inhibiting quorum sensing was suggested as a possible treatment against biofilms when some marine weeds were observed to resist the formation of bacterial coatings. Secreted, halogenated furanones were identified to be the cause of this phenomenon. These compounds were subsequently shown to have an inhibitory effect on quorum sensing. Likewise, Certain antibiotics have been identified as having an inhibitory effect on quorum sensing and may be helpful in the treatment of PA infection. Azithromycin, a macrolide antibiotic, is one promising possibility [5,34]. Trials of this drug in CF patients and mouse models have thus: showing a significant reduction in bacterial load [34,35]. However, as macrolides are antibiotics, the problem of bacterial resistance again becomes a concern for long-term use. Ideally, if QSI’s can be developed that have no inhibition of bacterial growth or bactericidal activity, resistance may become less of an issue [5,34]. Therefore, QSI’s show significant potential as a future treatment in the fight against chronic PA infections.
Antimicrobial peptides
Antimicrobial peptides (AMP) are highly attractive options in the development of anti-PA therapy. AMP’s are naturally occurring components of the innate immune system [21,30]. AMP’s may confer their effects by several mechanisms including: disruption of the bacterial cell wall; targeting metabolic processes and; destroying components of the cytoplasm. One of the most attractive features of the AMP’s is the non-specific mode of antimicrobial action. This non-specific action appears to confer a broad spectrum of antimicrobial activity and is even effective against highly resistant strains of bacteria. However, AMP’s are not without some disadvantages. Current data suggests that AMP’s are more effective against planktonic bacteria than biofilms. Despite this issue, studies have shown AMP’s are able to reduce the sustainability of PA biofilms. Another problem of AMP’s is a very low bioavailability. Therefore, acquisition of therapeutically useful quantities is a major issue. Despite these concerns, the use of AMP’s does still hold significant promise in the fight against PA infections in the CF patient.
Bacteriophages
Bacteriophages are viruses that specifically infect and destroy bacteria. The popularity and success of current antibiotic treatments has seen a distinct lack of research into the use of bacteriphage therapy over the last century. As such, use of bacteriophages as a therapeutic option has fallen behind [13]. The use of bacteriophage therapy against PA has shown much promise. Studies have shown that the use of bacteriophages in mice was able to reduce PA levels to undetectable levels [13]. A major advantage of bacteriophage therapy is the ability of the virus to replicate at the infection site, increasing the effectiveness of treatment [13]. However, bacteriophage therapy is susceptible to bacterial resistance and antibody attack from the host immune system [36]. One possible solution against the development of bacterial resistance is the simultaneous use of many different bacteriophages. The mode of action for each bacteriophage is different; therefore a bacterial mechanism against each and every phage is required before substantial resistance can be established.
Conclusion
Treating PA infections within the lungs of CF patients is indeed a very complex issue. PA by its very nature is a resilient organism, capable of survival in hostile environments with minimal nutritional and oxygen requirements. PA is an opportunistic pathogen, easily transmissible by patient-topatient contact and infects around 80% of all CF patients by early adulthood. Most CF patients still succumb to respiratory failure caused by the damaging effects of PA infection in the long term. PA is capable of forming resistance against antimicrobial agents and can evade functions of the host immune system. These resistive mechanisms are due to the highly adaptive nature of PA and the ability of PA to form biofilms. An array of resistive mechanisms offered by biofilm formation enhances these resistive capabilities. Biofilms also actively participate in the spread of PA infection throughout the lung. Once a mature biofilm is formed within the lungs, removal is next to impossible with all currently available pharmaceutical treatments. Due to the persisting nature of biofilms, all current CF treatments against PA are reduced to an array of suppressive and preventative antibiotic therapies. The relative ineffectiveness of all current PA therapies is highlighted by the use of preventative measures (such as isolation of infected and non-infected individuals) still being regarded as one of the most successful treatments on offer. Several new therapies are showing promise and include: quorum sensing inhibitors; antimicrobial peptides and; bacteriophage therapy. However, PA infections are complex and possess many resistive mechanisms. Most authors agree that any successful future treatments against PA will probably be multi-factorial in nature. No matter what treatments may prove successful; elimination of PA infections and bio-films remains one of the most important aspects of current and future CF treatment. Careful management of the chronic infection and inflammation caused by PA can improve the quality of life and extend the life expectancy of CF patients. However, without full eradication of PA from the lungs of CF patients; no current treatment can be viewed as ideal. However, without full eradication of PA from the lungs of CF patients; no current treatment can be viewed as ideal. Therefore, the development of new strategies against PA infection is paramount to the general health and life expectancy of CF patients.
References
- El Solh, AA, Alhajhusain A (2009) Update on the treatment of Pseudomonas aeruginosa pneumonia. J Antimicrob Chemother 64(2): 229-238.
- Cohen Cymberknoh M, Shoseyov D, Kerem E (2011) Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med 183(11): 1463-1471.
- Kipnis E, Sawa T, Wiener Kronish J (2006) Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Mal Infect 36(2): 78-91.
- Cornelis P (2008) Pseudomonas: Genomics and molecular biology. Caister Academic Press, Norfolk, UK.
- Hoiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, et al. (2011) The clinical impact of bacterial biofilms. Int J Oral Sci 3(2): 55-65.
- Fricks Lima J, Hendrickson CM, Allgaier M, Zhuo H, Wiener Kronish JP, et al. (2011) Differences in biofilm formation and antimicrobial resistance of Pseudomonas aeruginosa isolated from airways of mechanically ventilated patients and cystic fibrosis patients. Int J Antimicrob Agents 37(4): 309-315.
- Bobadilla JL, Macek M , Fine JP, Farrell PM (2002) Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum Mutat 19(6): 575-606.
- Lenney W (2015) Pseudomonas aeruginosa in cystic fibrosis is potentially serious, and more than merely a marker for disease severity. Paediatr Respir Rev 16 Suppl 1: 35-36.
- Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418): 1318- 1322.
- Pier GB (2007) Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol 297(5): 277-295.
- Davies JC (2002) Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev 3(2): 128-134.
- Hoiby N (2011) Recent advances in the treatment of pseudomonas aeruginosa infections in cystic fibrosis. BMC Med 9(32): 1-7.
- Alemayehu D, Casey PG, McAuliffe O, Guinane CM, Martin JG, et al. (2012) Bacteriophages phiMR299-2 and phiNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. MBio 3(2): e00029-00012.
- Asadpour L (2018) Antibacterial Resistance, Biofilm Forming Ability, and Virulence Potential of Pseudomonas aeruginosa Isolated from Burn Patients in Northern of Iran. J Glob Antimicrob Resist: S2213- 7165(18)30020-30011.
- Drenkard E (2003) Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect 5(13): 1213-1219.
- Maurice NM, Bedi B, Sadikot RT (2018) Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am J Respir Cell Mol Biol p. 13-16.
- Lee K, Yoon SS (2017) Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J Microbiol Biotechnol 27(6): 1053-1064.
- Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms. Trends Microbiol 13(1): 34-40.
- Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. The Lancet 358(9276): 135-138.
- Golovlev EL (2002) The mechanism of formation of pseudomonas aeruginosa biofilm, a type of structured population. Microbiology 71(3): 249-254.
- Kapoor R, Wadman MW, Dohm MT, Czyzewski AM, Spormann AM, et al. (2011) Antimicrobial peptoids are effective against pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 55(6): 3054-3057.
- Chen L, Wen YM (2011) The role of bacterial biofilm in persistent infections and control strategies. International Journal of Oral Science 3(2): 66-73.
- Veesenmeyer JL, Hauser AR, Lisboa T, Rello J (2009) Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med 37(5): 1777-1786.
- Mulcahy LR, Burns JL, Lory S, Lewis K (2010) Emergence of pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol 192(23): 6191-6199.
- Venkatesan N, Perumal G, Doble M (2015) Bacterial resistance in biofilm-associated bacteria. Future Microbiol 10(11): 1743-1750.
- Hoiby N (2017) A short history of microbial biofilms and biofilm infections. APMIS 125(4): 272-275.
- Hoiby N (2014) A personal history of research on microbial biofilms and biofilm infections. Pathog Dis 70(3): 205-211
- Stewart PS (2001) Multicellular resistance: biofilms. Trends Microbiol 9(5): 204.
- Drenkard E, Ausubel FM (2002) Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416(6882): 740-743.
- Pompilio A, Crocetta V, Scocchi M, Pomponio S, Di Vincenzo V, et al. (2012) Potential novel therapeutic strategies in cystic fibrosis: antimicrobial and anti-biofilm activity of natural and designed alpha-helical peptides against Staphylococcus aureus, pseudomonas aeruginosa and Stenotrophomonas maltophilia. BMC Microbiol 12(1): 145.
- Dorotkiewicz Jach A, Augustyniak D, Olszak T, Drulis Kawa Z (2015) Modern Therapeutic approaches against Pseudomonas aeruginosa infections. Curr Med Chem 22(14): 1642-1664
- Talwalkar JS, Murray TS (2016) The Approach to Pseudomonas aeruginosa in Cystic Fibrosis. Clin Chest Med 37(1): 69-81.
- Tan KH, Mulheran M, Knox AJ, Smyth AR (2003) Aminoglycoside prescribing and surveillance in cystic fibrosis. Am J Respir Crit Care Med 167(6): 819-823.
- Van Delden C, Kohler T, Brunner Ferber F, Francois B, Carlet J, et al. (2012) Azithromycin to prevent Pseudomonas aeruginosa ventilatorassociated pneumonia by inhibition of quorum sensing: a randomized controlled trial. Intensive Care Medicine 38(7): 1118-1125.
- Moreau Marquis S, Stanton BA, O Toole GA (2008) Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther 21(4): 595-599.
- Saeidi N, Wong CK, Lo TM, Nguyen HX, Ling H, et al. (2011) Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol 7: 521.






























