Plant Anthocyanins: Biosynthesis, Bioactivity and in vitro Production from tissue cultures
Tanya Biswas and Archana Mathur*
Plant Biotechnology Division, Central Institute of Medicinal & Aromatic Plants, India
Submission: July 26, 2017; Published: August 24, 2017
*Corresponding author: Archana Mathur, Plant Biotechnology Division, Central Institute of Medicinal & Aromatic Plants, Council of Scientific & Industrial Research, PO CIMAP, Lucknow-226015, India, Tel: +91-522-2359623/2357134; Fax: +91-522-2342666; Email: archnacimap@gmail.com
How to cite this article: Tanya B, Archana M. Plant Anthocyanins: Biosynthesis, Bioactivity and in vitro Production from tissue cultures. Adv Biotech & Micro. 2017; 5(5): 555672. DOI: 10.19080/AIBM.2017.05.5556672
Abstract
Anthocyanins are a major class of colorful plant pigments, with the exception of chlorophylls, that have long attracted the attention of chemists and biologists, investigating their biosynthesis patterns, metabolism and physiological roles. Belonging to the group of "flavonoids”, these anthocyanins accumulated in the vacuoles, are mainly responsible for the bright and distinct coloration to fruits, vegetables and flowers. Besides attracting pollinators, this particular class of compounds is often considered as potent "anti-oxidants”, largely impacting human health maintenance. Due to their immense importance as dietary neutraceuticals, enhanced production of these anthocyanins from cell/tissue cultures have been extensively explored since the last 2 decades. This review summarizes the different types of anthocyanins, their basic chemistry and biosynthesis, in vivo bioactivities and concludes with collation of major reports regarding in vitro production of anthocyanins.
Keywords: Anthocyanin; Flavonoid; Phenylalanine; Cardio protection; Elicitation
Introduction
Anthocyanins, one of the most important plant metabolites, are a group of naturally occurring pigments responsible for red- blue coloration in most fruits and vegetables. Belonging to the "flavonoid family", their structures (more than 500 in number) have been intensively elucidated [1,2]. Anthocyanins are of immense human interest due to their potential implications in maintenance of human health.
These pigments are present in different plant organs such as fruits, flowers and leaves etc [3]. Present exclusively in the vacuoles and for some species in the vacuolar compartment- the anthocyanoplasts, their main sources are red apples, red grapes, berries (blackberry, blueberry, cranberry, raspberry, strawberry), pomegranates, vegetables (red cabbage, red onion, red radish) and purple maize in amounts ranging from 20-1800mg/100g [4]. Flavonoids are a group of secondary metabolites which belong to the class of phenylpropanoids, They are primarily responsible for the red- blue colors found in many flowers, leaves and fruits [5]. Betalains (yellow-to-red) are nitrogen-containing compounds derived from tyrosine. They are also water-soluble and stored in vacuoles, present exclusively in Caryophyllalles. Carotenoids are isoprenoids and are found universally in plants and microorganisms, imparting yellow- to-red coloration to flowers and fruits, besides being important components of plant photosystems. Anthocyanins are believed to be functioning as photo protective pigments for the plant, and preventing oxidative damage. Anthocyanins are found to be induced via stresses such as UV radiation, pathogen attack etc [6].
The Structure and Biosynthesis: The Flavonoid Synthesis Pathway
Chemically, anthocyanins primarily possess anaglycone backbone, to which monosaccharides are attached at different positions, resulting in wide variety of flavonoids and colors (pale-yellow to blue) observed in nature. The aglycone forms of anthocyanins are categorized as "anthocyanidins”. A dozen of them have been described, but based on the different hydroxyl/ methoxy substitutes, 6 of them are widely present in nature, in fruits and vegetables [7]. The anthocyanidin is typically a flavilium ion (2-phenylbenzopyrilium). The 6 major classes of anthocyanidins are shown in Figure 1 namely cyanidin, pelargonidin, delphinidin and their methylated derivatives malvidin, peonidin and petunidin. These anthocyanidins differ at the hydroxyl/methoxy groups present at the 3' and 5' position. More the number of hydroxyl groups, the bluer the color. More the methoxy group addition, redder the color

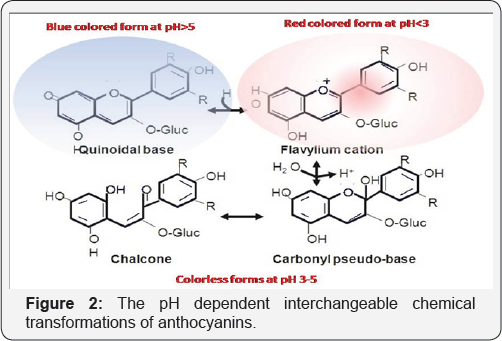
Anthocyanins are extremely water soluble. However, they exhibit a very interesting chemistry in aqueous solutions, with 4 major inter-convertible species, with varying relative amounts at a particular pH. At low pH, flaviliumcation is most prominent with a deep red color. As pH increases, they convert to colorless forms such as pseudobases and chalcones and at a pH more than 5; it changes to a blue colored quininoidal form Figure 2 [8]. These anthocyanidins are further attached to sugars such as glucose, galactose and rhamnose, via α/β linkage exclusively at position 3 of the aglycon. Alternatively they can also be acylated by cinnamic acid, caffeic, ferulic, malic, oxalic and succinic acid, to name a few [9].
The biosynthetic pathway elucidating the major enzyme systems and chemical transformations are shown in Figure 3. Synthesized cytosolically from phenylalanine, the enzyme systems have been hypothesized to form a "supra-molecular" complex, anchored in the endoplasmic reticulum [10]. Chalcone synthase (CHS) catalyzing formation of chalcones (Naringenin/ erodictoylchalcone) from coumaryl CoA or caffeoyl CoA along with 3 molecules of malonylCoA, is the first committed step of the pathway. Subsequently, they are isomerized to yield the typical flavanonesienaringenin and erodictoyl (Figure 3). Flavonoid 3' hydroxylase (FHT), is a 2-oxoglutarate-dependent dioxygenase, which catalyzes the formation of dihydroflavanols such as dihydrokaempferol, dihydroquercetin and dihydromyrecitin from the flavanones, naringenin, erodictoyl and pentahydroxylflavanones, respectively. Alternatively as shown in Figure 3, dihydrokaempferol can by hydroxylated at 3' (F3'H) and 3'-5' (F 3'5'H) to yield the twodihydroflavanols. Flavonoid 3' hydroxylase and flavonoid 3'5'hydroxylase are CYT P450 enzymes and are necessary for cyanidin and delphinidin synthesis.
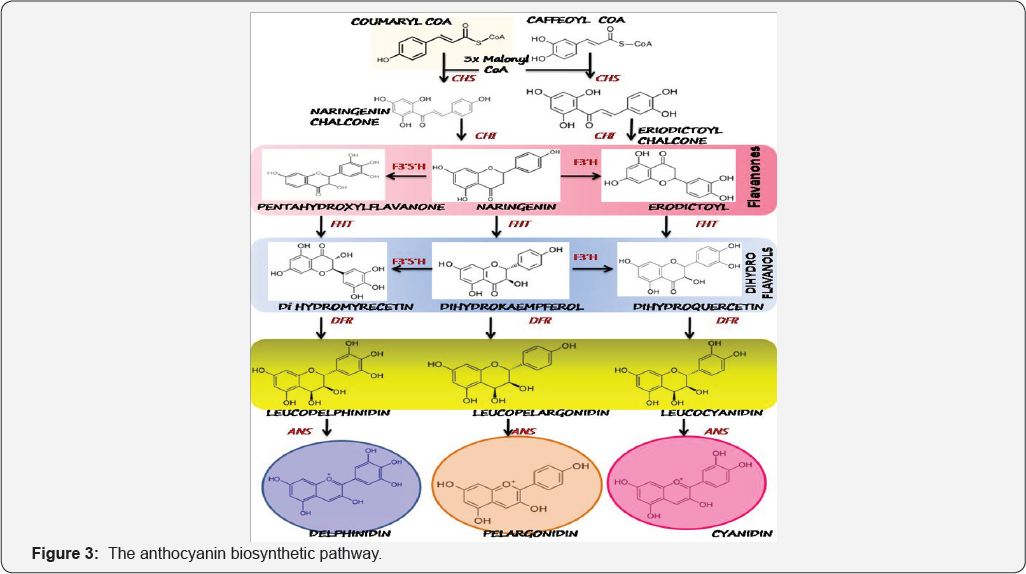
(CHS: Chalcone synthase; CHI: Chalconeisomerase; F3'H: Flavanol 3' hydroxylase; F3'5'H: Flavanol 3'5' hydroxylase: DFR: Dihydroflavanolreductase; ANS : Anthocyanidin synthase).
The dihydroflavanols are further catalyzed by dihydroflavanolreductase (DFR), which leads to synthesis of leucoanthocyanidins. In some species of Petunia, DFR has been observed to exhibit extreme substrate specificity, rejecting dihydrokaempferol as substrate. They consequently lack the brick-red coloration of pelargonidin in their flowers [8]. Anthocyanin synthase (ANS) again a dioxygenase, finally leads to production of the coloredanthocyanidins. These anthocyanidins can further attach a sugar at the 3 position to yield the corresponding glucosides, catalyzed by glycosyltransferases which belongs to the UFGT family (UDP glycosyltransferase).
Many factors seem to operate at regulating anthocyanin accumulation in plants, the most important being environmental conditions such temperature, light intensity etc. Fruits of grape, strawberry and lychee have been reported to show enhanced anthocyanin accumulation by increasing exposures to sunlight [11-13]. Decreased anthocyanin accumulation was observed when grapes were shaded (light intensity decreased) at veraison [14]. Low temperatures have long been known to promote anthocyanin synthesis [15]. Studies in apples have shown that they accumulate more anthocyanin when irrigated with micro sprinkler system at sunset and sunrise [16]. An increased PAL and CHS activity was reported in berries grown at low night temperature [17]. Plant hormones such as ABA [14], ethylene [18] and ethephon [19] have also been shown to increase anthocyanin accumulation. Since it is known that anthocyanin synthesis continues even after harvest, postharvest storage conditions such as maintenance of low temperatures, apt carbon dioxide concentrations, also have a prominent effect on the anthocyanin amount and quality in the food source.
Anthocyanins and Biological Activity
Anthocyanins are primarily antioxidants, exhibit free radical scavenging activity and are reported to manifest a range of bioactivities.
Anthocyanins and cardiovascular protection
Among one of the most studied effects of anthocyanins, they have been observed to have a "heart friendly" tendency. Atherogenesis can be attributed to MCP 1 protein release and anthocyanins have been shown to exert a protective effect against its secretion in human endothelial cells [20]. Similarly anthocyanins have also been shown to prevent release of VEGF (vascular endothelial growth factor), a pro-atherosclerotic factor in vascular cells [21]. In a different study, rats were treated with isoproterenol to induce post infarction remodeling and were fed with red wine which showed a protective effect on hearts by repressing hypertrophy-associated increased phosphorylation of protein kinase C (PKC) α/β II and by activating Akt/protein kinase B (Akt). Anthocyanins also are reported to have an effect on cholesterol distribution, protecting endothelial cells from CD40-induced proinflammatory signaling. It has been shown that the anthocyanin delphinidin decreases the extent of both necrotic and apoptotic cell death in cultured cardiomyocytes and reduces infarct size after ischemia in rats [4].
Anthocyanins and cancer
Chemo preventive properties have been reported extensively for anthocyanins. Different proteins related to cell cycle and cell death are attractive targets for anthocyanin based cancer prevention. It has been shown that red wine is capable of reducing proliferation of human colon cancer cell line and gastric adeno carcinoma [22,23]. Liu and team also reported prevention of human liver carcinoma cell line proliferation by raspberry extracts [24] the apoptosis inducing effect of anthocyanin glucosides have been reported in leukemia cell lines [25] and hepatoma cell lines [26]. Angiogenesis i.e. blood vessel system development in cancer cells, is a major factor responsible for proliferation of cancer cells. Its inhibition by black raspberry has been found to inhibit tumor development [27]. Even mutagenesis induced by methyl methane sulfonate and benzopyrene has been reported to be significantly inhibited by juices from anthocyanin rich fruits [28]. Further Marko and group (2004) demonstrated the inhibitory effect of anthocyanidins in human vulva carcinoma and colon carcinoma cell proliferation [29]. Clinical trials have shown that the consumption of pomegranate juice can significantly delay the reoccurrence and metastasis of prostate cancer following radical surgery or radiation therapy [30]. Berry (strawberry, raspberry, blackberry, blueberry etc) components, such as anthocyanidins, proanthocyanidins, flavonols, flavanols, stilbenoids, terpenoids, ellagitannins, and ellagic acid target oxidative and UV radiation stress-induced DNA damage and are known to act as chemo preventive agents [31]. Recently it has been reported that consumed blackberries alters innate cell trafficking in esophageal cancer [32]. A decreased expression of the proinflammatory cytokine IL1β followed with an increased expression of the anti-inflammatory cytokine IL10 was observed. Additionally they also increased the expression of IL12, a cytokine that activates both cytolytic natural killer and CD8+ T cells.
Other bioactivities reported
Anthocyanins have been shown to demonstrate an antidiabetic effect in rats by Jayaprakasam and group who observed stimulation of insulin secretion under the effect of monoglucosides of cyaniding and pelargonidin [33]. They have also been implicated in protection from hepatic injury [34]. Even ocular defects such as myopic conditions, are reported to improve after anthocyanin administration, however the use of anthocyanins for night vision improvement is still controversial [35]. Anthocyanins have even been reported to exert a beneficial effect on Alzheimer’s disease in a transgenic mouse model [36]. They have been reported to demonstrate anti-microbial and anti-oxidative effects [37,38].
In vitro Production of Anthocyanins and their Elicitation
Anthocyanin production has been reported to be biosynthesized in vitro in a number of plant systems as elucidated in Table 1 [39-61]. The most extensively studied system is the Vitis vinifera, also known as the Cabarnet Sauvignon or the humble black grape. Strawberry culture systems have also been investigated for their in vitro anthocyanin production potential. The three most important factors that decide the anthocyanin biosynthetic capacity of a particular plant system are sugar concentration, temperature and light irradiation. Light irradiations in Perilla frutescens cell suspension culture have been observed to be a positive regulator for anthocyanin biosynthesis with a 1.6g/L anthocyanin yield obtained [38]. In a separate study on methyl jasmonate elicited Vitis vinifera, 13.2 fold enhanced anthocyanin accumulation was reported when kept under continuous light irradiation [53]. Maier and team have reported that light irradiation provides stability to two small crucial proteins, Pap 1 and Pap 2, in Arabidopsis, which get degraded in dark conditions. Light stabilized Pap 1 and Pap 2 further activate the transcriptional factors that induce anthocyanin pathway structural gene expression [62]. Low temperatures have also been observed to have a stimulatory effect on anthocyanin biosynthesis. However, as observed by [44] biomass accumulation was maximum at 30 °C, after which strawberry cell suspensions were shifted to 20 °C, at which a 4 fold increase in anthocyanin content was observed. Similar phenomenon was also observed with Perilla frutescens cell suspensions, with maximum biomass at 28 °C, however with reduced anthocyanin synthesis. Maximum pigment volume could only be attained at 25 °C [42]. The most important and the most extensively studied factor, is the sucrose concentration, which seems to have a significant impact on in vitro plant anthocyanin synthesis. Increase in sucrose has been observed to a have a direct co-relation with increased anthocyanin accumulation [42,48,52,54,59]. Vitrac et al. [52] conducted a series of experiments to determine the involvement of calcium and calmodulin in sugar signal perception. They concluded that hexokinase, which phosphorylates the glucose, plays an important role in the sugar sensing. Calcium and calmudulin mediated activation of a cascade of protein kinase/phosphatase activities may help in transferring the sugar signal to the genomic encoded machinery for anthocyanin synthesis [52]. Elicitation strategies have also been employed to enhance anthocyanin biosynthesis, most predominantly in Vitis. Abiotic elicitations are frequent as opposed to biotic mode of elicitation. Methyl jasmonate is a fruitful elicitor for Vitis vinifera cell suspensions [54,57], with enhancement to the tune of 2.8-4.1 folds. Methyl jasmonate has also been reported to have a positive effect on anthocyanin production in Rosa hybrida [51]. They observed that although MeJA had a negative impact on biomass accumulation, but with highest frequency for color response in callus lines (97.25%). Pectins and ABA additions have also been observed to have a stimulatory effect on Vitis cell suspensions, in terms of anthocyanin production [56,55]. Precursor feeding mostly phenylalanine has also been reported to have a positive effect on anthocyanin accumulation. Repetitive phenylalanine feeding to strawberry cell suspensions led to an enhanced anthocyanin production, as opposed to cultures which were not fed with the precursor [46]. They observed an 81% increase over the nonfed cultures and a 30% increase over a single fed culture. The authors, however, did not observe any growth inhibition, as is likely with higher doses of phenylalanine. Precursor feeding can also be clubbed with elicitation to modulate the anthocyanin biosynthetic pathway. Qu et al. [57] reported a 3.4 fold increase in anthocyanin yield in Vitis vinifera cell suspensions, when treated with 5mg/L phenylalanine and 50mg/L MeJA [57]. Reports on anthocyanin enhancement via biotic elicitations are scanty. Rajendran et al. [40] observed a 27.4% DW anthocyanin content in Daucus carota cell suspensions when elicited with mycelial extract of Aspergillusflavus. Cai et al. [63] reported a 7 fold increase in resveratrol production in Vitis cultures from a biotic elicitor prepared from insect salivae (Manduca sexta).

Our team at CSIR-CIMAP has also been extensively involved towards in vitro production of anthocyanins since the past 15 years. The team has been working in the field of Panax biotechnology especially in unexplored Indian ginseng congeners from the North-East. One such ginseng congener i.e. Panaxsikkimensis from Sikkim, India has been explored for its in vitro secondary metabolite production. The team has been the first group in India to develop and patent an anthocyanin producing red colored cell line of Panaxsikkimensis that could co-accumulate secondary metabolites ieginsenosides as well as anthocyanins [64]. The anthocyanins stably accumulated in this particular cell line were found to be of the "peonidine" type [60]. Cell suspensions developed from this cell line was observed to be a potentially rich source of ginsenosides (77mg/L) and anthocyanins (199mg/L) at the shake-flask level [56]. Studies have been conducted to identify elicitors, combined with precursor feeding strategies that successfully led to 3-4 fold enhancements in productions of anthocyanins from these cell suspensions. These cell suspensions exhibited enhanced biochemical activities of PAL and UFGT enzyme systems in a crude cell free preparation. Alternatively enhanced expression of these genes was also observed when these treatments were subjected to Real Time PCR analysis [61]. Bench level up scaling of these treatments in a 3-5 litre bioreactor is underway
Conclusion
Anthocyanins as a group of chemical compounds are pretty much well understood in terms of their biosynthesis, enzyme families and regulation. Reports are being constantly generated regarding novel bioactivities, different classes of antioxidants exhibit, in vivo. Production of anthocyanins via cell cultures has been investigated since a long time. Efforts to enhance their production have constantly been examined via elicitation and precursor feeding strategies. Effects of physical factors such as light, temperature and postharvest conditions are well defined in terms of anthocyanin accumulation. However, a definitive gap still remains in terms of mechanisms involved in sequestering and vacuolar compartmentalization, cellular trafficking of anthocyanins and their regulation. Answers to these key missing elements will contribute significantly in successful metabolic pathway engineering/flux diversion for plant pigment biosynthesis. Extensive biochemical characterization of putative " molecular protein complexes" , determining their structures and use of bioinformatics tools to predict structure- function relationship may also help to explore systems capable of synthesis of novel compounds, difficult to process chemically. Such information may also help in developing biochemical enzyme systems capable of customized plant pigment synthesis with "cellular editing" such as hydroxylation, de-glycosylation etc for desired chemical transformation.
Acknowledgement
The authors are grateful to the Director, CSIR-CIMAP for providing the infrastructure required to carry out the experimentation. Tanya B is also grateful to the University Grants Commission for award of a Senior Research Fellowship grant during the course of the study
References
- Andersen ØM (2001) Anthocyanins. In: Encyclopedia of life sciences. John Wiley & Sons Ltd Chichester, UK.
- Andersen ØM, Markham KR (2006) Flavonoids: chemistry biochemistry, and applications. CRC Taylor & Francis Boca Raton, USA.
- Brouillard R (1982) Chemical structure of anthocyanins. In: Markakis P (Ed.), Anthocyanins as Food Colors Academic Press, New York, USA.
- De Pascual-Teresa S, Moreno DA, García-Viguera C (2010) Flavanols and anthocyanins in cardiovascular health: a review of current evidence. International J Mo Scl 11(4): 1679-1703.
- Tanaka Y, Sasaki N, Ohmiya N (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54(4): 733-749.
- Li J, Ou-Lee T, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5(2): 171-179.
- Pascual-Teresa S, Sanchez Ballesta MT (2008) Anthocyanins: From Plant to Health. Phytochem Rev 7(2): 281-299.
- Brouillard R, Dangles O (2000) Flavonoids and flower colour. In: Harborne JB (ed) The Flavonoids. Advances in Research since 1986. Chapman & Hall, London 1993 Clifford MN. Anthocyanins nature, occurrence and dietary burden. J Sci Food Agric 80: 1063-1072.
- Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126(2): 485-493.
- Matsuzoe N, Yamaguchi M, Kawanobu S, Watanabe Y, Higashi H, et al. (1999) Effect of dark treatment of the eggplant on fruit skin color and its anthocyanin component. J Jpn Soc Hortic Sci 68(1): 138-145.
- Spayd SE, Tarara JM, Mee DL, Ferguson JC (2002) Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am J Enol Vitic 53: 171-182.
- Jia HJ, Araki A, Okamoto G (2005) Influence of fruit bagging on aroma volatiles and skin coloration of "Hakuho" peach (Prunuspersica Batsch). Postharvest BiolTechnol 35(1): 61-68.
- Jeong ST, Goto-Yamamoto N, Kobayashi S, Esaka M (2004) Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci 167(2): 247-252.
- Leng P, Itamura H, Yamamura H, Deng XM (2000) Anthocyanin accumulation in apple and peach shoots during cold acclimation. Sci Hortic 83: 43-50.
- Iglesias I, Salvia J, Torguet L, Cabus C (2002) Orchard cooling with over tree micro-sprinkler irrigation to improve fruit color and quality of 'Topred Delicious' apples. Sci Hortic 93: 39-51.
- Mori K, Sugaya S, Gemma H (2005) Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. SciHort 105(3): 319-330.
- Faragher JD, Brohier RL (1984) Anthocyanin accumulation in apple skin during ripening-regulation by ethylene and phenylalanine ammonia-lyase. SciHortic 22(1-2): 89-96.
- Li ZH, Gemma H, Iwahori S (2002) Stimulation of 'Fuji' apple skin color by ethephon and phosphorus-calcium mixed compounds in relation to flavonoid synthesis. SciHortic 94(1-2): 193-199.
- Garcia-Alonso M, Rimbach G, Rivas-Gonzalo JC, de Pascual-Terese S(2004) Antioxidant and cellular activities of anthocyanins and their corresponding vitisins A-studies in platelets, monocytes, and human endothelial cells. J Agric Food Chem 52(11): 3378-3384.
- Oak MH, Bedoui JE, Madeira SV, Chalupsky K, Schini-Kerth VB (2006) Delphinidin and cyanidin inhibit PDGF(AB)-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. British J Pharmacol 149(3): 283-290.
- Kamei H, Hashimoto Y, Koide T, Kojima T, Hasegawa M (1998) Antitumor effect of methanol extracts from red and white wines. Cancer BiotherRadiopharm 13(6): 447-452.
- Shih PH, Yeh CT, Yen GC (2005) Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem Toxicol 43: 1557-1566.
- Liu M, Li XQ, Weber C, Lee CY, Brown J, et al. (2002) Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem 50(10): 2926-2930.
- Fimognari C, Berti F, Nusse M, Cantelli-FortiG, Hrelia P (2004) Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-beta-glucopyranoside. Biochem Pharmacol 67(11): 2047-2056.
- Yeh CT, Yen GC (2005) Induction of apoptosis by the Anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. J Agric Food Chem 53(5): 1740-1749.
- Liu Z, Schwimer J, Liu D, Greenway FL, Anthony CT, et al. (2005) Black raspberry extract and fractions contain angiogenesis inhibitors. J Agric Food Chem 53(10): 3909-3915.
- Hope Smith S, Tate PL, Huang G, Magee JB, Meepagala KM, et al. (2004) Antimutagenic activity of berry extracts. J Med Food 7(4): 450-455.
- Marko D, Puppel N, Tjaden Z, Jakobs S, Pahlke G (2004) The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol Nutr Food Res 48(4): 318-325.
- Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, et al. (2006) Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clinical Cancer Research 12(13): 4018-4026.
- Folmer F, Basavaraju U, Jaspars M, Hold G, El-Omar E, et al. (2014) Anticancer effects of bioactive berry compounds. Phytochemistry Rev 13(1): 295-322.
- Riaz M, Zia-Ul-Haq M, Saad B (2016) Anthocyanins Effects on Carcinogenesis, Immune System and the Central Nervous System. In: Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects. Springer International Publishing, Switzerland, pp. 125-138.
- Jayaprakasam B, Vareed SK, Olson LK, Nair MG (2005) Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem 53(1): 28-31.
- Han KH, Hashimoto N, Shimada K, Sekikawa M, Noda T, et al. (2006) Hepatoprotective effects of purple potato extract against D-galactosamine- induced liver injury in rats. Biosci Biotechnol Biochem 70(6): 1432-1437.
- Lee J, Lee HK, Kim CY, Hong YJ, Choe CM, et al. (2005) Purified highdose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. British J Nutr 93(6): 895-899.
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, et al. (1999) Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci 19(18): 8114-8121.
- Werlein HD, Kutemeyer C, Schatton G, Hubbermann EM, Schwarz K (2005) Influence of elderberry and blackcurrant concentrates on the growth of microorganisms. Food Control 16(8): 729-733.
- Silva S, Costa EM, Mendes M, Morais RM, Calhau C, et al. (2016) Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J Applied Microbiol 121(3): 693-703.
- Zhong JJ, Yoshida M, Fujiyama K, Seki T, Yoshida T (1993) Enhancement of anthocyanin production by Perillafrutescens cells in a stirred bioreactor with internal light irradiation. J Fermentation Bioengg 75(4): 299-303.
- Rajendran L, Ravishankar GA, VenkataramanLV, Prathiba KR (1992) Anthocyanin production in callus cultures of Daucuscarota as influenced by nutrient stress and osmoticum. BiotechnolLett 14(8): 707-712.
- Rajendran L, Suvarnalatha G, Ravishankar GA, Venkataraman LV (1994) Enhancement of anthocyanin production in callus cultures of Daucuscarota L. under the influence of fungal elicitors. Appl Microbiol Biotechnol 42(2-3): 227-231.
- Sudha G, Ravishankar GA (2003) Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiol Plant 25(3): 249-256.
- Zhong JJ, Yoshida T (1995) High-density cultivation of Perillafrutescens cell suspensions for anthocyanin production: effects of sucrose concentration and inoculum size. Enzyme Microbial Tech 17(12):1073- 1079.
- Sato K, Nakayama M, Shigeta JI (1996) Culturing conditions affecting the production of anthocyanin in suspended cell cultures of strawberry. Plant Sci 113(1): 91-98.
- Zhang W, Seki M, Furusaki S (1997) Effect of temperature and its shift on growth and anthocyanin production in suspension cultures of strawberry cells. Plant Sci 127(2): 207-214.
- Mori T, Sakurai M, Sakuta M (2001) Effects of conditioned medium on activities of PAL, CHS, DAHP synthase (DS-Co and DS-Mn) and anthocyanin production in suspension cultures of Fragariaananassa. Plant Sci 160(2): 355-360.
- Edahiro J, Nakamura M, Seki M, Furusaki S (2005) Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of L-Phenylalanine into the medium. J Bioscience Bioengg 99(1): 43-47.
- Simöes-Gurgel C, da Silva Cordeiro L, de Castro TC, Callado CH, Albarello N, et al. (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tiss Org Cult 106: 537-545.
- Pasqua G, Monacelli B, Mulinacci N, Rinaldi S, Giaccherini C, et al.(2005) The effect of growth regulators and sucrose on anthocyanin production in Camptothecaacuminata cell cultures. Plant Physiol Biochem 43(3): 293-298.
- Sakamoto K, Iida K, Sawamura K, Hajiro K, Asada Y, et al. (1994) Anthocyanin production in cultured cells of Aralia cordata Thunb. Plant Cell Tiss Organ Cult 36(1): 21-26.
- Fang Y, Smith MAL, Pépin MF (1999) Effects of exogenous methyl jasmonate in elicited anthocyanin-producing cell cultures of ohelo (Vacciniumphalae). In Vitro Cellular Develop Biol Plant 35(1): 106113.
- Ram M, Prasad KV, Singh SK, Hada BS, Kumar S (2013) Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrid L. Plant Cell Tiss Org Cult 113(3): 459-467.
- Vitrac X, Larronde F, Krisa S, Decendit A, Deffieux G (2000) Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry 53(6): 659-665.
- Zhang W, Curtin C, Kikuchi M, Franco C (2002) Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant Sci 162(3): 459-468.
- Belhadj A, Telef N, Saigne C, Cluzet S, Barrieu F, et al. (2008) Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol Biochem 46(4): 493-499.
- Gagne S, Cluzet S, Merrilion JM, Geny L (2011) ABA initiates anthocyanin production in grape cell cultures Plant Growth Regul 30(1): 1-10.
- Cai Z, Kastell A, Mewis I, Knorr D, Smetanskal (2012) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tiss Org Cult 108(3): 401-409.
- Qu J, Zhang W, Yu XA (2011) A combination of elicitation and precursor feeding leads to increased anthocyanin synthesis in cell suspension cultures of Vitis vinifera. Plant Cell Tiss Org Cult 107(2): 261-269.
- Saw NM, Riedel H, Cai Z, Kütük O, Smetanska I (2012) Stimulation of anthocyanin synthesis in grape (Vitis vinifera) cell cultures by pulsed electric fields and ethephon. Plant Cell Tiss Organ Cult 108(1): 47-54.
- Mathur A, Mathur AK, Gangwar A, Verma P, Sangwan RS (2010) Anthocyanin production in a callus line of Panax sikkimensis Ban. In Vitro Cell Develop Biol Plant 46(1): 13-21.
- Biswas T, Singh M, Mathur AK, Mathur A (2015) A dual purpose cell line of an Indian congener of ginseng-Panaxsikkimensis with distinct ginsenoside and anthocyanin production profiles. Protoplasma 252(2): 697-703.
- Biswas T (2016) Elicitation of in vitro secondary metabolite production and its transcript expression profiling in Panax species, Phd Thesis. Jawaharlal Nehru University, New Delhi, India.
- Maier A, Schrader A, Kokkelink L, Falke C, Welter B, et al. (2013) Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. The Plant J 74(4): 638-651.
- Cai Z, Knorr D, Smetanska I (2012) Enhanced anthocyanins and resveratrol production in Vitis vinifera cell suspension culure by indanoyl-isoleucine, N-linolenoyl-L-glutamine and insect saliva. Enzyme Microbial Tech 50(1): 29-34.
- Mathur A, Gangwar A, Mathur AK, Sangwan RS, Jain DC (2002) A procedure for the development of an anthocyanin producing callus line of Panaxsikkimensis (Indian species of ginseng). US PATENT 6368860.






























