Mechanisms of Charge Transfer during Bio-Cathodic Electro-Synthesis of CO2-Neutral Methane
Sreemoyee Ghosh Ray1 and Makarand M Ghangrekar2*
1PKSinha Center for Bioenergy, Indian Institute of Technology, India
2Department of Civil Engineering, Indian Institute of Technology, India
Submission:May 15, 2017; Published: June 27, 2017
*Correspondence Address: M Ghangrekar, Department of Civil Engineering, Indian Institute of Technology, Kharagpur-721302, India, Tel: +91-3222-283440; Fax: 91-3222-282254; Email: ghangrekar@civil.iitkgp.ernet.in
How to cite this article: Sreemoyee G R, Makarand M G. Mechanisms of Charge Transfer during Bio-Cathodic Electro-Synthesis of CO2-Neutral Methane. Adv Biotech & Micro. 2017; 3(5): 555619. DOI: 10.19080/AIBM.2017.03.555625.
Abstract
Exploiting fossil-fuels to meet the increasing per capita energy consumption is leading to generation of green house gasses. Massive CO2 emission causes an alarming impact on global warming. Sustainable solutions are hence required to be explored to capture and re-utilize CO2 to valuable products. Application of Bio-electrochemical systems (BES) is a novel approach based on electrochemical redox processes, capable of converting the chemical energy stored in biodegradable organic matter by catalytic activity of microorganisms to electrical energy or using electrical energy it can synthesis organic compounds from CO2. This review briefly discusses the salient features of different electron transfer mechanisms and microbial pathways for cathodic generation of bio-methane in BES.
Keywords: 3Bio-electrochemical systems; Bio-methane; Extra-cellular electron transfer; Microbial electro-synthesis; Redox reactions
Introduction

Bioelectrochemical approaches provide an attractive solution for microbial electro-synthesis of bio-methane, which is having a greater prospect to become an energy source and an energy carrier as well [1]. Methane-producing bio-electrochemical system (BES) offers advantages of producing such CO2-neutral methane (Figure 1), where the process is independent of biomass. Energy from the (excess) renewable electricity can be stored in the form of produced methane. One of the key principles of BES systems is the use of microorganisms as bio-catalyst, which helps in executing diversified bio-chemical reduction and oxidation reactions [2,3]. A more recent application of electron transfer from electrode to microorganisms, where current is getting consumed, enables the possibilities of biological reduction of oxidized pollutants in bioremediation systems [4-6], biological reduction of nitrate to nitrogen gas [7] and microbial electrosynthesis for production of a wide array of valuable fuels and reduced bio-chemical compounds [8,9].
Conversion of CO2 to methane in bio-electrochemical system
Methane producing BES consists of both anodic and cathodic compartments, which were comprised of respective anodic and cathodic electrode and separated by proton exchange membrane. Oxidation of organic substrates takes place in the anodic chamber and the fate of oxidation reaction depends on the type of substrates used. For example, upon acetate oxidation, protons and electrons get liberated and the process generates two moles of carbon di-oxide (Equation 1).
CH,COO<>SUP- + 2H2o → 2CO2- + 8H + 8e-(E0 ≈ -0.2Vvs.SHE) (Equation 1)
Electrons are transferred by redox active mediators or conductive bacterial pilli to anode and flow through the external circuit to cathode; whereas, protons are transferred through membrane to maintain the electro-neutrality of the system. Application of bio-cathode in microbial electrolysis cells (MEC), a variant of BES, enables the growth of microorganisms, which catalyses the reduction of CO2 generated in the anodic chamber, combined with electrons and protons, to generate methane. Methane generation by hydrogenotrophic methanogens takes places via two classical pathways of extracellular electron transfer (EET) [10].
Mechanisms of Extracellular Electron Transfer for Bio-Cathodic Methane Generation
Mediated electron transfer
In microbial electro-synthesis process, during mediated electron transfer, hydrogenotrophic methanogenesis is dependent on biotic/abiotic H2 production (Figure 2). The intermediate H2 production can be achieved electrochemically (Equation 2) or bio-electrochemically i.e., by the activity of hydrogenase enzyme present in electro-active H2 producing microorganisms. The produced hydrogen then can be consumed by H2-utilizing methanogens in presence of excess CO2 to produce methane under applied cathode potential of around - 0.5 V vs. SHE (Equation 3) [3,11].
Probiotics encapsulation
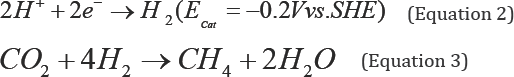
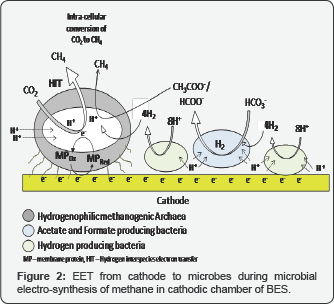
The major disadvantage of this process was found to be the use of expensive cathode catalyst for achieving enhanced electro-catalytic activity for H2 evolution. Moreover, considering overpotential and internal resistance developed during electrohydrogenesis it requires 0.5-1V to carry out the process effectively. Bio-methane production can also be facilitated by acetate and formate producing bacteria (Figure 2), where the intermediate products are again re-utilized by methanogenic bacteria to produce methane as reaction end-product [12].
Microbial electrochemical synthesis
An investigation on direct metabolic pathways for EET driven methane production was first reported by Cheng et al. [13] where the ability of microorganisms to produce methane from CO2 reduction by using an electrode as direct electron donor was depicted while using mixed methanogenic inoculum and referred to as electro-methanogenesis process (Figure 2). Reaction of electron transport is catalyzed by membrane-bound compounds, which can use the energy difference between donor and acceptor (depending on the difference in redox potentials, AE) and facilitate the establishment of ion-gradient across the membrane. Trans-membrane ion transport is assisted by membrane-localized protein complexes (such as cytochrome c and terminal oxidases/ reductases) or bacterial conductive pilli (nano-wires), which can transport electrons to the final electron acceptors [14,15]. The standard potential for methane production via electro-methanogenesis (ECat =- 0.24 V vs. SHE at pH of 7) is lower than the H2 production via indirect EET (electro-hydrogenesis, ECat = - 0.41 V vs. SHE), which makes the former reaction to be energetically more efficient to occur [10]. Hence, electro-methanogenesis can be carried out in a singlechamber anaerobic systems like UASB reactor to construct localized methane producing MECs, which can be regarded as a potentially applicable device to increase the overall methane yield [16].
The CO2 and electrons generated in the anodic chamber during biological oxidation of organic matter can be reutilized during the cathodic generation of methane. The possible electrochemical reduction of CO2 to CH4 in the cathodic chamber happens according to the following equation (Equation 4):
CO2 + 8H+ + 8e- ^ CH4 + 2H2O(E0 * -0.44Vvs.SHE) (Equation 4)
Methane production at more negative potential within the range of - 0.65 to - 0.9 V vs. SHE, both via direct EET and abiotically produced H2 gas via hydrogenotrophic methanogenesis, was compared and the relative contributions of these two mechanisms were reported to be highly dependent on the set cathodic potential [17]. It was also found that the biotic cathode with mixed enriched culture of biocatalyst enhanced current densities compared to the abiotic cathode and generated small amount of abiotic H2. However, the study could not reveal the inter-species H2 transfer between electro-active H2 producing microorganisms and H2 utilizing methanogens.
Direct interspecies electron transfer
A more recent interest has been revealed on mechanisms by which exchange of electrons happens through electrically conductive biological connections of filamentous appendages and conductive extra-cellular polymeric substances (EPS) present in the intercellular spaces of syntrophic microbial partners in the form of aggregates [8,18,19]. Such bacteria can therefore participate in direct interspecies electron transfer (DIET), which can be attributed by their ability to form extracellular electrical connections. The knowledge cultivation on DIET mechanism, which is yet to be explored completely, is important to understand the energy exchange and to expand the metabolic capabilities of anaerobic microbial community
The well-known mechanism of hydrogen interspecies electron transfer (HIT) depicts the ability of electron donating microbial species (hydrogen producing bacteria) to reduce protons to H2, which is consumed by electron accepting partner species (methanogens) for reduction of any electron acceptor, such as CO2 [20]. DIET has been documented among Geobactor sp. and between co-culture of Geobactor and methanogenic species [21,22].
Conclusion
Research on cathodic reaction in BESs exploiting bio- cathodic electro-synthesis of methane, as alternative fuel, has been intensified over the last few decades. However, detailed understanding on charge transfer mechanisms are immensely needed to bring an insight of bio-electrochemical approaches for methane generation and recovery. Moreover, many scientific and technical challenges are yet to be addressed to make this technology more eco-friendly, economical and feasible for commercial applications, which can be regarded as an important step to mitigate the future requirement of clean energy.
Acknowledgement
Authors sincerely acknowledge the Ministry of New and Renewable Energy, Government of India (Ministry sanction letter no. 10/14/2010-P&C, dated 26/03/2012) for providing fellowship to the first author.
References
- Pant D, Singh A, Van Bogaert G, Olsen SI, Nigam PS, et al. (2012) Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv 2(4): 1248-1263.
- Clauwaert P, Van der Ha D, Boon N, Verbeken K, Verhaege M, et al. (2007) Open air biocathode enables effective electricity generation with microbial fuel cells. Environ Sci Technol 41(21): 7564-7569.
- Rozendal RA, Hamelers HV, Euverink GJ, Metz SJ, Buisman CJ (2006) Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int J Hydrogen Energy 31(12): 1632-1640.
- Aulenta F, Canosa A, Majone M, Panero S, Reale P, et al. (2008) Trichloroethenedechlorination and H2 evolution are alternative biological pathways of electric charge utilization by a dechlorinating culture in a bioelectrochemical system. Environ Sci Technol 42(16): 6185-6190.
- Aulenta F, Canosa A, Reale P, Rossetti S, Panero S, et al. (2009) Microbial reductive dechlorination of trichloroethene to ethene with electrodes serving as electron donors without the external addition of redox mediators. Biotechnol Bioeng 103(1): 85-91.
- Aulenta F, Canosa A, De Roma L, Reale P, Panero S, et al. (2009) Influence of mediator immobilization on the electrochemically assisted microbial dechlorination of trichloroethene (TCE) and cis- dichloroethene (cis- DCE). J Chem Technol Biotechnol 84(6): 864-870.
- Clauwaert P, Rabaey K, Aelterman P, de Schamphelaire L, Pham TH, et al. (2007) Biological denitrification in microbial fuel cells. Environ Sci Technol 41(9): 3354-3360.
- Choi O, Sang BI (2016) Extracellular electron transfer from cathode to microbes: application for biofuel production. Biotechnol. Biofuels 9(1): 11.
- Kumar G, Saratale RG, Kadier A, Sivagurunathan P, Zhen G, et al. (2017) A review on bio-electrochemical systems (BES) for the syngas and value added biochemicals production. Chemosphere 177: 84 -92.
- Geppert F, Liu D, van Eerten-Jansen M, Weidner E, Buisman C, et al. (2016) Bioelectrochemical power to gas: State of the art and future perspectives. Trends Biotechnol 34(11): 879-894.
- Cheng S, Logan BE (2007) Sustainable and efficient biohydrogen production via electro hydrogenesis. Proc Natl Acad Sci 104(47): 18871-18873.
- Xafenian N, Mapelli V (2014) Performance and bacterial enrichment of bioelectrochemical systems during methane and acetate production. Int J Hydrogen Energy 39(36): 21864 - 21875.
- Cheng S, Xing D, Call DF, Logan BE (2009) Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol 43(10): 3953 - 3958.
- Kracke F, Vassilev I, Kromer JO (2015) Microbial electron transport and energy conservation - the foundation for optimizing bioelectrochemical systems. Front Microbiol 6: 575.
- Patil SA, Hagerhall C, Gorton L (2012) Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal Rev 4(2-4): 159-192.
- Clauwaert P, Verstraete W (2009) Methanogenesis in membraneless microbial electrolysis cells. Appl Microbiol Biotechnol 82(5): 829.
- Villano M, Aulenta F, Ciucci C, Ferri T, Giuliano A, et al. (2010) Bioelectrochemical reduction of CO2 to CH 4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresource Technol 101(9): 3085-3090.
- Mao X, Stenuit B, Polasko A, Alvarez-Cohen L (2015) Efficient metabolic exchange and electron transfer within a syntrophictrichloroethene- degrading coculture of Dehalococcoidesmccartyi 195 and Syntrophomonaswolfei. Appl Environ Microbiol 81(6): 2015-2024.
- Lovley DR (2011) Reach out and touch someone: potential impact of DIET (direct interspecies energy transfer) on anaerobic biogeochemistry, bioremediation, and bioenergy. Rev Environ Sci Biotechnol 10: 101-105.
- Shrestha PM, Rotaru AE, Aklujkar M, Liu F, Shrestha M, et al. (2013) Syntrophic growth with direct interspecies electron transfer as the primary mechanism for energy exchange. Environ Microbiol Rep 5(6): 904-910.
- Rotaru AE, Shrestha PM, Liu F, Markovaite B, Chen, S, et al. (2014) Direct interspecies electron transfer between Geobactermetallireducens and Methanosarcinabarkeri. Appl Environ Microbiol 80: 4599-4605.
- Rotaru AE, Shrestha PM, Liu FH, Shrestha M, Shrestha D, Embree M, et al. (2014) A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci 7(1): 408-415.






























