Effect of in feed Supplementation of Trans cinnamaldehyde and Caprylic Acid on Cecal Microbiome of Layer Hens
Indu Upadhyaya1, Abhinav Upadhyay2, Chi Hung Chen3, Hsin Bai Yin3, Meera S Nair4, Kendra Maas5, Abraham J Pelliserry6, and Kumar Venkitanarayanan2*
1Department of Extension, University of Connecticut, Storrs, USA
2Department of Animal Science, University of Connecticut, Storrs, USA
3Food Safety Laboratory at USDA-Beltsville Agricultural Research, USA
4Veterinary and Biomedical Sciences, Penn State University, University Park, USA
5Microbial Analysis, Resources, and Services, University of Connecticut, Storrs, USA
6Department of Comparative Diagnostic and Population Medicine, College of Veterinary Medicine, University of Florida, USA
Submission: January 06, 2024;Published: January 11, 2024
*Corresponding author: Kumar Venkitanarayanan, Department of Animal Science, University of Connecticut, Storrs, CT- 06269, USA
How to cite this article: Indu U, Abhinav U, Chi Hung C, Hsin Bai Y, Meera S N, et al. Effect of in feed Supplementation of Trans cinnamaldehyde and Caprylic Acid on Cecal Microbiome of Layer Hens. Arch Anim Poult Sci. 2024; 2(4): 555592. DOI: 10.19080/AAPS.2024.02.555592
Abstract
In this study, the effect of in-feed supplementation of two phytochemicals, Trans-cinnamaldehyde (TC) and Caprylic acid (CA) on the cecal microbiome of 40-wk-old layer chickens in the presence of Salmonella Enteritidis (SE) was determined. Single-comb, White Leghorn hens (N=126) were randomly assigned to 6 groups (n = 21/group): a negative control (no SE, no compound), a positive control (SE, no compound), 2 compound controls (no SE, 1% vol/ wt TC or 1% vol/wt CA), TC treatment (SE, 1% TC) and CA treatment (SE, 1% CA). On the day of arrival, birds were tested for any inherent Salmonella (n = 3/experiment), and in-feed phytochemical supplementation was provided for the entire duration of the study (60 days). On day 8, birds in the positive controls and phytochemical supplemented groups were orally challenged with SE @~10 log10 cfu/bird by crop gavage.
Cecal contents collected from birds on days 0, 1, 7, 10, 20, 30 and 60 were subjected to 16S rRNA sequencing by Illumina Miseq. In-feed phytochemical supplementation did not affect the cecal population of the major bacterial phylotypes, including Firmicutes, Bacteroidetes and Proteobacteria (p>0.05). Moreover, phytochemical supplementation decreased SE in the cecum, yolk and eggshell of birds when compared with controls (p<0.05), potentially indicating that TC and CA could be used as feed additives to reduce foodborne salmonellosis in chickens without adversely affecting the endogenous cecal microflora of chickens.
Keywords: Layers; Salmonella; Cecal Microbiome; Trans cinnamaldehyde; Caprylic acid
Introduction
According to USDA-Economic Research Service, the US is the major poultry producer and ranks as the second largest exporter of poultry products worldwide. The US poultry sector is valued over $20 billion, primarily from broiler production, followed by eggs, turkey and other poultry products [1-2]. Despite progress in production standards, poultry food safety concerns continue to be a challenge for the poultry production sector. Of the total foodborne outbreaks reported, 13% of the cases have been attributed to contaminated poultry products, accounting for at least 32 poultry-related outbreaks in the past decade and a half that have resulted in illness to over a million people [3-5]. Foodborne pathogens can cause gastrointestinal dysfunction and account for 48 million illnesses, 128,000 hospitalizations, and 3,000 deaths annually in the United States [3,4,6]
Among the foodborne pathogens, Salmonella enterica serovar Enteritidis (SE) is one of the major bacteria in the United States responsible for causing enteric illnesses in humans, with eggs as the primary source of human infections [7]. Approximately 109 billion eggs were produced in 2018, and the demand of eggs has grown in the United States over the last decade, with an annual consumption of eggs estimated at 293 per person [2,8]. Thus, poultry egg safety is a major concern to the government, stakeholders and consumers from the public health and economic standpoints. From epidemiological studies, it has been established that chickens are asymptomatic carriers of SE, promoting environmental dissemination and leading to possible human outbreaks and illness via consumption of contaminated, raw or undercooked eggs [9]. Various interventions aimed to decrease the incidence of egg-associated foodborne illnesses are generally employed post-harvest, such as washing with chlorine and iodine-based sanitizers. In addition, on-farm strategies to reduce SE at different phases of the production process prior to processing have been previously employed [10,11].
In this regard, pre-harvest interventions, especially the application of feed additives to modulate the gastrointestinal microbial community (the gut microbiome) with minimal impact, have been the focus of growing interest. Following the 2006 European Union ban on the use of prophylactic antibiotic-feed additives as growth promoters (Regulation (EC) No 1831, 2003) and calls for similar regulation in the U.S. [12-16] have motivated the industry and federal regulatory agencies to act upon the current crisis of antibiotic resistance. Numerous strategies for reducing pathogen colonization in poultry have been explored with variable efficacy including, chemical targets such as oligosaccharides, organic acid and antibiotics, as well as biological agents such as competitive exclusion bacteria and bacteriophages [17-24]. Limited efficacy of previously mentioned methods coupled with concerns over chemical toxicity and possible evolution of multi-drug resistance in bacteria has heightened interest to investigate the potential for alternative, naturally derived antimicrobials for controlling foodborne pathogens [25,26].
The major factors to evaluate effectiveness of natural antimicrobial as a potential feed additive include a) testing its efficacy in controlling the foodborne pathogen of interest and b) its impact on the host’s gut microbiome [27]. The importance of gut microbiome for the health and nutrition of the host has been well established [27-30], and antibiotic withdrawal from feed based on the FDA guidance for poultry industry has previously been reported to modulate the chicken microbiome [31-34]. Therefore, identifying and developing antibiotic alternatives will require evaluating their effect on specific pathogens and the host gut microbiome before recommending for use in poultry.
Historically, plant extracts and their purified derivative compounds have been extensively used in herbal medicine, either prophylactically or therapeutically, to prevent infections or as treatment against diseases, respectively [35]. Many studies have previously reported the identification of plants that produce a wide array of phytochemical actives with antimicrobial activity [36-38]. Trans-cinnamaldehyde (TC) or trans-3-phenylprop- 2-enal, a major bioactive component derived from cinnamon (Cinnamomum zeylandicum), has already been proven to display antibacterial properties against both Gram-negative and Gram-positive bacteria [38]. It is a member of the cinnamaldehyde group of compounds and considered a GRAS (generally regarded as safe) chemical approved for use as a food additive by the U.S. FDA (approval TC-21CFR182.60). Previous research conducted in our research group identified that TC was capable of reducing S. Enteritidis in chicken cecal contents in vitro and in various internal organs in broilers [39].
Furthermore, chickens supplemented with TC in-feed had reduced egg yolk and shell contamination with SE without adversely affecting egg production parameters or consumer acceptability of eggs obtained from TC-treated birds [40]. In addition, functional mechanistic inferences derived from follow-up cell culture and gene expression analysis studies revealed that TC reduced S. Enteritidis colonization in primary oviduct epithelial cells and survival in chicken macrophage cell line by downregulating critical virulence genes in the bacterium [40].
In addition to plant-derived compounds such as TC, researchers have extensively studied the antimicrobial properties of lipids and their esters [41,42]. Medium-chain fatty acids (MCFAs) classified under the group of free fatty acids have shown bactericidal activity against Gram-positive and Gram-negative bacteria [43,44]. Caprylic acid (CA) (octanoic acid) is a naturally occurring MCFA that is commonly found in coconut oil, breast milk, and bovine milk [45,46]. It also has a GRAS status (CFR 184.1025) and can be added to food as an additive. Previous research investigations conducted in our laboratory as well as other researchers indicated that in-feed CA supplementation decreased Campylobacter jejuni and SE carriage in broiler chickens [47-49]. Additionally, CA as an antimicrobial feed additive was effective in controlling egg-borne transmission of SE in layers [50].
With current developments in next generation “omics” technology, extensive research has been directed towards chicken gut functionality for enhancing poultry productivity and resistance against various enteric pathogens [51]. Several researchers have investigated the effects of a variety of alternative antimicrobial feed additives on gut microbial communities in poultry [31,52-57]. Prior trials conducted in layers have mainly focused on the reduction of Salmonella and improving growth performance [40,58]. Similarly, a few studies investigating the microbiome of broilers fed with essential oils and prebiotic fiber supplements have been conducted [59-63], but, to the best of our knowledge, this is the first study to investigate the microbiome of layer chicken that have been challenged with Salmonella and fed with natural compounds such as TC and CA. The objective of the current study was primarily to determine the relative effects of TC and CA on layer cecal microbiome at the community level using 16S rRNA sequencing by Illumina MiSeq platform. In addition, a comprehensive assessment of dataset related to the taxonomic composition of the cecal microbiome in 40-wk old layer hens was evaluated.
Materials and Methods
Ethics Statement
All the animal work described in this study was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Connecticut, and all experiments were performed in accordance with the approved guidelines and regulations.
Bacterial strains and dosing
A four-strain cocktail of SE (SE-12 (chicken liver, phage type 14b), SE-21 (chicken intestine, phage type 8), SE-28 (chicken ovary, phage type 13a) and SE-31 (chicken gut, phage type 13a); source:- Connecticut Veterinary Diagnostic Laboratory, University of Connecticut) was used to inoculate the birds. To facilitate selective enumeration of S. Enteritidis from the necropsy samples, each strain was pre-induced for nalidixic acid (NA; Sigma Aldrich, St. Louis, MO) resistance (up to 50μg/mL) [64]. In four separate sterile tubes containing 10 mL tryptic soy broth (TSB; Difco, Becton Dickinson, Sparks, MD), 100 μL of each NA-resistant SE isolate was separately cultured overnight. Further, the respective cultures were later transferred to separate flasks containing 100 mL TSB supplemented with 50μg/mL of NA and incubated overnight in a shaking incubator at 37°C (100 rpm).
The four SE cultures were pooled equally into sterile tubes and centrifuged at 3600 g for 15 min at 4°C. The pellet was washed and resuspended in 100 mL of phosphate-buffered saline (PBS, pH 7.0), and used as the inoculum. Bacterial count confirmation of the individual SE cultures and the four-strain cocktail was performed by serial dilution and plating of 0.1-mL portions of appropriate dilutions on xylose lysine desoxycholate agar (XLD; Difco) plates containing NA (XLD-NA) and incubating the plates at 37°C for 24 h [58].
Experimental birds and housing
Forty-week-old, Single comb, White Leghorn layer hens (Salmonella– free) were procured from the University of Connecticut poultry farm and housed in bedded floor pens, with age-appropriate ambient conditions (16 hours light-8 hours dark cycle, 21℃) at the Isolation Facility of the University of Connecticut. The birds were provided nonmedicated feed ad-libitum, and Salmonella-free water. The hens were randomly allocated to 6 treatments (N=126, n=21): (a) negative control (no S. Enteritidis challenge and no supplemental TC or CA), (b) TC control (no S. Enteritidis challenge but 1.0% supplemental TC [vol/wt]), (c) CA control (no S. Enteritidis challenge but 1.0% supplemental CA [vol/wt]), (d) a positive control (S. Enteritidis challenge but no supplemental TC or CA), (e) a 1% TC treatment (S. Enteritidis challenge), and (f) a 1% CA treatment (S. Enteritidis challenge). TC was supplemented in the feed for 60 days, starting on day 0. Appropriate amounts of TC and CA were added to feed and mixed thoroughly to obtain 1% concentration. Three birds from each treatment group were sacrificed and ceca samples were collected on day 0, 1, 7, 10, 20, 30 and 60 days. On day 8, birds in the positive control, TC, and CA treatment groups were challenged with SE (10 log10 CFU/bird) by crop gavage. From day 20, (10 days post challenge), eggs were collected from each treatment group on day 20, 30 and 60 and tested for the presence or absence of SE. At the end of 60 days, the birds from all treatment groups were euthanized by CO2 asphyxiation. Cecum samples from birds were collected (1g/10 mL of PBS) at each time point for SSE detection [40].
Detection of S. Enteritidis in cecal contents
Enumeration of SE in the cecal contents were determined as described previously [40]. Whole ceca with their contents collected on day 10, 20, 30 and 60, were weighed and homogenized. The respective homogenate was serially diluted (1:10) in PBS, and suitable dilutions were plated on XLD-NA plates for SE enumeration. Representative colonies from XLD-NA plates were confirmed as Salmonella by use of a Salmonella rapid detection kit (Microgen Bioproducts Ltd.). When colonies were absent via direct plating, samples were enriched in 100 mL selenite cysteine broth (Oxoid) to test for surviving Salmonella, and the samples were enriched by enrichment in 100 ml selenite cysteine broth (Oxoid) and incubated at 37°C for 48 hours. Subsequent to enrichment, the samples were streaked on XLD-NA plates to check for the presence of SE [40].
Detection of S. Enteritidis on egg surfaces and in egg contents
The presence of S. Enteritidis on eggshell surfaces and in egg contents was determined according to the method of [65]. Sterile stomacher bags filled with 50 mL of selenite cysteine broth supplemented with NA (50 ng/mL) was used to wash individual eggs for a period of 2 min. The eggs were removed after rinsing and the broth was incubated at 37°C for 48 h. Subsequently, the enriched broth was streaked on XLD-NA plates to detect the presence of S. Enteritidis colonies which were confirmed as Salmonella by use of a Salmonella rapid detection kit (Microgen Bioproducts Ltd., Camberley, United Kingdom). Eggs rinsed in the selenite cysteine broth were subsequently surface disinfected with 70% ethanol, dried and, aseptically cracked open to void the egg contents into separate stomacher bags containing 50 ml of selenite cysteine broth with NA. The stomacher bags with the egg contents or shells were stomached for 1 min to ensure uniform homogenization. The bags were subsequently incubated at 37°C for 24-48 h to detect Salmonella present inside the egg. Confirmation of SE colonies from the enriched broth was determined as previously mentioned.
DNA extraction, PCR amplification, and sequencing of taxonomic marker
DNA from 0.25 g of fecal sample was extracted using the MoBio PowerMag Soil 96 well kit (MoBio Laboratories, Inc) in accordance with the manufacturer’s protocol for the Eppendorf ep Motion liquid handling robot [66]. Extracts of DNA were quantified using the Quant-iT PicoGreen kit (invitrogen, ThermoFisher Scientific). Amplification of partial bacterial 16S rRNA genes (V4) was carried out by using 30ng DNA extracted as template. Amplification of the V4 region was done using 515F and 806R using Illumina adapters and dual indices (8 basepair golay on 3’ (Caporaso 2012), and 8 basepair (bp) on the 5’ [67].
Triplicate samples were amplified using Phusion High- Fidelity PCR master mix (New England BioLabs) with the addition of 10μg BSA (New England BioLabs). Incubation of the PCR reaction was done at 95˚C for 3.5 minutes, the 30 cycles of 30 s at 95.0°C, 30 s at 50.0°C and 90 s at 72.0°C, followed by final extension as 72.0°C for 10 minutes. PCR products were pooled for quantification and visualization using the QIAxcel DNA Fast Analysis (Qiagen). Normalization of PCR products was done based on the concentration of DNA from 250-400 bp then pooled with the QIAgility liquid handling robot. Pooled PCR products were cleaned using the Gene Read Size Selection kit (Qiagen) according to the manufacturer’s protocol. The cleaned pool was sequenced on the MiSeq using v2 2x250 base pair kit (Illumina, Inc [66].
Sequence Analysis
The microbiome analysis was performed as a completely randomized design with treatments done in replicates of four, following published protocol [66]. Mothur 1.36.1 was used to filter and cluster the sequences based on published protocol with slight modifications [67]. Briefly, Operational taxonomic units (OTUs) were clustered at 97% sequence similarity. Downstream analysis of samples was conducted using R version 3.2. To calculate alpha diversity, inverse Simpson was used to measure the richness and evenness of the OTUs.
Tukey’s test was used to analyze the effect of both treatment and day on the alpha diversity. Beta-diversity was estimated as the difference in bacterial composition based on treatment and time by coupling Bray-Curtis Dissimilarity with non-metric multidimensional scaling (NMDS) for ordination from any resemblance matrix. A permutational multivariate analysis (PERMANOVA, adonis function, 999 permutations) was done to analyze the effect of various treatments on the bacterial community composition. Finally, the relative abundance of OTUs of major phyla, order, and genera were determined to assess the effect of treatment [66]. To identify changes in groups of bacteria based on treatment, Tukey’s test was used, and the significance was detected at P < 0.05.
Results and Discussion
The cecum constitutes the largest reservoir of bacteria in the poultry gasatrointestinal tract (GI). Before the advent of modern-day sequencing, research that focused on studying the poultry GI bacteriome depended on classical cultivation techniques [68]. However, during the past two decades, the 16S rRNA gene has been regarded as the primary biomarker to identify bacteria in numerous environments, including the poultry GI tract. Studies that used individual 16S rRNA gene clone libraries offered valuable insight into the diversity of poultry GI bacteriome by generating high-quality sequences. But these studies were limited to relatively few sequences that were affordable to scientists. Hence, a comprehensive effort investigating the diversity and composition of the poultry GI bacteriome was not possible until next-generation sequencing (NGS) became available [68].
High-throughput NGS technologies have been established as powerful tools for comprehensive analysis of complex bacteriomes [69,70]. Previously, GI bacteriomes of chickens and turkeys have been compared using such NGS technologies employing either QIIME or Mothur packages [68] However, the effect of in-feed supplementation of a natural antibiotic alternative has not been well studied in a comparative platform. Although previous studies have identified that both tools are relatively similar in identifying the most abundant genera, despite the database [71], there is insufficient knowledge with respect to chicken microbiome. Additionally, the Mothur package assigns OTUs to a larger number of genera and in larger relative abundance for less frequent microorganisms. Mothur is also believed to have more favorable rarefaction curves and a larger analytic sensitivity when used with a suitable microbial gene database [71]. Therefore, this platform was chosen to read the sequenced samples from chicken microbiota.
Effects of treatments on SE survival in cecum and on egg:
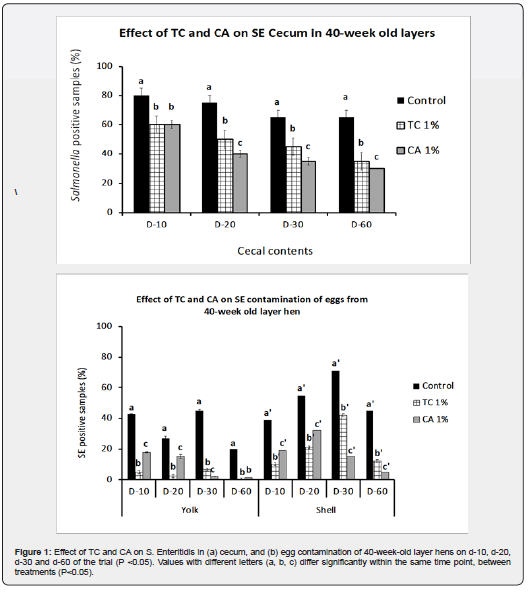
The dietary supplementation of TC and CA at 1% significantly decreased the amounts of SE on cecal contents and on eggshells and in yolks (p<0.05). As observed in Figure 1a, TC and CA at 1% consistently decreased the amounts of Salmonella Figure 1a from cecal contents on Day 10, 20, 30 and 60 of the study periods (p<0.05). These results were similar to previous reports [40-58] wherein, TC and CA, fed to layers at different concentrations, were able to reduce the SE colonization of various organs including cecum. While the controls still had 65% of the samples positive for SE in cecum, 1% TC and CA reduced the pathogen to 35% and 30% respectively (p<0.05). Moreover, in-feed supplementation of 1% TC and CA decreased Salmonella prevalence on egg yolk and in eggshell (p<0.05) consistently until day 60, as seen in Fig 1b. By the end of the trial, 1% TC and CA significantly reduced SE contamination of yolk and eggshell to 0 and 12% and to 1.5% and 4.8%, respectively, compared to that for control birds, which produced 20% positive eggs based on yolk and 45 % positive eggs based on shell Figure 1b.
Enteritidis primarily colonizes the chicken cecum [72,73] and spreads to the spleen and liver by lymphatic or circulatory routes followed by subsequent colonization and spread to the reproductive organs in layers, thereby contaminating the yolk. These results indicated that in-feed administration of TC and CA significantly decreased SE colonization in layer chickens and reduced the egg-borne transmission of the bacterium. In addition to reducing SE levels on eggshell and in the yolk, TC supplementation decreased pathogen populations in the cecum when compared to those in control birds (p<0.05).
Temporal changes in poultry cecal microbiome fed with phytochemicals.
We found minimal temporal changes in the cecal microbiome, which were observed across treatments. The inverse Simpson diversity index for abundance and evenness from each bird for 60 days showed variation in dominant species between control and treatments, as depicted in Figure 2a. On day 1, both CA and TC treatments had a wide range of bacterial communities when compared with limited variation in controls. In comparison with microbial diversity from day 7 through 30, there were changes observed by the end of day 60 between controls and treatments, especially in CA which had a wider diversity. Moreover, CA also exhibited more richness and abundance in the bacterial communities by end of day 60 when compared to controls as observed in Shannon even alpha diversity indices.
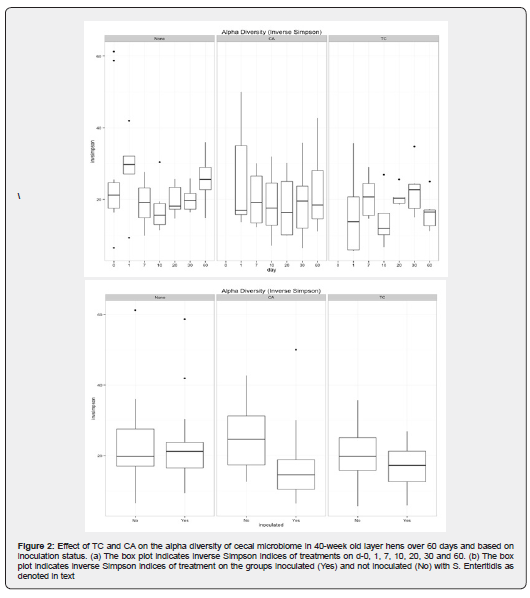
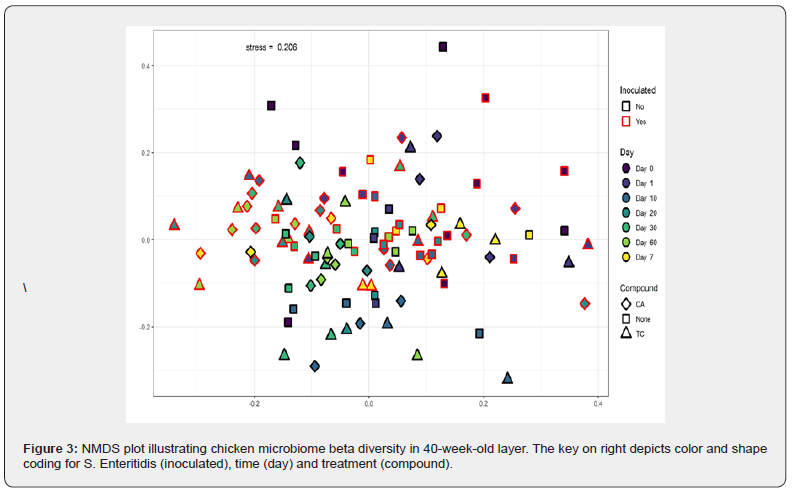
Trans-cinnamaldehyde treatment on day 7 was the most significantly different community when compared with other time points within the group including day 1, 20, and day 60. In addition, the same time point on day 7 was significantly different from the control treatment. This time point may denote a critical change in the microbial diversity occurring in chicken fed with TC which may be responsible for the antimicrobial effect of the phytochemical against pathogenic bacteria such as Salmonella. However, when clustering of the cecal microbiome from birds at the OTU level was conducted using NMDS as described in the methods, the results did not represent clear groupings by time. To test the relative effects of time on experimental treatments affecting the cecal microbiome, permutational MANOVA was employed. Although there were outliers, there was no significant difference in the beta diversity of different samples over a period of time.
In order to represent the cecal microbiota composition and determine potential changes through time, clustering, hypothesis testing and taxonomic classifications of sequences to the genus level was conducted using the RDP classifier in Mothur platform with OTU clustering at 97% sequence similarity. No significant difference was observed in the different bacterial communities over time. From day 0 through day 60, the cecal community was dominated by three major groups of bacteria namely Bacteroides, Firmicutes and an unclassified group which did not contain a sequence similarity to known microflora. There was no significant difference between Proteobacteria due to time, this shows that the compounds did not influence the bacterial community in the chicken cecum over time. These data are consistent with previous results that identify various members of GI microbiome of poultry [27].
However, exhaustive sequencing with modern methods can establish valuable new information on the generic composition of microbial community in the chicken ceca and how it varies through time. In a feed supplement study, it is important to have proper understanding and management of temporal changes in the GI microbiome in response to the supplement, so that bird health can be maintained, and productivity can be improved.
Effects of phytochemical treatment versus inoculation on cecal microbiome
Treatment effects on Salmonella in the cecal microbiome were non-significant. The inverse Simpson diversity index for abundance and evenness and the Shannon even alpha diversity indices for richness from birds fed with control vs treatment showed no significant variation in dominant species between control and treatments as depicted in Figure 2b. Similarly, the clustering of the cecal microbiome from birds at the OTU level, using NMDS did not reveal clear groupings by inoculation meaning there was no significant difference in the beta diversity of samples inoculated with Salmonella or free of the pathogen. Moreover, there was no significant difference in the bacterial communities. Similar to the temporal spread of the cecal community, three major groups of bacteria namely Bacteroides, Firmicutes and the unclassified group were abundant. Interestingly, there was no significant change observed in the Gammaproteobacteria which encompass Salmonella across the groups. We observed that TC and CA at 1% concentration are effective in reducing Salmonella in cecum Figure 1a &1b.
However, various factors can affect the occurrence of a specific group of bacteria in the analysis, including bird type i.e., whether it is a broiler or a layer hen; age of the bird; DNA extraction and the platform utilized for the sequencing itself. Therefore, any of the above conditions could potentially contribute to lack of abundant Salmonella or absence of a significant difference in the Gammaproteobacterial across groups even after inoculation. Similarly, researchers have observed that higher colonization of Salmonella and Campylobacter in poultry tend to occur without significant shifts in the native microbiome. and observed that the alpha diversity remained conserved with moderate changes in beta diversity among chicken that were colonized with Salmonella and Campylobacter jejuni, respectively [74,75].
Overall composition of cecal microbiota in layers
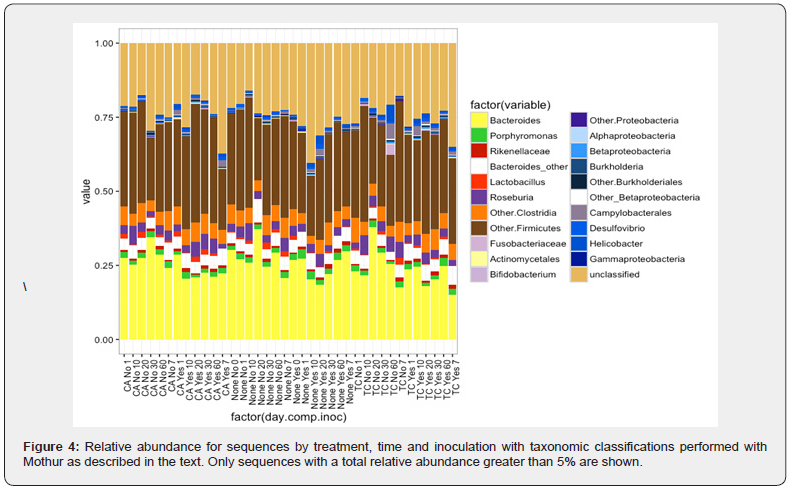
The comprehensive clustering of the cecal microbiome from birds at the OTU level was conducted using NMDS, but with the exception of a few outliers, the results did not represent clear groupings overall Figure 3. This was in line with the results from the beta diversity of different samples over a period of time and with inoculation. The cecal microbiota of layer hens was primarily dominated by Bacteroides, Firmicutes and the unclassified group of bacteria Figure 4. Other minor abundant taxonomic groups included Clostridia (not included in Firmicutes), Roseburia, Proteobacteria and Lactobacillus. The abundance levels of these groups did not show significant difference (p > 0.05); however, Lactobacillus growth was favored in birds supplemented with 1% CA over a period of time when compared to control and 1% TC treatment.
As observed in the temporal changes in inverse Simpson and Shannon even indices, CA showed a wider alpha diversity by end of day 60 including more richness and abundance in the bacterial communities when compared to controls Figure 2b. The increased prevalence of Lactobacillus could potentially have led to the difference observed in the alpha diversity. Similarly, TC treatment on day 7 was the most significant in alpha diversity when compared with other time points within the group including day 1, 20, and day 60. A surge in Lactobacillus at this particular time point is observed in the non- challenged treated group with 1% TC Figure 4. This may have caused the change in alpha diversity and the time point may denote a critical change in the microbial diversity occurring in chicken fed with TC. Furthermore, taxa considered as putative pathogens were a minor component of the community with no significant difference among various groups over a period of time.
Conclusion
In-feed supplementation of TC and CA did not harm the population of the major chicken cecal bacterial phylotypes, including Firmicutes, Bacteroidetes and Proteobacteria (p>0.05). Moreover, TC and CA supplementation decreased S. Enteritidis in the cecum, yolk and eggshell of birds when compared with controls (p<0.05). These results suggest that TC and CA could potentially be effective as feed additives in decreasing S. Enteritidis colonization in chickens without unfavorably affecting the endogenous cecal microflora of chickens. In addition, the cecal microbiome evaluation done in this study warrants further research to identify unclassified taxonomic groups.
References
- Upadhyaya I, Upadhyay A, Venkitanarayanan K (2019) Applications of “Omics” Technologies to Study Gut Health in Poultry. In K. Venkitanarayanan, S Thakur, S C Ricke EdT’s Food Safety in Poultry Meat Production, Springer International Publishing p. 211-234.
- USDA (2020) USDA ERS Poultry & Eggs. United States Department of Agriculture: Economic Research Service.
- Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM (2011) Foodborne illness acquired in the United States-Unspecified agents. Emerg Infect Dis 17(1): 7-15.
- Dewey Mattia D (2018) Surveillance for Foodborne Disease Outbreaks—United States, 2009-2015. MMWR. Surveillance Summaries 67.
- CDC (2022) Salmonella Homepage, CDC.
- Scharff RL (2012) Economic burden from health losses due to foodborne illness in the United States. J Food Prot 75(1): 123-131.
- CDC (2010) CDC - Outbreak of Enteritidis Infections, Salmonella.
- Trejo Pech CJO, White S (2020) Capital Budgeting Analysis of a Vertically Integrated Egg Firm: Conventional and Cage-Free Egg Production. Applied Economics Teaching Resources (AETR) 2(4): 34-46.
- Heredia N, García S (2018) Animals as sources of food-borne pathogens: A review. Animal Nutrition 4(3): 250-255.
- Doyle MP, Erickson MC (2012) Opportunities for mitigating pathogen contamination during on-farm food production. Int J Food Microbiol 152(3): 54-74.
- Rajan K, Shi Z, Ricke SC (2017) Current aspects of Salmonella contamination in the US poultry production chain and the potential application of risk strategies in understanding emerging hazards. In Critical Reviews in Microbiology 43(3): 370-392.
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition (Text with EEA relevance), 268 OJ L (2003).
- Weber JT, Courvalin P (2005) An emptying quiver: Antimicrobial drugs and resistance. In Emerging Infectious Diseases Centers for Disease Control and Prevention 11(6): 791-793.
- Love DC, Davis MF, Bassett A, Gunther A, Nachman KE (2011) Dose imprecision and resistance: Free-choice medicated feeds in industrial food animal production in the United States. Environmental Health Perspectives 119(3): 279-283.
- Oakley BB, Carbonero F, Dowd SE, Hawkins RJ & Purdy KJ (2012) Contrasting patterns of niche partitioning between two anaerobic terminal oxidizers of organic matter. ISME Journal 6(5): 905-914.
- Seal BS, Lillehoj HS, Donovan DM, & Gay CG (2013) Alternatives to antibiotics: A symposium on the challenges and solutions for animal production. Anim Health Res Rev 14(1): 78-87.
- Spring P, Wenk C, Dawson KA, Newman KE (2000) The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poult Sci 79(2): 205-211.
- Byrd JA, Hargis BM, Caldwell DJ, Bailey RH, Herron KL, et al. (2001) Effect of Lactic Acid Administration in the Drinking Water During Preslaughter Feed Withdrawal on Salmonella and Campylobacter Contamination of Broilers. Poultry Science 80(3): 278-283.
- Stern NJ, Cox NA, Bailey JS, Berrang ME& Musgrove MT (2001) Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. Colonization in broiler chickens. Poult Sci 80(2): 156-160.
- Fernandez F, Hinton M, Van Gils B (2002) Dietary mannan-oligosaccharides and their effect on chicken caecal microflora in relation to Salmonella Enteritidis colonization. Avian Pathol J WVPA 31(1): 49-
- Chadfield MS, Hinton MH (2004) Effects of furazolidone pretreatment of Salmonella enteritidis PT4 at sub- and suprainhibitory concentrations on phagocytosis and intracellular survival in chicken macrophages. Vet Immunol Immunopathol 100(1-2): 81-97.
- Heres L, Engel B, Urlings HAP, Wagenaar JA, Van Knapen F (2004) Effect of acidified feed on susceptibility of broiler chickens to intestinal infection by Campylobacter and Salmonella. Vet Microbiol 99(3-4): 259-267.
- Higgins SE, Erf GF, Higgins JP, Henderson SN, Wolfenden AD, et al. (2007) Effect of probiotic treatment in broiler chicks on intestinal macrophage numbers and phagocytosis of Salmonella enteritidis by abdominal exudate cells. Poultry Science 86(11): 2315-2321.
- Fiorentin L, Vieira ND, Barioni W (2005) Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol 34(3): 258-263.
- Salamci E, Kordali S, Kotan R, Cakir A, Kaya Y (2007) Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. Chiliophyllum. Biochem Syst Ecology 35(9): 569-581.
- Sakkas H, Economou V, Gousia P, Bozidis P, Sakkas VA, et al. (2018) Antibacterial Efficacy of Commercially Available Essential Oils Tested Against Drug-Resistant Gram-Positive Pathogens. Applied Sciences 8(11): 2201.
- Oakley BB, Buhr RJ, Ritz CW, Kiepper BH, Berrang ME, et al. (2014) Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet Res 10: 282.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, et al. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122): 1027-1031.
- McKenna P, Hoffmann C, Minkah N, Aye PP, Lackner A, et al. (2008) The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. Plos Pathog 4(2): e20.
- Greenblum S, Turnbaugh PJ, Borenstein E (2012) Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci USA 109(2): 594-599.
- Knarreborg A, Simon MA, Engberg RM, Jensen BB, Tannock GW (2002) Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl Environ Microbiol 68(12): 5918-5924.
- Wise M, Siragusa G, Gregory Siragusa CR (2007) Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J Appl Microbiol 102(4): 1138-1149.
- Fairchild AS, Smith JL, Idris U, Lu J, Sanchez S, et al. (2005) Effects of orally administered tetracycline on the intestinal community structure of chickens and on tet determinant carriage by commensal bacteria and Campylobacter jejuni. Appl Environ Microbiol 71(10): 5865-5872.
- Danzeisen, JL, Kim HB, Isaacson RE, Tu ZJ, Johnson TJ (2011) Modulations of the Chicken Cecal Microbiome and Metagenome in Response to Anticoccidial and Growth Promoter Treatment. Plos One 6(11): e27949.
- Wollenweber E (1988) Occurrence of flavonoid aglycones in medicinal plants. Prog Clin Biol Res 280: 45-55.
- Dixon RA (2001) Natural products and plant disease resistance. Nature, 411: 843-847.
- Burt S (2004) Essential oils: Their antibacterial properties and potential applications in foods-A review. Int J Food Microbiol 94(3): 223-253.
- Holley RA, Patel D (2005) Improvement in shelf-life and safety of perishable foods by planting essential oils and smoke antimicrobials. Food Microbiol 22(4): 273-292.
- Kollanoor-Johny A, Mattson T, Baskaran SA, Amalaradjou MA, Babapoor S (2012) Reduction of Salmonella enterica serovar enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl Environ Microbiol 78(8): 2981-2987.
- Upadhyaya I, Upadhyay A, Kollanoor-Johny A, Mooyottu S, Baskaran SA, et al. (2015) In-feed supplementation of trans-cinnamaldehyde reduces layer-chicken egg-borne transmission of Salmonella enterica serovar enteritidis. Appl Environ Microbiol 81(9): 2985-2994.
- Bergsson G, Arnfinnsson J, Karlsson SM, Steingrímsson Ó, Thormar H (1998) In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother 42(9): 2290-2294.
- Nair KM, Joy J, Vasudevan P, Hinckley L, Hoagland TA, Venkitanarayanan KS (2005) Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J Dairy Sci 88(10): 3488-3495.
- Nakai SA, Siebert KJ (2003) Validation of bacterial growth inhibition models based on molecular properties of organic acids. Int J Food Microbiol 86(3): 249-255.
- Dierick NA, Decuypere JA (2004) Influence of lipase and/or emulsifier addition on the ileal and faecal nutrient digestibility in growing pigs fed diets containing 4% animal fat. J Sci Food Agri 84(12): 1443-1450.
- Jensen RG (2002) The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci Am Associat 85(2): 295-350.
- Sprong RC, Hulstein MF, Meer D Van R (2001) Bactericidal activities of milk lipids. Antimicrob Agents Chemother 45(4): 1298-1301.
- Solis De los santos F, Donoghue AM, Venkitanarayanan K, Metcalf JH, Reyes-Herrera I, et al. (2009) The natural feed additive caprylic acid decreases Campylobacter jejuni colonization in market-aged broiler chickens. Poult Sci 88(1): 61-64.
- Solis De Los Santos F, Donoghue AM, Venkitanarayanan K, Dirain ML, Reyes-Herrera I, et al. (2008) Caprylic acid supplemented in feed reduces enteric Campylobacter jejuni colonization in ten-day-old broiler chickens. Poult Sci 87(4): 800-804.
- Johny, AK, Baskaran SA, Charles AS, Amalaradjou MAR, Darre MJ, et al. (2009) Prophylactic supplementation of caprylic acid in feed reduces salmonella enteritidis colonization in commercial broiler chickst. J Food Protection 72(4): 722-727.
- Upadhyaya I, Upadhyay A, Yin HB, Nair MS, Bhattaram VK, et al. (2015) Reducing colonization and eggborne transmission of Salmonella Enteritidis in layer chickens by in-feed supplementation of caprylic acid. Foodborne Pathog Dis 12(7): 591-597.
- Stanley D, Hughes RJ, Moore RJ (2014) Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl Microbiol Biotech 98(10): 4301-4310.
- Bjerrum L, Pedersen K, Engberg RM (2005) The influence of whole wheat feeding on salmonella infection and gut flora composition in broilers. Avian Dis 49(1): 9-15.
- Gong J, Yu H, Liu T, Gill JJ, Chambers JR, et al. (2008) Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens. J Appl Microbiol104(5): 1372-1382.
- Geier MS, Torok VA, Allison GE, Ophel-Keller K, Gibson RA, et al. (2009) Dietary omega-3 polyunsaturated fatty acid does not influence the intestinal microbial communities of broiler chickens. Poult Sci 88(11): 2399-2405.
- Oviedo-Rondón EO, Hume ME, Barbosaa NA, Sakomura NK, Weber G, et al. (2010) Ileal and caecal microbial populations in broilers given specific essential oil blends and probiotics in two consecutive grow-outs. Avian Biol Res 3(4): 157-169.
- Torok VA, Allison GE, Percy NJ, Ophel-Keller K, Hughes RJ (2011) Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl Environ Microbiol 77(10): 3380-3390.
- Tillman GE, Haas GJ, Wise MG, Oakley B, Smith MA, et al. (2011) Chicken intestine microbiota following the administration of lupulone, a hop-based antimicrobial. FEMS Microbiology Ecology 77(2): 395-403.
- Upadhyaya I, Upadhyay A, Yin HB, Nair MS, Bhattaram VK, et al. (2015) Reducing colonization and eggborne transmission of Salmonella enteritidis in layer chickens by in-feed supplementation of caprylic acid. Foodborne Pathog Dis 12(7): 591-597.
- Pathak M, Mandal GP, Patra AK, Samanta I, Pradhan S, et al. (2016) Effects of dietary supplementation of cinnamaldehyde and formic acid on growth performance, intestinal microbiota and immune response in broiler chickens. Animal Product Sci 57(5): 821-827.
- Yang C, Kennes YM, Lepp D, Yin X, Wang Q, et al. (2019) Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult Sci 99(2): 936-948.
- Gomez-Osorio LM, Yepes-Medina V, Ballou A, Parini M, Angel R (2021) Short and Medium Chain Fatty Acids and Their Derivatives as a Natural Strategy in the Control of Necrotic Enteritis and Microbial Homeostasis in Broiler Chickens. Front Vet Sci 8: 773372.
- Micciche AC, Foley SL, Pavlidis HO, McIntyre DR, Ricke SC (2018) A Review of Prebiotics Against Salmonella in Poultry: Current and Future Potential for Microbiome Research Applications. Front Vet Sci 5: 191.
- Johnson LP, Walton GE, Psichas A, Frost GS, Gibson GR et al. (2015) Prebiotics Modulate the Effects of Antibiotics on Gut Microbial Diversity and Functioning in Vitro. Nutrients 7(6): 4480-4497.
- Kollanoor-Johny A, Mattson T, Baskaran SA, Amalaradjou MA, Hoagland TA, et al. (2012) Caprylic acid reduces Salmonella Enteritidis populations in various segments of digestive tract and internal organs of 3- and 6-week-old broiler chickens, therapeutically. Poultry Sci 91(7): 1686-1694.
- Miyamoto T, Baba E, Tanaka T, Sasai K, Fukata T, Arakawa A (1997) Salmonella enteritidis contamination of eggs from hens inoculated by vaginal, cloacal, and intravenous routes. Avian Dis 41(2): 296-303.
- Fancher SM (2015) Efficacy of Plant-derived Antimicrobials in Reducing Foodborne Pathogens in Agricultural Soil and Their Effect on Soil Nutrients and Microbiome, Master’s Thesis, University of Connecticut.
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79(17): 5112-5120.
- Wei S, Lilburn M, Yu Z (2016) The Bacteriomes of Ileal Mucosa and Cecal Content of Broiler Chickens and Turkeys as Revealed by Metagenomic Analysis. Int J Microbiol.
- Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J (2009) Metagenomic pyrosequencing and microbial identification. Clin Chem 55(5): 856-866.
- Krause L, Diaz NN, Goesmann A, Kelley S, Nattkemper TW, et al. (2008) Phylogenetic classification of short environmental DNA fragments. Nucleic Acids Res 36(7): 2230-2239.
- López-García A, Pineda-Quiroga C, Atxaerandio R, Pérez A, Hernández I, et al. (2018) Comparison of mothur and QIIME for the analysis of rumen microbiota composition based on 16S rRNA amplicon sequences. Front Microbiol 9: 3010.
- Cerquetti MC, Gherardi MM (2000) Orally administered attenuated Salmonella enteritidis reduces chicken cecal carriage of virulent Salmonella challenge organisms. Vet Microbiol 76(2): 185-192.
- Van Immerseel F, De Buck J, De Smet I, Pasmans F, Haesebrouck F, et al. (2004) Interactions of butyric acid- and acetic acid-treated Salmonella with chicken primary cecal epithelial cells in vitro. Avian Dis 48(2): 384-391.
- Videnska P, Sisak F, Havlickova H, Faldynova M, Rychlik I (2013) Influence of Salmonella enterica serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Vet Res 9(140).
- Thibodeau A, Fravalo P, Yergeau E, Arsenault J, Lahaye L, et al. (2015) Chicken Caecal Microbiome modifications induced by campylobacter Jejuni colonization and by a non-antibiotic feed additive. Plos One 10(7): e0131978.






























