- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Longitudinal Multicenter Randomized Controlled Trial on the ASSYST Individual Treatment Intervention Provided to Adult Females with Adverse Childhood Experiences
Mainthow Nicolle*, Zapién Rosa, Givaudan Martha and Jarero Ignacio
Department of Research, Mexican Association for Mental Health Support in Crisis, Mexico
Submission: March 27, 2023; Published: April 29, 2023
*Corresponding author: Nicolle Mainthow, Department of Research, Mexican Association for Mental Health Support in Crisis, Mexico.
How to cite this article:Mainthow N, Zapién R, Givaudan M, Jarero I. Longitudinal Multicenter Randomized Controlled Trial on the ASSYST Individual Treatment Intervention Provided to Adult Females with Adverse Childhood Experiences. Psychol Behav Sci Int J. 2023; 20(3): 556040. DOI: 10.19080/PBSIJ.2023.20.556040.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Abstract
The aim of this longitudinal multicenter randomized controlled trial with intention-to-treat analysis was to evaluate the effectiveness, efficacy, and safety of the Acute Stress Syndrome Stabilization Individual (ASSYST-I) treatment intervention in reducing posttraumatic stress disorder (PTSD), depression, and anxiety symptoms in adult females with adverse childhood experiences. A total of 43 adult females met the inclusion criteria and participated in the study. Participants’ ages ranged from 18 to 80 years old (M =33.74 years). A two-arm randomized controlled trial (RCT) design was applied. PTSD, anxiety, and depression symptoms were measured in four-time points for all participants in the study. Results showed that the ASSYST-I treatment intervention had a significant effect in the different comparisons carried out through ANOVA for repeated measurements for these variables.
In regard to posttraumatic stress disorder (PTSD), results showed that the intervention had a significant effect for time on this variable with a large effect size (F (3,123) = 196.07 p <.000, η² = .827), and a significant interaction between time and group, (F (3, 123) = 25.67, p <.000, η² = .385). A significant effect for group was also found (F (1, 41 = 9.17, p<.005, η² = .183). The significant differences of the means over time and comparing the groups are presented including the size effect.
The statistical analysis and the clinical reports confirmed that the ASSYST-I is an effective, safe, and efficient treatment intervention to reduce PTSD, anxiety, and depression symptoms caused by pathogenic memories produced by adverse childhood experiences in the adult female general population. No adverse effects or events were reported by the participants during the treatment procedure administration or at sixty-day follow-up. None of the participants showed clinically significant worsening/exacerbation of symptoms after treatment. All participants received the intervention treatment according to the research design, fulfilling our ethical criteria.
Keywords: Acute Stress Syndrome Stabilization; ASSYST; Posttraumatic stress disorder (PTSD); Anxiety; Depression; Adverse Childhood Experiences
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Introduction
The prevalence of Adverse Childhood Experiences (ACEs) and their detrimental effects has been consistently correlated with long-term adult negative consequences. The US Center for Disease Control and Prevention (CDC) reports 61% of adults had at least one ACE and 16% had four or more types of ACEs, with females at greater risk for experiencing four or more ACEs [1]. ACEs include, but are not limited to childhood physical, verbal, or sexual abuse, witnessing parental domestic violence, parental divorce, and living with someone who was depressed, abused drugs or alcohol, or had been incarcerated [2]. Adults who retroactively report experiencing ACEs have been found to be more at risk of diabetes, heart attack, obesity, cardiovascular and respiratory diseases, and cancer, also demonstrating the influence of ACEs on mortality, showing that individuals who have experienced six or more ACEs die approximately 20 years earlier than those who have not experienced ACEs [3-8].
ACEs also negatively impact mental, emotional, and social wellbeing in adults. The number of ACEs exposure corresponds with increased mental health risks, with those with four or more ACEs at higher risk of drug abuse, depression and anxiety, being a victim and perpetrator of violence, and suicide attempts than those without ACEs [9,10]. The long-term social impact of adults with exposure to ACEs is reflected in a research study that showed that offenders report nearly four times as many ACEs than nonoffenders, with eight of ten ACEs reported at significantly higher levels in offender populations, with those convicted of sexually based crimes more likely to have experienced child sexual abuse [11]. A study of female offenders showed that females were ten times more likely to have experienced sexual abuse than male offenders, and 46.7% reported witnessing or experiencing family violence 46.7% [12].
The number of ACEs experienced are exponentially interrelated, with one study displaying that the presence of one ACE category had a 65-93% probability of experience of ACEs in another category and a 40–74% probability of experiencing two or more additional categories, and an additional study which showed that experiencing one ACE type had 78–98% probability of exposure to another type, and a 58–90% probability of exposure to two or more additional types [6,13].
Females are at greater risk for experiencing four or more ACEs, which also has an effect on their children. One study showed that the mother’s higher number of ACEs exposure was related children’s higher scores on internalizing and externalizing behavior problems measures, lower levels of positive behavior, and high association with attention deficit hyperactive disorder (ADHD) and emotional disturbance, while another study found a correlation between mothers with exposure to ACEs and depressive symptoms in their children, suggesting that higher level of cortisol due to maternal ACEs may affect the development of the fetal brain [10,14].
A literature review, in which 99 published articles met inclusion criteria, found that Cognitive Behavior Therapy (CBT) was the most effective treatment intervention for adults who have reported exposure to ACEs, but also concluded that there are significant gaps in the literature, resulting in the lack of determination of a “gold standard” for the treatment of adults who have been exposed to ACEs. [15]. Other proposed interventions for adults who have had exposure to ACEs are Trauma-Focused Cognitive Behavioral Therapy (TF-CBT) and Eye Movement Desensitization and Reprocessing (EMDR) Therapy, as well as Prolonged Exposure Therapy (PE), yoga, mindfulness, psychoeducation, and neurofeedback, but with less empirical evidence [16]. These interventions require temporal and specialist resources, creating a barrier in efficiency and accessibility to those who are at higher risk of being exposed to ACEs, especially those in developing countries, lower socio-economic status (SES), or of specific racial/ethnic minorities. Therefore, finding an efficient, cost-effective, and accessible treatment intervention for the adult female population with ACEs is of particular interest for this study, as well as to fill the deficit of evidence for this population.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Acute Stress Syndrome Stabilization Individual Treatment Intervention
The Acute Stress Syndrome Stabilization (ASSYST) Individual treatment intervention was born during humanitarian fieldwork and is an Adaptive Information Processing (AIP)-informed, evidence-based, carefully field-tested, and user-friendly psychophysiological algorithmic approach, whose reference is the EMDR Protocol for Recent Critical Incidents and Ongoing Traumatic Stress (EMDR-PRECI) [17-27].This treatment intervention is specifically designed to provide in-person or online support to clients who present Acute Stress Disorder (ASD) or Posttraumatic Stress Disorder (PTSD) intense psychological distress and/or physiological reactivity caused by the disorders’ intrusion symptoms associated with the memories of the adverse experience(s) [28]..
The objective of this treatment intervention is focused on the patient’s Autonomic Nervous System sympathetic branch hyperactivation regulation through the reduction or removal of the activation produced by the sensory, emotional, or physiological components of the pathogenic memories of the adverse experience(s) to achieve optimal levels of Autonomic Nervous System activation, stop the three major stress hormones [adrenaline (epinephrine), noradrenaline (norepinephrine), and cortisol] secretion, and reestablish the Prefrontal Cortex functions (e.g., processing of information); thus, facilitating the AIP-system and the subsequent adaptive processing of information [29].
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Previous Assyst Treatment Intervention Studies
Five previous studies on the ASSYST treatment interventions have proven their efficacy and safety with different populations: (I) General population in lockdown and with ongoing traumatic stress during the COVID-19 Pandemic. (II) TeleMental Health counseling to the general population after adverse experiences. (III) Mental Health Professionals working during the COVID-19 Pandemic with patients suffering from trauma-related disorders and stressors. (IV) General population with non-recent pathogenic memories. (V) Adult Syrian refugees living in Lebanon [30-34].
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Objective
The objective of this longitudinal multicenter randomized controlled trial was to evaluate the effectiveness, efficacy, and safety of the Acute Stress Syndrome Stabilization Individual (ASSYST-I) treatment intervention in reducing posttraumatic stress disorder (PTSD), depression, and anxiety symptoms in adult females with adverse childhood experiences.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Method
Study Design
To measure the effectiveness of the ASSYST-I on the dependent variables PTSD, Anxiety, and Depression, this study with intention to-treat analysis used a two-arm longitudinal multicenter randomized controlled trial (RCT) design. For ethical reasons (to provide therapy to all participants), we selected a waitlist/ delayed treatment control group design, comparing immediate treatment and waitlist/delayed treatment groups. PTSD, anxiety, and depression symptoms were measured in four-time points for all participants in the study: Time 1. Baseline assessment; Time 2. Immediate treatment group (ITG) post-treatment assessment; Time 3. Waitlist/delayed treatment control group (DTG) posttreatment assessment, and Time 4. Follow-up assessment.
Ethics and Research Quality
The research protocol was reviewed and approved by the EMDR Mexico International Research Ethics Review Board (also known in the United States of America as an Institutional Review Board) in compliance with the International Committee of Medical Journal Editors recommendations, the Guidelines for Good Clinical Practice of the European Medicines Agency (version 1 December 2016), and the Helsinki Declaration as revised in 2013. The research quality of this study was based on the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement and the Standard Protocol Items Recommendation for Interventional Trials (SPIRIT) 2013 checklist [35,36].
Participants
This study was conducted at two different inpatient centers in the cities of Puebla and Toluca in Mexico, from September 2022 to March 2023, with the Mexican (Latina) adult general female population with pathogenic memories from adverse childhood experiences. Forty-eight potential participants were recruited. Inclusion criteria were: (a) being an adult female, (b) having pathogenic memories from adverse childhood experiences causing current distress, (c) voluntarily participating in the study, (d) not receiving specialized trauma therapy, (e) not receiving drug therapy for PTSD symptoms, (f) having a PCL- 5 total score of 30 points or more. Exclusion criteria were: (a) ongoing self-harm/suicidal or homicidal ideation, (b) diagnosis of schizophrenia, psychotic, or bipolar disorder, (c) diagnosis of a dissociative disorder, (d) organic mental disorder, (e) a current, active chemical dependency problem, (f) significant cognitive impairment (e.g., severe intellectual disability, dementia), (g) presence of uncontrolled symptoms due to a medical illness. Five of the 48 potential participants were excluded. One due to ongoing self-harm/suicidal or homicidal ideation, one who did not provide informed consent, and three due to having PCL-5 scores under 30 points. A total of 43 adult females met the inclusion criteria and participated in the study. Participants’ ages ranged from 18 to 80 years old (M =33.74 years). Participation was voluntary with the participants’ signed informed consent in accordance with the Mental Capacity Act 2005.
Instruments for Psychometric Evaluation
a) The study participants used the Childhood Trauma Questionnaire-Short Form (CTQ-SF; translated and backtranslated to Spanish) to choose their worst pathogenic memory from their adverse childhood experiences causing current distress. The CTQ-SF is a retrospective measure of childhood trauma that has been psychometrically assessed in diverse populations, cultures, and languages and is one of the most widely used scales in the world. It has shown high validity and performed consistently across different populations with various abuse histories. These include having good convergent validity with structured interviews that assess childhood trauma. Of the 28 questions on the CTQ-SF, 25 items are split into five subscales: Emotional Abuse, Physical Abuse, Sexual Abuse, Physical Neglect, and Emotional Neglect. The questions ask respondents to make statements about their childhood and their understanding of their trauma as an adolescent or adult. There are five statements under each subscale. Respondents are asked to give a number rating of between 1 and 5 for each statement. These numbers correspond to truth statements, with 1 being Never True and 5 being Very Often True [37,38].
b) To measure PTSD symptom severity and treatment response, we used the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5) provided by the National Center for PTSD (NCPTSD) with the time interval for symptoms to be the past week. The instrument was translated and back-translated to Spanish. It contains 20 items, including three new PTSD symptoms (compared with the PTSD Checklist for DSM-IV) [39,40]: blame, negative emotions, and reckless or self-destructive behavior. Respondents indicated how much they have been bothered by each PTSD symptom over the past week (rather than the past month), using a 5-point Likert scale ranging from 0=not at all, 1=a little bit, 2=moderately, 3=quite a bit, and 4=extremely. A total symptom score of zero to 80 can be obtained by summing the items. The sum of the scores yields a continuous measure of PTSD symptom severity for symptom clusters and the whole disorder. Psychometrics for the PCL-5, validated against the Clinician- Administered PTSD Scale-5 (CAPS-5) diagnosis, suggest that a score of 31-33 is optimal to determine probable PTSD diagnosis, and a score of 33 is recommended for use at present [41,42].
c) To measure anxiety and depression symptom severity and treatment response, we used the Hospital Anxiety and Depression Scale (HADS), which has been extensively used to evaluate these psychiatric comorbidities in various clinical settings at all levels of healthcare services and with the general population. The instrument was translated and back-translated to Spanish. It is a 14-item self-report scale to measure the Anxiety (7 items) and Depression (7 items) of patients with both somatic and mental problems using a 4-point Likert scale ranging from 0 to 3. The response descriptors of all items are Yes, definitely (score 3); Yes, sometimes (score 2); No, not much (score 1); No, not at all (score 0). A higher score represents higher levels of Anxiety and Depression: a domain score of 11 or greater indicates Anxiety or Depression; 8–10 indicates borderline case; 7 or lower indicates no signs of Anxiety or Depression [43,44].
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Procedure
Randomization, Allocation Concealment Mechanism, and Blinding Procedure
A computer-generated simple randomization with a 1:1 allocation ratio was used. Two independent assessors blind to treatment conditions conducted the randomization process to avoid allocation influence. The treatment random allocation sequence was concealed using sequentially numbered, opaque, sealed, and stapled envelopes who were open only after they were irreversibly assigned to the participants. The safekeeping of the envelopes and the assignment of participants to each arm of the trial (implementation of the random allocation sequence) was overseen by a person not involved in the research study and independent of the enrollment personnel. The treatment allocation of the participants was blinded for the research assistants who conducted the intake interview, initial assessment, and enrollment, and for the independent assessors who conducted the follow-up assessments. Participants were instructed to not reveal their treatment allocation to the persons conducting the assessments. Twenty-three participants were allocated in the immediate treatment group (ITG) and twenty participants in the delayed treatment group (DTG). See Flow Diagram in Figure 1.

Enrollment, Assessments Times, Blind Data Collection, and Confidentiality of Data
Immediate treatment group (ITG) and waitlist/delayed treatment control group (DTG) participants completed the instruments in person and on an individual basis during distinct assessment moments. During Time 1, research assistants (all mental health professionals) formally trained in all of the instruments’ administration, who were not blind to the study, but blind to the treatment allocation, conducted the intake interview, collected demographic data (e.g., name, age, gender, and contact information), assessed potential participants for eligibility based on the inclusion/exclusion criteria, obtained signed informed consent from the participants, conducted the pre-treatment/baseline application of instruments, enrolled participants in the study, and randomly assigned each participant to one of the nine clinicians formally trained in the ASSYST-I that participated in this study. The research assistants also assisted the participants in identifying the pathogenic memory of their worst adverse childhood experience to be treated with the ASSYST-I. Each identified memory was written down by the research assistants on the Memory Record Sheets that were utilized by the clinicians during the ASSYST-I treatment intervention and utilized by participants during the four assessments times to ensure participants were focusing on the same memory when they received the treatment intervention, as well as the specific assessment time when they completed the assessment tools.
During Time 2 (post-treatment assessment 15 days after Time 1 baseline assessment), Time 3 (follow-up assessment 45 days after Time 1 baseline assessment), and Time 4 (followup assessment 60 days after Time 1 baseline assessment), assessments were conducted for all participants by blind to treatment allocation independent assessors with a master’s degree in clinical psychology and formal training in administration of all of the instruments. The data safe keeper independent assessor received the participant’s assessment instruments that were answered during Times 1, 2, 3, and 4. All data was collected, stored, and handled in full compliance with the EMDR Mexico International Research Ethics Review Board requirements to ensure confidentiality. Each study participant gave their consent for access to their data, which was strictly required for study quality control. All procedures for handling, storing, destroying, and processing data were in compliance with the Data Protection Act 2018. All persons involved in this research project were subject to professional confidentiality.
Withdrawal from the Study and Missing Data
All research participants had the right to withdraw from the study without justification at any time and with assurances of no prejudicial result. If participants decided to withdraw from the study, they were no longer followed up in the research protocol. There were no withdrawals or missing data during this study.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Treatment
Clinicians and Treatment Fidelity
The ASSYST-I was provided in-person to individual participants by nine licensed clinicians formally trained in this treatment intervention. Clinicians received on-going supervision and clinical feedback from the research project Clinical Director through daily group supervision and completing detailed session summary forms for each session with each participant that they were assigned that were designed specifically for the ASSYST-I treatment intervention to guide, elicit, monitor, and facilitate clinicians’ treatment adherence.
Treatment Description and Treatment Safety
Participants’ treated memories were an average of 17.69 years old and received an average of 2.1 in-person sessions, with an average length of 46 minutes per session. The ASSYST-I treatment intervention focused only on the pathogenic memory produced by the worst adverse childhood experience that was selected during T1 pre-treatment assessment. To ensure the continuity and congruency of the intervention and measurement of its efficiency and efficacy, as mentioned above, during the intake interview after the memory was selected, the research assistants conducting the intake interview wrote down the specific memory on a Memory Record Sheet, which was used during each session of the ASSYST-I treatment procedure, and was referred to in order to answer all T1, T2, T3, and T4 assessments. At the beginning of the first treatment session, the participants were asked to run a mental movie of the specific previously selected memory, and then to choose the worst part. The treatment intervention was considered complete when the participant’s subjective levels of disturbance associated with the pathogenic memory decreased to zero or one (ecological/realistic). The ASSYST treatment intervention was provided to all participants in an intensive treatment modality with two 60 minutes (max) sessions provided per day over three consecutive days. No participant required more than the maximum six sessions.
Treatment safety was defined as the absence of adverse effects, events, or symptoms worsening. Therefore, participants were instructed by their clinicians to immediately report any adverse effects (e.g., dissociative symptoms [derealization/ depersonalization], fear, panic, freeze, shut down, collapse, fainting); events (e.g., suicidal ideation, suicide attempts, selfharm, homicidal ideation); or symptoms worsening during the entire study timeframe. The research project Clinical Director monitored attrition, adverse effects, events, or worsening of symptoms during the study. No adverse effects or events were reported by the participants during the treatment procedure administration or at sixty-day follow-up. None of the participants showed clinically significant worsening/exacerbation of symptoms on the PCL-5 or HADS after treatment.
Examples of the Pathogenic Memories Treated with the ASSYST-I
Examples of pathogenic memories treated during the ASSYST-I sessions were: a) sexual assault perpetrated by a brother-in-law, and familial blame and rejection placed on the participant for the sexual assault and her sister and brother-in-law’s divorce; b) an accident which killed several members of a family and the image of the bodies; c) repeated rape perpetrated by a family member; d) a sexual assault at age 5 and being blamed by the mother for provoking the assault; e) the day a parent migrated to the US and never returned; f) the murder of a family member and being followed and receiving threats of murder by the same people; g) a near fatal asthma attack; h) being told a family member had died while the patient was on the way to see the family member; i) being told the patient’s father was not the patient’s biological father; j) being sexually assaulted by a father while sleeping.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Statistical Analyses
Analyses of variance (ANOVA) for repeated measurements comparing the two groups Immediate Treatment Group (ITG) vs Delayed Treatment Group (DTG) was applied to analyze the effects of the treatment across time through four-time measurements: Time 1. Baseline assessment; Time 2. Immediate treatment group (ITG) post-treatment assessment; Time 3. Waitlist/delayed treatment control group (DTG) post-treatment assessment, and Time 4. Follow-up assessment. To analyze the effect of the ASSYST-I treatment on PTSD, Anxiety, and Depression; eta squared (η²) is reported to show the effect size. Cohen´s d for independent samples t test, comparing between groups and Cohen´s d for paired (repeated) samples in a within group design was carried out.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
PTSD
Results showed that the intervention had a significant effect for time on PTSD with a large effect size (F (3,123) = 196.07 p <.000, η² = .827). A significant effect for group was also found (F (1, 41 = 9.17, p<.005, η² = .183), and a significant interaction between time and group, (F (3, 123) = 25.67, p <.000, η² = .385). Means comparison between groups did not show significant differences for Time 1. Baseline assessment (M = 38.30, SD = 12.32 vs M = 20.16, SD = 15.52). For Time 2. ITG post-treatment assessment, significant differences between the Immediate Treatment Group (ITG) and Delayed Treatment Group (DTG) group were found, t (41) = - 5.46, p=.000, d = -1.72, (M= 38.30, SD= 12.32 vs M=20.16, SD=15.52). No significant differences were found on the following measurements in the intergroup comparisons.
Intragroup means comparisons for ITG showed significant differences between Time 1. Baseline and Time 2. ITG posttreatment assessment with a large effect, t (22) = 9.96, p=.000, d = 1,57; between Time 2. ITG post-treatment assessment and Time 3. DTG post-treatment assessment, t (22) = 3.32, p =.000, d = .25 and between Time 3. DTG post-treatment assessment and Time 4. Follow-up assessment, t (22) =3.27, p=.003, d= .21, with a smaller effect. Intragroup means comparisons for DTG showed significant differences between Time 2. ITG post-treatment assessment and Time 3. DTG post-treatment assessment with a large effect, t (19) = 8.30, p =.000, d = 1.34, as well as for between Time 3. DTG post-treatment assessment and Time 4. Follow-up assessment with medium effect, t (19) = 4.16, p=.001, d = .51. See Table 1 and Figure 2.

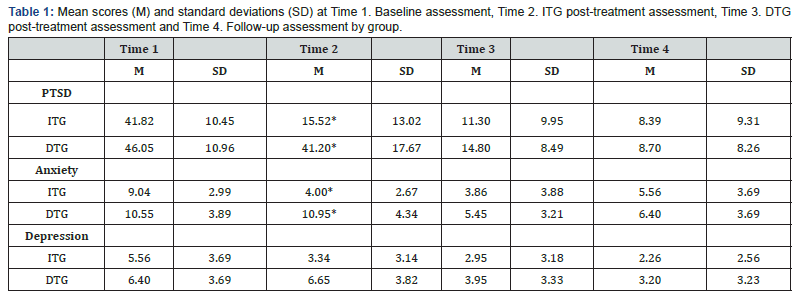
ITG=Immediate Treatment Group DTG= Delayed Treatment Group
*Statistically significant differences between groups.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Anxiety
Repeated-measures ANOVA determined that mean scores on Anxiety differed significantly across time points with (F (2, 123), 54.24, p= .000, η² =.570, β-1=1). Significant effect for group was also observed (F (1, 41 = 13.67, p<.001, η² = .258) and a significant interaction between time and group, (F (3, 123) = 14.24, p <.000, η² = .258).
Means comparison between groups for Anxiety did not show significant differences for Time 1. Baseline assessment (M = 9.04, SD = 2.99 vs M =10.55, SD =3,89). For Time 2. ITG post-treatment assessment, significant differences between the Immediate Treatment Group (ITG) and Delayed Treatment Group (DTG) group were found, t (41) = - 6,40, p =.00, d = -2.05, (M = 4.00, SD = 2.67 vs M = 10.95, SD = 4.34). No significant differences were found on the following measurements for inter-group comparisons. Intragroup means comparisons for ITG showed significant differences between Time 1. Baseline assessment and Time 2. ITG post-treatment assessment, with a large effect, t (22) = .24, p =.00, d = 1.25. Intragroup means comparisons for DTG showed significant differences between Time 2 and Time 3, with a large effect, t (19) = 6.74, p=.000, d= 1.01. See Table 1 and Figure 3.

- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Depression
Statistical analyses using ANOVA for repeated measurements revealed that that mean scores on Depression differed significantly across time points with (F (2, 123), 18.87, p= .000, η² =.315, β-1=1). Significant interaction between time and group, (F (3, 123), 3.92, p =.050, η² =. 070) was also observed. Inter-subject comparisons did not show significant effect for this variable.
Means comparison between groups for Depression did not show significant differences for pre-test Time 1. Baseline assessment (M= 5.56, SD= 3.69 vs M= 6.40, SD= 3.69). For Time 2. ITG post-treatment assessment, significant differences between the Immediate Treatment Group (ITG) and Delayed Treatment Group (DTG) group were found, t (41) = - 3.10, p =.00, d =. -0.92, (M = 3.34, SD = 3.14 vs M = 6.65, SD = 3.82). No significant differences were found on the following measurements for intergroup comparisons. Intragroup means comparisons for ITG showed significant differences between Time 1. Baseline and Time 2. ITG post-treatment assessment, t (22) = .768, p=.00, d = .45 and between Time 3 and Time 4, t (22) = 2.03, p=.00, d= .16. Intragroup means comparisons for DTG showed significant differences between Time 2 and Time 3, t (19) = 3.50, p=.002, d= .53 and between Time 3. DTG post-treatment assessment and Time 4. Follow-up assessment, t (19) = 2.11, p< .05, d = .17. See Table 1 and Figure 4.

- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Discussion
The aim of this longitudinal multicenter randomized controlled trial with intention-to-treat analysis was to evaluate the effectiveness, efficacy, and safety of the Acute Stress Syndrome Stabilization Individual (ASSYST-I) treatment intervention in reducing posttraumatic stress disorder (PTSD), depression, and anxiety symptoms in adult females with adverse childhood experiences. A total of 43 adult females met the inclusion criteria and participated in the study. Participants’ ages ranged from 18 to 80 years old (M =33.74 years).
Repeated-measures ANOVA analyses showed that the intervention had a significant effect for time on PTSD with a large effect size (F (3,123) = 196.07 p <.000, η² = .827). A significant effect for group was also found (F (1, 41 = 9.17, p<.005, η² = .183), and a significant interaction between time and group, (F (3, 123) = 25.67, p <.000, η² = .385). Anxiety differed significantly across time points with (F (2, 123), 54.24, p= .000, η² =.570, β-1=1). Significant effect for group was observed (F (1, 41 = 13.67, p<.001, η² = .258) as well as a significant interaction between time and group, (F (3, 123) = 14.24, p <.000, η² = .258). Regarding Depression, significant effects across time points (F (2, 123), 18.87, p= .000, η² =.315, β-1=1) and significant interaction between time and group, (F (3, 123), 3.92, p =.050, η² =. 070) were observed. Means comparisons at baseline confirm the random selection of subjects with no differences between groups at this point. Largest effects were found after the ASSYST-I intervention treatment in both groups in the three evaluated variables.
The research design using a two-arm longitudinal multicenter randomized controlled trial (RCT) with intention-to-treat analysis was a powerful scientific way to confirm the ASSYST-I large effect size treatment effect in both groups. The randomization, allocation concealment, blinding procedure, and groups with no significant differences at baseline in any variable significantly reduced the risk of bias. It is important to notice that the treatment effect extended beyond the post-treatment assessment as we can see in the follow-up measurements.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Conclusion
Public policy, organizations, and programs advocate for and endorse interventions directed at prevention and early intervention to treat children who have experienced ACEs, however, there is little research and information on clinical and primary care interventions for adults. While prevention and early intervention in relation to ACEs for children is crucial for health, treatment interventions for adults who have been exposed to ACEs is essential, but often overlooked, and adults are frequently unassessed or underassessed for exposure to ACEs. The negative adult outcomes previously mentioned, when experienced by a parent, particularly the mother, then create exposure to ACEs for their children. However, there is a scarcity of research on effective treatment interventions for adult females who have been exposed to ACEs, and no “gold standard” intervention exists for the treatment of ACEs in adult females. Finding a safe, effective, and efficient treatment intervention for adult females who have been exposed to ACEs is of particular importance because females are more likely to experience a higher number of ACEs, and in particular those ACEs categorized as sexual abuse. The present study shows that the ASSYST-I is an effective, safe, and efficient treatment intervention to reduce PTSD, anxiety, and depression symptoms caused by pathogenic memories produced by adverse childhood experiences in the adult female general population.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Limitations, and Future Directions
The small sample size and the follow-up assessment at sixty days are limitations of this study. We recommend future longitudinal multicenter randomized controlled trials with intention-to-treat analysis, a larger sample, follow-up assessment at six months, and following the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement and the Standard Protocol Items Recommendation for Interventional Trials (SPIRIT) 2013 checklist.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Conflict of Interest and Funding
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
Acknowledgment
a) We want to express our gratitude to all the clinicians and research assistants that participated in this study: Lara María Gómez Trejo, Sofía Gabriela Tirado, María Pía Rodríguez Regordosa, Yazmín Maldonado Aviles, and Javier Alonso Alonso.
- Research Article
- Abstract
- Introduction
- Acute Stress Syndrome Stabilization Individual Treatment Intervention
- Previous Assyst Treatment Intervention Studies
- Objective
- Method
- Procedure
- Treatment
- Statistical Analyses
- PTSD
- Anxiety
- Depression
- Discussion
- Conclusion
- Limitations, and Future Directions
- Conflict of Interest and Funding
- Acknowledgment
- References
References
- US Center for Disease Control and Prevention (2021) Vital Signs. Cdc.gov.
- US Center for Disease Control and Prevention (2022) Fast Facts: Preventing Adverse Childhood Experiences.
- Monnat M, Chandler RF (2015) Long-Term Physical Health Consequences of Adverse Childhood Experiences. The Sociology Quarterly 56(4): 723-752.
- Fujiwara T (2022) Impact of adverse childhood experience on physical and mental health: a life-course epidemiology perspective. Psychiatry Clin Neurosci 76(11): 544-551.
- Ross N, Gilbert R, Torres S, Dugas K, Jefferies P, et al. (2020) Adverse childhood experiences: assessing the impact on physical and psychosocial health in adulthood and the mitigating role of resilience. Child Abuse Negl 103:104440.
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, et al. (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine 14(4): 245–258.
- Riedl D, Lampe A, Exenberger S, Nolte T, Trawöger I, et al. (2020) Prevalence of adverse childhood experiences (ACEs) and associated physical and mental health problems amongst hospital patients: results from a cross-sectional study. Gen Hosp Psychiatry 64: 80-86.
- Novais M, Henriques T, Vidal-Alves MJ, Magalhães T (2021) When problems only get bigger: the impact of adverse childhood experience on adult health. Front Psychol 12: 693420.
- Merrick M, Ports KA, Ford DC, Afifi TO, Gershoff E, et al. (2017) Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse Negl 69: 10-19.
- Fujiwara T (2022) Impact of adverse childhood experience on physical and mental health: a life-course epidemiology perspective. Psychiatry Clin Neurosci 76(11): 544-551.
- Reavis JA, Looman J, Franco KA, Rojas B (2013) Adverse childhood experiences and adult criminality: how long must we live before we possess our own lives? Perm J 17(2): 44-48.
- Rossegger A, Wetli N, Urbaniok F, Thomas E, Franca C, et al. (2009) Women convicted for violent offenses: Adverse childhood experiences, low level of education and poor mental health. BMC Psychiatry 9: 81.
- Dong M, et al (2004) The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl 28(7): 771-784.
- Schickedanz A, Halfon N, Sastry N, Chung P (2018) Parents’ adverse childhood experiences and their children’s behavioral health problems. Pediatrics 142(2): e20180023.
- Korotana LM, Dobson KS, Pusch D, Josephson T (2016) A review of primary care interventions to improve health outcomes in adult survivors of adverse childhood experiences, Clinical Psychology Review 46: 59-90.
- Navalta CP, McGee L, Underwood J (2018) Adverse childhood experiences, brain development, and mental health: a call for neurocounseling. Journal of Mental Health Counseling 40(3): 266–278.
- Shapiro F (2018) Eye movements desensitization and reprocessing. In: (3rd edn), Basic principles, protocols, and procedures Guilford Press, USA.
- Hase M, Balmaceda UM, Ostacoli L, Liebermann P, Hofmann A, et al. (2017) The AIP Model of EMDR Therapy and Pathogenic Memories. Front Psychol 8: 1578.
- Jarero I, Artigas L, Luber M (2011) The EMDR protocol for recent critical incidents: Application in a disaster mental health continuum of care context. Journal of EMDR Practice and Research, 5(3): 82-94.
- Jarero I, Uribe S (2011) The EMDR protocol for recent critical incidents: Brief report of an application in a human massacre situation. Journal of EMDR Practice and Research, 5(4): 156–165.
- Jarero I, Uribe S (2012) The EMDR protocol for recent critical incidents: Follow-up Report of an application in a human massacre situation. Journal of EMDR Practice and Research 6(2): 50-61.
- Jarero I, Amaya C, Givaudan M, Miranda A (2013) EMDR Individual Protocol for Paraprofessionals Use: A Randomized Controlled Trial Whit First Responders. Journal of EMDR Practice and Research 7(2): 55-64.
- Jarero I, Uribe S, Artigas L, Givaudan M (2015) EMDR protocol for recent critical incidents: A randomized controlled trial in a technological disaster context. Journal of EMDR Practice and Research 9(4): 166-173.
- Jarero I, Schnaider S, Givaudan M (2019) EMDR Protocol for Recent Critical Incidents and Ongoing Traumatic Stress with First Responders: A Randomized Controlled Trial. Journal of EMDR Practice and Research 13(2): 100-110.
- Encinas M, Osorio A, Jarero I, Givaudan M (2019) Randomized Controlled Clinical Trial on the Provision of the EMDR-PRECI to Family Caregivers of Patients with Autism Spectrum Disorder. Psychology and Behavioral Science International Journal 11(1), 1-8.
- Estrada BD, Angulo BJ, Navarro ME, Jarero I, Sánchez-Armass O, et al. (2019) PTSD, Immunoglobulins, and Cortisol Changes after the Provision of the EMDR- PRECI to Females Patients with Cancer-Related PTSD Diagnosis. American Journal of Applied Psychology 8(3): 64-71.
- Jiménez G, Becker Y, Varela C, García P, Nuño MA, et al. (2020) Multicenter Randomized Controlled Trial on the Provision of the EMDR-PRECI to Female Minors Victims of Sexual and/or Physical Violence and Related PTSD Diagnosis. American Journal of Applied Psychology 9(2): 42-51.
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. In: (5th edn), Arlington, VA.
- Jarero I (2021) ASSYT Treatment Procedures Explanation. Technical Report.
- Becker Y, Estévez ME, Pérez MC, Osorio A, Jarero I, et al. (2021) Longitudinal Multisite Randomized Controlled Trial on the Provision of the Acute Stress Syndrome Stabilization Remote for Groups to General Population in Lockdown During the COVID-19 Pandemic. Psychology and Behavioral Science International Journal 16(2): 1-11.
- Smyth-Dent K, Becker Y, Burns E, Givaudan M (2021) The Acute Stress Syndrome Stabilization Remote Individual (ASSYST-RI) for TeleMental Health Counseling After Adverse Experiences. Psychology and Behavioral Science International Journal 16(2): 1-7.
- Magalhães SS, Silva CN, Cardoso MG, Jarero I, Pereira Toralles MB, et al. (2022) Acute Stress Syndrome Stabilization Remote for Groups Provided to Mental Health Professionals During the Covid-19 Pandemic. Journal of Medical and Biological Science 21(3): 637-643.
- Mainthow N, Pérez MC, Osorio A, Givaudan M, Jarero I, et al. (2022) Multisite Clinical Trial on the ASSYST Individual Treatment Intervention Provided to General Population with Non-Recent Pathogenic Memories. Psychology and Behavioral Science International Journal 19(5).
- Smith S, Todd M, Givaudan M (2023) Clinical Trial on the ASSYST for Groups Treatment Intervention Provided to Syrian Refugees living in Lebanon. Psychology and Behavioral Science International Journal 20(2): 1-8.
- (2010) Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement. BMJ 340: c869.
- (2013) Standard Protocol Items Recommendation for Interventional Trials (SPIRIT) 2013 Checklist.
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, et al. (2003) Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27(2): 169–190.
- Bernstein DP, Fink L (2011) Childhood Trauma Questionnaire. A retrospective self-report. Manual – Svensk version [Swedish version]. Stockholm: Pearson Assessment.
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, et al. (2013) The PTSD Checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD.
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, et al. (2016) Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders- Firth edition (PCL-5) in veterans. Psychol Assess 28(11): 1379-1391.
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, et al. (2013a) Clinician-administered PTSD Scale for DSM-5. National Center for Posttraumatic Stress Disorder, Boston.
- Franklin CL, Raines AM, Cucurullo L-A, Chambliss JL, Maieritsch KP, et al. (2018) 27 ways to meet PTSD: Using the PTSD-checklist for DSM-5 to examine PTSD core criteria. Psychiatry Res 261: 504-507.
- Zigmond AS, Snaith RP (1983) The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 67: 361-370.
- Ying LC, Pakpour AH (2017) Using Hospital Anxiety and Depression Scale (HADS) on patients with epilepsy: Confirmatory factor analysis and Rasch models. Seizure 45: 42-46.






























