Expansion of Magallana Gigas Colonization: Northernmost and Southernmost Invasion on Saltmarsh Ecosystems in the SW Atlantic
Lucas M Molina1,2*, Martin A Luna1,2, Leonel Luppi1 and Daniel A Barrio1,2
1Universidad Nacional de Río Negro, Argentina
2CIT Rio Negro (UNRN-CONICET), Argentina
Submission:June 19, 2023; Published:July 14, 2023
*Correspondence author: Lucas M Molina, Universidad Nacional de Río Negro, Argentina Email: lmolina@unrn.edu.ar
How to cite this article: Lucas M M, Martin A L, Leonel L, Daniel A B. Expansion of Magallana Gigas Colonization: Northernmost and Southernmost Invasion on Saltmarsh Ecosystems in the SW Atlantic. Oceanogr Fish Open Access J. 2023; 16(3): 555938. DOI: 10.19080/OFOAJ.2022.16.555938
Abstract
This study investigates the colonization of saltmarshes by the invasive species Magallana gigas in the South West Atlantic region. The research was conducted in the Bahía Blanca and Río Negro estuaries in Argentina. Oyster densities were assessed in different areas of the saltmarshes. The results show varying densities of M. gigas in different locations, with higher densities observed at the edges of vegetated areas in Bahía Blanca and in non-vegetated areas, particularly saltmarsh channels, in the Río Negro estuary. The mechanisms of oyster arrival and their impact on native species and ecosystem functioning are discussed. This research highlights the need for monitoring and managing invasive species to protect saltmarsh ecosystems.
Keywords:Invasive species; Magallana gigas; Saltmarsh ecosystems; Spartina
Introduction
Intertidal marshes, characterized by strong plant zonation and low species diversity but exceptionally high primary and secondary production [1-4], provide valuable ecosystem services such as raw materials, food, coastal protection, erosion control, water purification, support for fisheries, carbon sequestration, and opportunities for tourism, recreation, education, and research [5-11]. Unfortunately, wetland loss worldwide, particularly in the form of marshes [12-14], has been accelerated in the past decade due to factors such as global climate change, sea-level rise, agricultural and industrial development, and sediment supply loss [15]. Although physical factors like wind action, wave energy, and tides contribute significantly to marsh erosion rates [16,17], biological factors interacting with these physical forces also play an important role in geomorphological processes. Autogenic ecosystem engineers and allogenic ecosystem engineers, along with herbivores and their predators, influence primary production and the stability of these environments [4,18-21]. Invasive species further complicate ecological systems by altering the evolutionary pathways of native species, modifying the structure of biological communities, and disrupting habitat complexity [22-25]. Invasive marine invertebrate species pose significant ecological and economic threats to marine ecosystems globally. These species, often unintentionally introduced through ballast water discharge, hull fouling, or aquaculture activities, establish populations in non-native habitats, where they lack natural predators or competitors [26]. Their rapid growth, high reproductive rates, and adaptability enable them to outcompete native species for resources, disrupt marine ecosystems, and cause declines in native biodiversity, impacting fisheries, aquaculture, and coastal communities. Managing and preventing these invasions require effective monitoring, early detection, rapid response strategies, and international cooperation to address this global issue [27].
The Pacific oyster, Magallana gigas, intentionally introduced for aquaculture purposes, often becomes invasive, resulting in significant alterations to coastal ecosystems [28]. In Argentina, Magallana gigas was illegally introduced in Anegada Bay (39º 50´ S to 40º 40´S and 62º 10´w) [29,30] around 1982 as part of an oyster culture project [31]. Since its introduction, the first oysters were discovered outside the bay in Los Pocitos in 1987 (40°26’37.08”S. 62°25’20.47”W) [32], and they have since spread both north and south, as reported by Dos Santos et al. [33] who found a few oysters on port docks at the Bahia Blanca estuary (38°52′00″S 62°07′00″W), and by Narvarte & Morsan [34] in the rocky intertidal of El Condor (41° 3’35.84”S 62°50’14.68”w).
(36°29′00″S 56°42′00″W approximately 20 km away from the location). However, [36] later determined that the oysters were another species Crasostrea talonata [37], which negatively impacted S. alterniflora. While the presence of the invasive species Magallana gigas, the Pacific oyster, may have a positive effect on some fish species, as described by [38], since it can be considered an autogenic ecosystem engineer [39], its effect on spartina species remains unknown. Twelve years after the population was established in Bahía Blanca, M. gigas is abundant and has colonized saltmarshes, with apparent significant impacts on ecosystem structure and functionality [38]. However, eighteen years after the population was found in El Condor, it has almost disappeared from the rocks, but it is starting to colonize the saltmarshes in the Rio Negro estuary, a few kilometers away (Molina, pers. obs., this work). In the South West (SW) Atlantic, between southern Brazil (32°S) and northern Patagonia (42°S), extensive saltmarshes dominated by cord grasses such as Spartina densiflora, Spartina alterniflora, and the chickenclaws Sarcocornia perennis are found [40]. These SW saltmarshes, where S. alterniflora is the only or dominant species, are daily flooded by tides (e.g., [38]) and support a high abundance of fishes [38,41].
The aim of this paper is to report the northernmost and southernmost colonization of saltmarshes by Magallana gigas.
While no presence of oysters has been registered in saltmarshes, [35] reported a small population of C. gigas in San Borombon Bay (36°00′S 57°12′W) attached to various structures such as the stems of Spartina alterniflora, plastic bags, wooden sticks, and possibly other mollusk shells, which indicates a population close to an experimental C. gigas farm in Las Toninas.
Materials and Methods
Study Area
The study was conducted in two saltmarshes located in the Bahía Blanca estuary (Villa del Mar, 38° 51’ S, 62° 6’ W) and the Río Negro estuary (40º 58´24” S 62º 48´65” W) in Argentina (Figure 1). These are large macrotidal embayments with tidal amplitudes of up to 4 meters, covering an area of 2300 km2 and 400 km2 respectively, and experiencing semidiurnal tides [42]. In both locations, Spartina alterniflora is the dominant marsh plant species occupying the lower intertidal zone. The middle zone is occupied by Spartina densiflora, while Sarcocornia perennis is restricted to a narrow strip on the higher part of the saltmarsh. During low tide, both the saltmarsh and a wide area of the tidal flat are exposed to air. Sampling was conducted in the summer of 2023 in the lower part of the saltmarsh where S. alterniflora dominates, including vegetated areas and the edge of vegetated areas, as well as in the adjacent tidal channels and tidal flats.
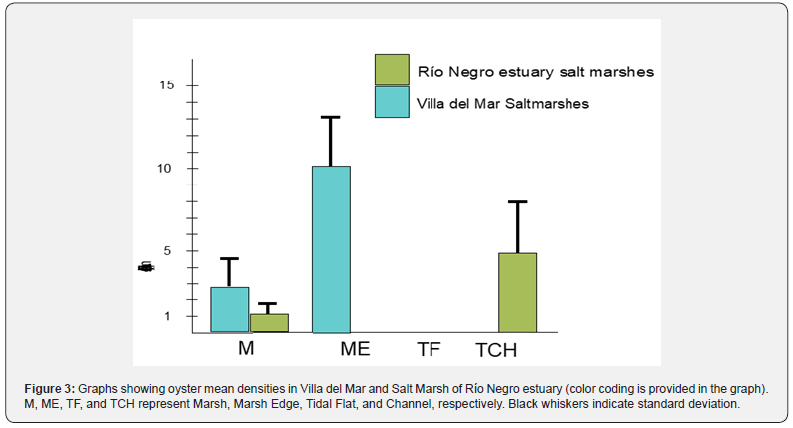
To estimate oyster densities in vegetated areas, channels, and bare sediments on the saltmarshes, we utilized a circular sampler of 0.28 m2 (N=20 per site, Figure 2). Mean densities were calculated and graphed for each site (Figure 3).
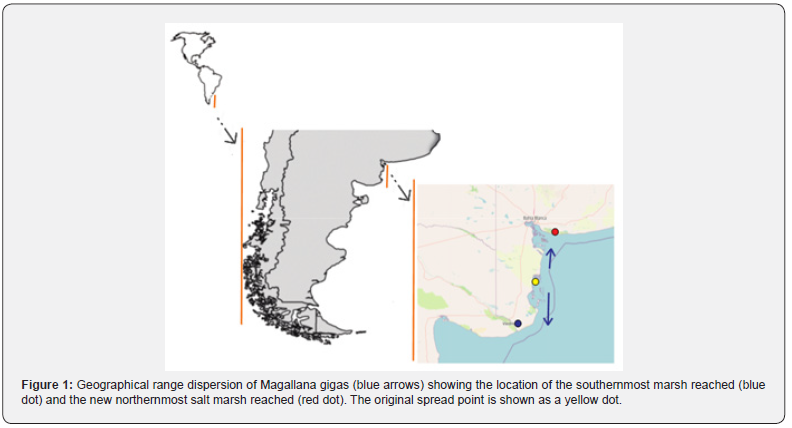

Results and Discussion
The density patterns vary between the different sites. In the Bahia Blanca salt marshes, oyster densities are higher at the edges of vegetated areas, while they are lower in the bare sediment areas or tidal flats. On the other hand, in the Rio Negro estuary salt marshes, oyster densities are higher in non-vegetated areas, specifically in the salt marsh channels, compared to the vegetated areas (Figure 3).
In the first location, the substrate consists of a mixture of sand and mud, with extensive bare flats covering most of the intertidal fringe. Despite the limited freshwater input relative to the size of the estuary, there are high densities of Magallana gigas covering the sediment surface, forming reefs. Vegetation is mainly restricted to the upper intertidal zone, with plant densities ranging from 100 to more than 300 ramets per square meter, following a strong seasonal pattern. Interestingly, numerous oysters can be found growing between the plants, resulting in a significant biogeomorphological impact (Molina pers. Obs.).
In the Rio Negro salt marshes, there are no significant differences in sediment characteristics and marsh plant structure compared to the previous location. However, there is a notable difference in the freshwater input to the system, which is considerably higher in this area. As a result, oysters show a preference for salt marsh tidal channels as their preferred habitat. The mechanism by which oysters arrived at both marshes remains unknown, with uncertainty whether it occurred naturally through larval dispersion or through intentional human activities. The lack of larval dispersal models for Non-Indigenous Species (NIS) along the Argentinian coast has led to a significant debate regarding the migration of the Pacific oyster. Additionally, the absence of systematic coastal observations, such as monitoring wind waves, nearshore currents, and other hydrographic features in this specific coastal region, presents a challenge in conducting an unbiased study based on observed parameters. Consequently, there is currently a shortage of peer-reviewed literature on coastal hydrodynamics or littoral processes in the study area due to the limited availability of data [43].
Conclusion
Invasive species have a significant impact on the distribution, abundance, and composition of native species groups. When considering the Pacific oyster (M. gigas) as an autogenic ecosystem engineer, there is an ongoing debate about its effects on biodiversity, with theoretical predictions suggesting both decreases and increases [38], and references therein. This controversy is particularly important in soft-bottom habitats, where M. gigas promotes spatial heterogeneity and complexity by creating reef structures through larval settlement and shell attachment [44].
The overall impact of M. gigas ecosystem engineering is influenced by various factors and interactions. It can facilitate primary producers [43,45], displace native species due to reef structures [46,47], promote further invasions [48,49], and increase sedimentation rates, potentially obstructing coastal areas [46,50]. Additionally, M. gigas can provide new habitat for cryptogenic fish species [38].
The introduction of Magallana gigas, which competes with existing habitat structures such as Spartina, may affect the availability of important refuge and foraging resources for estuarine fishes, while increasing habitat complexity [51,52]. Further studies are necessary to understand the impacts and modifications occurring within communities and their habitats.
This knowledge will enable the prediction of new dynamics and the improvement of management strategies, especially considering that many species inhabiting these habitats may be negatively affected. The loss of stability in saltmarshes, crucial for coastal defense, nurseries, and carbon storage, is a potential consequence that should be taken into account [53,54].
Acknowledgment
We thank the authorities of the university of Río Negro for support all authors. LMM is supported by UNRN and CONICET, but this research was carried out with LMM personal financing.
References
- Traut BH (2005) The role of coastal ecotones: a case study of the salt marsh/upland transition zone in California. Journal of Ecology 93(2): 279-290.
- Lortie CJ, Callaway RM (2006) Re‐analysis of meta‐analysis: support for the stress‐gradient hypothesis. Journal of Ecology 94(1): 7-16.
- Ríos I, Bouza PJ, Bortolus A, Del Pilar AM (2018) Soil-geomorphology relationships and landscape evolution in a southwestern Atlantic tidal salt marsh in Patagonia. Argentina. Journal of South American Earth Sciences 84: 385-398.
- Hughes Z, Farron SJ, Fitz GDM (2022) Differential Headward Erosion of Marsh Tidal Creeks: Ecological and Physical Causes, p. 60.
- Crosby SC, Sax DF, Palmer ME, Booth HS, Deegan LA, et al. (2016) Salt marsh persistence is threatened by predicted sea-level rise. Estuar Coast Shelf Sci 181: 93-99.
- Baker R, Taylor MD, Able KW, Beck MW, Cebrian J, et al. (2020) Fisheries rely on threatened salt marshes. Science 370 (6517): 670-671.
- Gedan KB, Silliman BR, Bertness MD (2009) Centuries of human-driven change in salt marsh ecosystems. Annual review of marine science 1: 117-141.
- Morgan PA, Burdick DM, Short FT (2009) The functions and values of fringing salt marshes in northern New England, USA. Estuaries and Coasts 32: 483-495.
- Wolanski E, Brinson MM, Cahoon DR, Perillo GM (2009) Coastal wetlands: a synthesis. Coastal wetlands an integrated ecosystem approach. Elsevier. Amsterdam, Países Bajos, p. 1-62.
- Bertness MD, Coverdale TC (2013) An invasive species facilitates the recovery of salt marsh ecosystems on Cape Cod. Ecology 94(9): 1937-1943.
- Battini N, Giachetti CB, Castro KL, Bortolus A, Schwindt E (2021) New invasive predator reduces the abundance of native prey in a cold‐temperate marine fouling community. Aquatic Conservation: Marine and Freshwater Ecosystems 31(10): 2842-2854.
- Escapa M, Perillo GME, Oscar I (2015) Biogeomorphically driven salt pan formation in Sarcocornia-dominated salt-marshes. Geomorphology 228:146-157.
- Molina LM, Nicolás L, Daniel EB (2019) Relative impacts of eutrophication and consumers on shoreline stability in a salt marsh. Congreso COLACMAR.
- Wilson CA, Hughes ZJ, FitzGerald DM (2022) Causal relationships among sea level rise, marsh crab activity, and salt marsh geomorphology. Proceedings of the National Academy of Sciences 119(9): e2111535119.
- Gilby BL, Weinstein MP, Baker R, Cebrian J, Alford SB, et al. (2021) Human actions alter tidal marsh seascapes and the provision of ecosystem services. Estuaries and Coasts 44(6): 1628-1636.
- Adam P (2002) Saltmarshes in a time of change. Environmental Conservation 29(1): 39-61.
- Van der Wal D, Pye K (2004) Patterns, rates and possible causes of saltmarsh erosion in the Greater Thames area (UK). Geomorphology 61(3-4): 373-391.
- Fei S, Phillips J, Shouse M (2014) Biogeomorphic impacts of invasive species. Annu Rev EcolEvol Syst 45: 69-87.
- Zapperi G, Pratolongo P, Piovan MJ, Marcovecchio J (2014) Benthic-pelagic coupling in an Intertidal Mudflat in the Bahía Blanca Estuary (SW Atlantic). Journal of Coastal Research.
- Houttuijn BLJ, FitzGerald DM, Hughes ZJ, Novak AB, Georgiou IY (2023) Reevaluating the wave power-salt marsh retreat relationship. Scientific Reports 13(1): 2884.
- Silliman BR, Bertness MD (2002) A trophic cascade regulates salt marsh primary production. Proceedings of the National Academy of Sciences 99: 10500-10505.
- Ren L, Jensen K, Porada P, Mueller P (2022) Biota-mediated carbon cycling - A synthesis of biotic-interaction controls on blue carbon. Ecology Letters 25(2): 521-540.
- Carlton JT, Cohen AN (2003) Episodic global dispersal in shallow-water marine organisms: The case history of the European shore crabs Carcinus maenas and C. aestuarii. Journal of Biogeography 30(12): 1809-1820.
- Schwindt E, Gappa JL, Raffo MP, Tatián M, Bortolus A, et al. (2014) Marine fouling invasions in ports of Patagonia (Argentina) with implications for legislation and monitoring programs. Marine Environmental Research 99: 60-68.
- Schwindt E, Bortolus A (2017) Aquatic invasion biology research in South America: Geographic patterns, advances and perspectives. Aquatic Ecosystem Health & Management 20(4): 322-333.
- Young AM, Elliott JA (2020) Life history and population dynamics of green crabs (Carcinus maenas). Fishes 5(1): 4.
- Pereyra PJ, De la Barra P, Saad JF, Gastaldi M, Arcángel AE, et al. (2021) Unravelling facilitation among introduced species, a mechanistic approach. Biological Invasions 23(11): 3483-3496.
- Carlton JT (2021) Patterns of transoceanic marine biological invasions in the Pacific Ocean. In: A Natural History of the Hawaiian Islands. University of Hawaii Press, pp. 504-518.
- Orensanz JM, Schwindt E, Pastorino G, Bortolus A, Casas G, et al. (2002) No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biological Invasions 4: 115-143.
- Darrigran G, Agudo PI, Baez P, Belz C, Cardoso F, et al. (2020) Non-native mollusks throughout South America: emergent patterns in an understudied continent. Biological Invasions 22: 853-871.
- Pascual MA, Orensanz JM, Parma AM, Saba SL (1998) The Patagonian challenge: melding conservation with development. Conservation Biology: For the Coming Decade pp. 410-425.
- Borges ME (2006) Ecology of oysters in southern Buenos Aires environments: Cultivation and management of their populations. National University of the South, Bahía Blanca, Argentina.
- Dos Santos EP, Fiori SM (2010) First record of the presence of Crassostrea gigas (Thumberg, 1793) in the Bahía Blanca Estuary, (Argentina).
- Narvarte M, Morsan EM (2005) Preliminary evaluation of the situation regarding the presence of concave oyster (Cassostrea gigas) in Balneario El Có Informe Técnico InBioMar. p. 21.
- Giberto D, Bremec C, Schejter L, Escolar M, Souto V, et al. (2012) The Pacific oyster Crassostrea gigas (Thunberg, 1793) in the Province of Buenos Aires: natural recruitments in Bahía Samborombon. Mar Fish Sci (MAFIS) 21: 21-30.
- Lomovasky BJ, Alvarez G, Addino M, Montemayor DI, Iribarne O (2014) A new non-indigenous Crassostrea species in Southwest Atlantic salt marshes affects mortality of the cordgrass Spartina alterniflora. Journal of Sea Research 90: 16-22.
- Cavaleiro NP, Lazoski C, Tureck CR, Melo CM, Do Amaral VS, et al. (2019) Crassostrea talonata, a new threat to native oyster (Bivalvia: Ostreidae) culture in the Southwest Atlantic. Journal of Experimental Marine Biology and Ecology 511: 91-99.
- Molina LM, Dubox MCG, Cazorla AL (2023) Southernmost occurrence of Gobiosoma hemigymnum (Eigenmann & Eigenmann, 1888) on salt marshes of the Bahia Blanca estuary, Argentina: an unusual finding. Journal of the Marine Biological Association of the United Kingdom 103: 31.
- Fei S, Phillips J, Shouse M (2014) Biogeomorphic impacts of invasive species. Annu Rev Ecol Evol Syst 45: 69-87.
- Isacch JP, Costa CSB, Rodríguez GL, Conde D, Escapa M, Gagliardini DA, Iribarne OO (2006) Distribution of saltmarsh plant communities associated with environmental factors along a latitudinal gradient on the south-west Atlantic coast. Journal of Biogeography 33(5): 888-900.
- Molina LM, Valiñas MS, Pratolongo PD, Elias R, Perillo GM (2017) Effect of “Whitemouth Croaker” (Micropogonias furnieri, Pisces) on the Stability of the Sediment of Salt Marshes - an Issue To Be Resolved. Estuaries and Coasts 40(6): 1795-1807.
- Piccolo MC, Perillo GM (1990) Physical characteristics of the Bahía Blanca estuary (Argentina). Estuarine, Coastal and Shelf Science 31(3): 303-317.
- Padilla DK (2010) Context-dependent impacts of a non-native ecosystem engineer, the Pacific oyster Crassostrea gigas. Integrative and Comparative Biology 50(2): 213-225.
- Troost K (2010) Causes and effects of a highly successful marine invasion: case-study of the introduced Pacific oyster Crassostrea gigas in continental NW European estuaries. Journal of Sea Research 64(3): 145-165.
- Herbert RJ, Humphreys J, Davies CJ, Roberts C, Fletcher S, et al. (2016) Ecological impacts of non-native Pacific oysters (Crassostrea gigas) and management measures for protected areas in Europe. Biodiversity and Conservation 25: 2835-2865.
- Diederich S, Nehls GJEE, Van Beusekom JE, Reise K (2005) Introduced Pacific oysters (Crassostrea gigas) in the northern Wadden Sea: invasion accelerated by warm summers? Helgoland Marine Research 59(2): 97-106.
- Faria J, Prestes ACL, Moreu I, Cacabelos E, Martins GM (2022) Dramatic changes in the structure of shallow-water marine benthic communities following the invasion by Rugulopteryx okamurae (Dictyotales, Ochrophyta) in Azores (NE Atlantic). Marine Pollution Bulletin 175: 113358.
- Rodriguez LF (2006) Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biological Invasions 8: 927-939.
- Braga AC, Camacho C, Marques A, Gago MA, Pacheco M, et al. (2018) Combined effects of warming and acidification on accumulation and elimination dynamics of paralytic shellfish toxins in mussels Mytilus galloprovincialis. Environmental Research 164: 647-654.
- Darrigran G, Agudo PI, Baez P, Belz C, Cardoso F, et al. (2020) Non-native mollusks throughout South America: emergent patterns in an understudied continent. Biological Invasions 22: 853-871.
- Castel J, Labourg PJ, Escaravage V, Auby I, Garcia ME (1989) Influence of seagrass beds and oyster parks on the abundance and biomass patterns of meio-and macrobenthos in tidal flats. Estuarine, Coastal and Shelf Science 28(1): 71-85.
- Grabowski JH, Hughes AR, Kimbro DL, Dolan MA (2005) How habitat setting influences restored oyster reef communities. Ecology 86(7): 1926-1935.
- Wörner S, Dragani WC, Echevarria ER, Carrasco M, Barón PJ (2019) An estimation of the possible migration path of the Pacific Oyster (Crassostrea gigas) along the northern coast of Patagonia. Estuaries and coasts 42(3): 806-821.
- Hosack GR, Dumbauld BR, Ruesink JL, Armstrong DA (2006) Habitat associations of estuarine species: comparisons of intertidal mudflat, seagrass (Zostera marina), and oyster (Crassostrea gigas) Estuaries and Coasts 29: 1150-1160.






























