Manifestations of Photosynthesis in the Evolution of the Global Carbon Cycle
A A Ivlev*
Russian State Agrarian University, Russia
Submission: January 21, 2019; Published: February 04, 2019
*Correspondence author: A Ivlev, Russian State Agrarian University, Timiryazev, Moscow, Russia
How to cite this article:A A Ivlev. Manifestations of Photosynthesis in the Evolution of the Global Carbon Cycle. Oceanogr Fish Open Access J. 2019; 9(1): 555755. DOI: 10.19080/OFOAJ.2019.09.555755
Abstract
The applicability of the photosynthesis equation to the analysis of the changes of global carbon cycle main participants (carbon dioxide, atmospheric oxygen, sedimentary organic carbon) is shown. Photosynthesis is manifested in antiphase changes in the average concentrations of CO2 and O2 in the atmosphere and in-phase changes in the concentration of oxygen in the atmosphere and the rate of burial of organic matter in the sediment. Due to the regulatory role of photosynthesis, the global carbon cycle spontaneously moves to a stationary state, what makes it possible to estimate the amount of buried organic matter in the earth’s crust. This parameter is the key in the study of the biosphere, its evolution, for the study of various geochemical correlations.
Keywords: Global Carbon Cycle; Photosynthesis; Sedimentary Organic Carbon; Carbon Dioxide; CO2 Assimilation; Photorespiration; Orogenic Cycles
Introduction
The well-known photosynthesis equation for a conventional photosynthetic organism can be written as follow
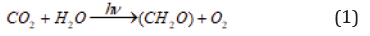
This equation establishes a formal relationship between reaction substrates CO2 and H2O assimilated from the environment and the reaction products, biomass and oxygen, produced in the light. The real kinetics of photosynthesis is complex and poorly studied. However, if to assume that the reaction rate is described by the first order kinetic equation when reaction rate is limited by СО2 entry and doesn’t depend on water concentration (the most common case, when water is in excess), the relationships between CO2 and the reaction products, (СН2О) and O2, in time are inversely proportional, whereas the relationship between the photosynthesis products (СН2О) and O2, in time are proportional Let›s try to show that equation (1) is applicable to the global photosynthesis. However, in this case it is necessary to find out what is the meaning of each of the participants of the photosynthesis reaction. Since there is an equilibrium in carbon chemical exchange “carbon dioxide - bicarbonate - carbonate” system, comprising atmosphere and hydrosphere, CO2 can be taken as the key inorganic substrate which determines concntrations of other components. According to the suggested global carbon cycle model, CO2 changed in time [1].
Consequently, the oxygen, associated with the CO2, changed as well for the considered period of time. Then, according to equation (1), there should be two antiphase curves, describing relationship of the CO2 and both products (СН2О and O2), and a curve, corresponding to proportional relationship linking the reaction products. Applying equation (1) to global photosynthesis, it is necessary to find out what is the sense of each participant of global photosynthesis reaction. Since CO2, as said, is the key component of the natural carbon chemical exchange system it remains to find out the sense of the reaction products biomass and oxygen (СН2О and O2). V.I. Vernadsky [2], developing the doctrine of the biosphere, introduced the concept of “living” matter, having defined it as the total biomass of all organisms living on the Earth. Formally, the “living” substance can be divided into parts, the biomass of photosynthetic organisms and the biomass of heterotrophic organisms:
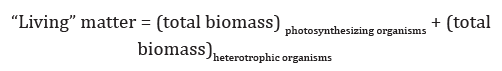
The first term is called the photosynthesis product of the first type. According to equation (1), this biomass corresponds to the equivalent amount of oxygen, produced by photosynthetic organisms, contributing to the atmosphere. The second term is called the photosynthesis product of the second type, since the total heterotrophic biomass is produced in food chains having as a source of carbon photosynthetic biomass. The thing is that the equivalent amount of oxygen corresponding to heterotrophic biomass was evolved into the atmosphere on the stage of photosynthetic assimilation. Thus the evolved oxygen and equivalent amount of heterotrophic biomass are isolated in time. Considering material balance in global carbon cycle, it should be noted that atmospheric oxygen is due to the contribution of the corresponding “living” matter of the biosphere and to the contribution of the biomass of organisms, which lived on the Earth in the past and turned into buried organic matter. According to this logic, the global photosynthesis equation for the carbon cycle can be written as follows
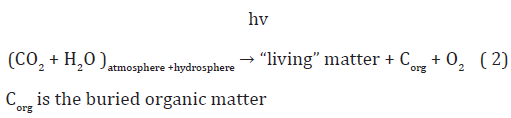
Where,
Since the amount of buried organic matter in the Earth’s crust is much greater then the amount of the “living” matter and, according to some data, is equal to a few hundredths of a percent [3], the global photosynthesis equation can be re-written

If the above logic and the approximations are correct, then equation (3) should detect the same relationships as equation (1), i.e. the concentrations of CO2 and O2 in the atmosphere should change antiphase during geological time, reflecting the “substrate - product” relationship, whereas the changes of the reaction products, Corg and O2, should be in-phase. Indeed, the results of model calculations, carried out by means of Geocarb III, show the inverse proportinality of average values of CO2 and O2 with time (Figure 1).
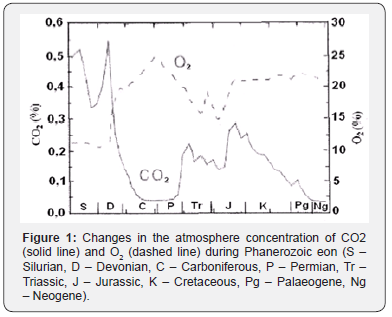
The first two periods (Cambrian and Ordovician) are not shown because of uncertainty in establishing the CO2 and O2 concentrations. The O2 estimates are based on Lenton (2001), the CO2 are from the Geocarb III model [4]. Figure was taken from work of Igamberdiev & Lea [5]. The in-phase correlation of organic carbon burial rate and atmospheric O2 concentration during Phanerozoic Figure 2 [6] also support the above conclusion. These results, obtained by independent researchers using modern geological models and based on significant experimental data, suggest that the equation of global photosynthesis exhibits the same links as the equation (1), describing the photosynthesis of an individual organism. This is consistent with the earlier assertion [7] that the amount of organic matter in the Earth’s crust has changed during geological time depending on the change of the ratio of CO2/O2 in the atmosphere and this relationship reflects the relationship of the biomass of the photosynthetic organism and mentioned ratio (Figure 2).
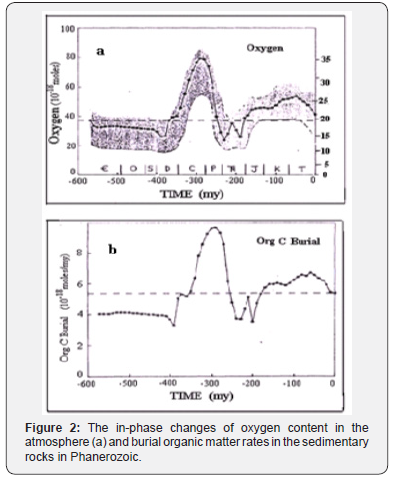
The shaded zone for oxygen designates the zone of possible errors based on sensitivity analysis. Another assertion also confirms the similar properties of conventional and global photosynthesis. It claims that carbon isotope composition of sedimentary organic matter and that of photosynthetic biomass depend on the ratio CO2/O2 as well. Both assertions are the result of a complex mechanism of photosynthesis, which, actually, consists of two reciprocal processes, CO2 assimilation and photorespiraration [8]. With the CO2 growth in the atmopshere the CO2 assimilation increases, with the growth of O2 concentration photorespiration increases. As shown [7], global photosynthesis has the same property. Applying this property in the framework of the global carbon cycle model [1], one can conclude that carbon isotope composition of sedimentary organic matter should periodically change in the course of orogenic cycles gradually getting enriched with isotope 13C.
Another important property of the global carbon cycle, associated with global photosynthesis, is its spontaneous strive to ecological compensation point, where the amount of reduced carbon produced in photosynthesis becomes equal to the amount of carbon returned back to inorganic oxidized forms. The carbon cycle gets a stationary state [9]. The oxidation occurs in sulfate reduction occurring in subduction zone. The amount of sedimentary organic matter in the earth’s crust and the equivalent amount of oxygen in the atmosphere become stationary. The presence of stationary state gives the possibility to estimate the amount of sedimentary organic matter in the Earth’s crust. The estimate is based on the equimolarity of production of Corg and atmospheric O2, according to the global photosynthesis equation. In other words, it is assumed that photosynthesis is a sole source of oxygen in the atmosphere, and the amount of oxygen, produced by the dissociation of water under the action of solar ultraviolet, can be neglected. Under the assumed approximations, the organic carbon, transformed into oxidized inorganic forms before it reaches the subduction zone, is not considered. At the same time, it was believed that if, after reaching a stationary state, all organic carbon in the earth’s crust was converted into an oxidized form, the Earth would acquire the original oxygen-free atmosphere [10]. In the frames of the same approximation it is assumed that the total organic carbon can be presented as “living” matter, i.e. as СН2О.
According to various encyclopedias (Russian geological encyclopedia, Wikipedia,) the total mass of air in the earth’s atmosphere (5.1 - 5.3) 1018 kg, and mass percent of oxygen is 23.1%. Using these data, it is easy to calculate the oxygen mass and through it the number of moles. It is amount to nO2 = 3, 75 1016 kgmole. The same number of organic matter moles is in the Earth’s crust. Despite the very approximate nature of the estimates, this figure is very important. It opens up the principal possibility of assessing the amount of natural derivatives of organic matter.
References
- Ivlev AA (2015) Global redox cycle of biospheric carbon: interaction of photosynthesis and earth crust processes. BioSystems 137: 1–11.
- Vernadsky VI (1926) Isotopes and “living” matter Dokl. AN SSSR December 215-220.
- Abelson P H (1978) Organic matter in the Earth‘s crust. Ann Rev Planet Sci 6: 325-351.
- Berner RA, Kothavala Z (2001) GEOCARB III: a revised model of atmospheric CO2 over Phanerozoic time. American Journal of Science 30: 184-204.
- Berner RA, Canfield DE (1989) A new model for atmospheric oxygen over Phanerozoic time. Am J Sci 289(4): 33-61.
- Ivlev AA (2017) Photosynthesis and its regulatory role in Natural Carbon Cycle. Journal of Ecosystem & Ecography 7:233.
- Andre MJ (2011) Modelling 18O and 16O unidirectional fluxes in plants: II. Analysis of Rubisco evolution. BioSystems 103(2): 252-265./a>
- Hunt J M (1979) Petroleum Geochemistry and Geology. In: Freeman WH, (2nd edn). Co-Publisher, San Francisco, US.
- Igamberdiev AU, Lea PJ (2006) Land plants equilibrate O2 and CO2 concentrations in the atmosphere. Photosynth Res 87: 177-94.
- Rosnedra (2010) Russian geological encyclopedia. VSEGEI Publ 1-3.






























