The Impact of Dichlorvos -Pesticide on African Catfish Clarias Gariepinus
Nwamba Helen O*, Achikanu Cosmas E and Chukwu Ginika P
Department of Applied Biology, Enugu State University of Science and Technology, Nigeria
Submission: November 13, 2018; Published: December 05, 2018
*Correspondence author: Nwamba Helen O, Department of Applied Biology, Enugu State University of Science and Technology, Enugu State, Nigeria.
How to cite this article: Nwamba H O, Achikanu C E, Chukwu G P. The Impact of Dichlorvos -Pesticide on African Catfish Clarias Gariepinus. Oceanogr Fish Open Access J. 2018; 8(4): 555745. DOI: 10.19080/OFOAJ.2018.08.555745
Abstract
The toxicity of dichlorvos (18 -20 mg/L) on Clarias gariepinus juveniles (mean weight 41.6±1.2(g) and mean length 18.5±2.5 (cm) was investigated in the present study using static bioassays over a period of 96 hours. The determined 96 hours LC50 of the exposed fishes was 17.21mg/L with lower and upper confidence limits of 15.78–18.19mg/L respectively. When the fishes were exposed there was strong evidence of stress responses characterised by hyperactive swimming with subsequent erratic with jerky movements before death which increases with time and concentration of exposure. The quality of water investigated in this study showed no change in dissolved oxygen, pH and temperature. The hepatosomatic indices (HSI) and condition factors (K) which are stress indices due to environmental pollutants decreased within 15 days of exposure and increase in concentration of dichlorvos indicating that it has detrimental effect on the liver of exposed fish with time.
Introduction
The useful state of water for humans and animals that is appropriate has become a rife concern [1]. There is a rapid decline in the quality of water from natural water resources arising from the demand of industrialisation which employ application of synthetic fertilisers and use of various insecticides and pesticides. Chemicals of agricultural or industrial origins have been reported to be source of contaminations to aquatic ecosystems by runoff and ground leaching through the area [2]. Dichlorvos (2,2- dichlorvinyl dimethyl phosphate) an agricultural insecticide is used to control household pests in public health and protecting crops and stored products. It is reported to be effective against mushroom flies, aphids, spiders, mites, caterpillars, whiteflies in greenhouse, outdoor fruits and vegetable crops [3]. It has contact and stomach insecticidal effect on food and non-food crop pests [4] and anticholinesterase action associated with the nervous systems of insects [3,5] stated that the volatility of dichlorvos will favour the vaporisation of a significant proportion of applied substance into the external atmosphere which is expected to dissipate rapidly through dilution, degradation and precipitation, reducing the atmospheric (concentrations well below levels. However, aquatic lives run a risk of toxicity if rain falls within 4 hours of application. Increased use of pesticides results in the excess inflow of toxic chemicals, mainly into the aquatic ecosystem [6,7]. This work aims at investigating the effects of dichlorvos on the wellbeing of aquatic organisms particularly on African catfish C. gariepinus. It belongs to the Claridae family and it is geographically located in Africa, the middle east, Brazil and Indonesia. They make fresh water, lakes, rivers and swamps and human made oxidative ponds and urban sewage system their habitats. The adult catfish can be 1-1.5m in length and weigh up to 60kg with flat body head, broad terminal mouth with four pairs of barbells and large accessory breathing organs made up of modified gill arches [8].
Materials and Methods
Source of experimental fish
One hundred juveniles of C. gariepinus were obtained from Rojenny tourist game village, Idemili LGA. Anambra state, Nigeria in 300 litre capacity plastic containers and transported to Heildin fisheries laboratory unit in Enugu state, Nigeria. The mean body weight and the length of the species were 41.6±1.2 (g) and 18.5±2.5 (cm) respectively.
Acclimatisation
The fish were acclimatised to laboratory conditions for 14 days and fed with top fish feed with crude protein of 38% on daily basis. The container was cleaned, and the water changed every morning. Less than 2% mortality was observed during the acclimatisation.
Range finding test
The preliminary tests to determine the range of concentrations used in this experiment were performed by exposing 10 juvenile catfish to 25 litres of dechlorinated tap water containing 18,20 and 22μl of dichlorvos respectively for 96 hours until suitable concentration that produced 100% mortality was obtained. The fish were not fed for 24 hours before and during the exposure time.
Experimental procedures
10 juvenile catfish were exposed to different concentrations of 18, 20 and 22μl dichlorvos in 10 litres of dechlorinated water from volume to volume (v/v) stock solution (10ml of concentrated dichlorvos in 1 litre of dechlorinated water) in triplicates. The mortality rate, behavioural characteristics of the catfish and the physicochemical properties of water such as pH, temperature, and dissolve oxygen were analysed every 24 hours for 96hour period [9]. The 96 hours lethal concentration (LC50) of dichlorvos was determined following the probity analysis method [10]. Dead fishes were removed from the experiment pond every morning to avoid contamination at every 24 hours interval for 96 hours. Based on the 96 hours LC50 value, the hepatosomatic indices (HSI) and condition factor (CF) of the Catfishes exposed to two sub-lethal concentrations of dichlorvos (21 and 43 mg/L) for 15 days were then determined according to White and Flecher (1985). A set of 10 fish were also maintained in dechlorinated tap water (0.00mg/l) as the control. The experiment was set in triplicates.
Statistics
Using the SPSS statistical package (version 17), the standard error mean (SEM) and Duncan’s multiple range test were used to determine the significance at 10% probability test. The significance between data were analysed with the one-way analysis of variance (ANOVA).
Result
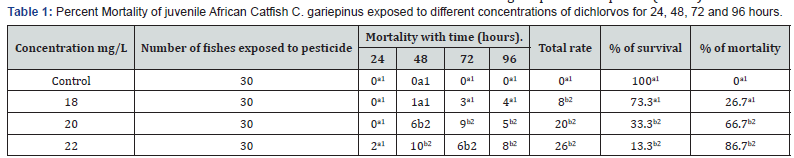
At the highest concentration of 22mg/L dichlorvos, 87 percent mortality of juvenile catfish was recorded within the 24 to 96 hours exposure. The least percentage mortality was observed in in the lowest concentration of 18mg/L. No death was recorded in the control during the period of exposure (Table1).

The following keys represent; - None, + Mild, ++ Moderate, +++ Strong
The percent mortality increased with increasing concentration. The behavioural responses of the juvenile catfish exposed to 18, 20 and 22mg/L dichlorvos for 24,48,72 and 96 hours respectively showed faster swimming, opercula activity, surfacing and gulping of air in almost all concentrations at exposure time compared to the control. However, at higher concentrations of 20 and 22mg/L, within 40 minutes of exposure the fish appeared to be hyperactive and swimming became erratic with jerky movements while at 18mg/L, the fish showed normal behaviour for the first 48hours then stopped swimming and remained static in response to the changes in the surrounding environment. There was mean pH 7.1, temperature 27 ⁰C and dissolve oxygen 5.1 for water quality in various treatments with dichlorvos. The lethal concentration of dichlorvos that will kill 10-90% (LC10-90) of the experimental organism at 24, 48, 72 and 96hours was significantly different (P<0.05). At the exposure period, the median LC50 ranges from 17.21 (15.78-18.19) to 17.98 (16.27-19.35). The hepatosomatic indices (HSI) with 95% confidence limits showed a significant difference across the sub-lethal concentrations for day 1 and 15. The condition factor (CF) of C.gariepinus exposed to sublethal concentrations of dichlorvos was significantly different throughout the exposure duration compared to control (Table 1-6) (Figure 1).
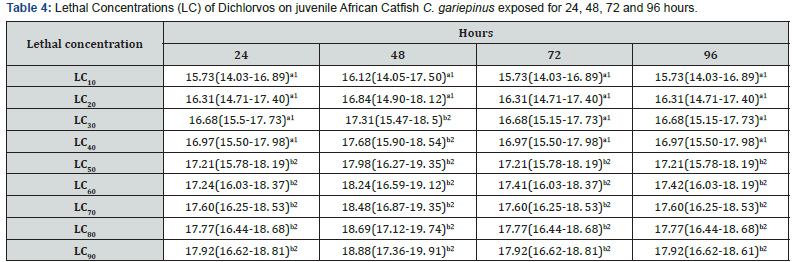
Values outside the brackets are Lethal Concentrations (LC). First value within the bracket is the lower confidence and the second value is the upper confidence respectively. Value with different numeric superscripts differ significantly (P<0.05) between different concentrations within the same exposure duration.
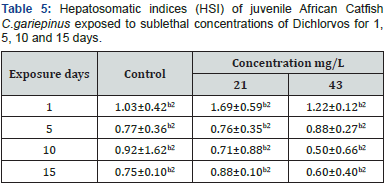
Values with different alphabetic (lower case) superscripts differ significantly (P < 0.05) between exposure periods within the same concentration. The values with different numeric superscripts differ significantly (P<0.05) between different concentrations within the same exposure duration.
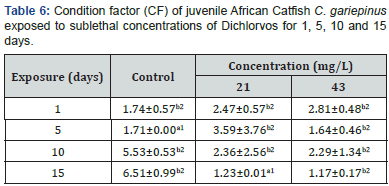
Values with different alphabetic (lowercase) superscripts differ significantly (P<0.05) between exposure periods within the same concentration. Value with different numeric superscripts differ significantly (P<0.05) between different concentrations within the same exposure duration
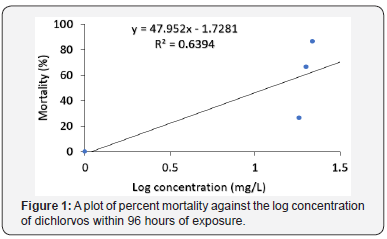
Discussion
The present study is to demonstrate the toxic effects of dichlorvos in the juveniles of the freshwater fish Clarias gariepinus. In this study, the survival rate of C. gariepinus decreased as the concentration of dichlorvos increased. At 96hour exposure to dichlorvos, the mortality percent for control, 18, 20 and 22mg/L test samples were 0, 27, 67 and 87 respectively. This finding agrees with earlier reports by Asuquo et al. Oti, Adakole [11-13]. Toxicity of dichlorvos to organisms has however been shown to be dependent on concentrations, sex, developmental stages and exposure periods [14].
Behaviour provides a unique perspective linking the physiology and ecology of an organism and its environment and allows the organism to adjust to external and internal stimuli to best meet the challenge of surviving in a changing environment [15]. The results showed that dichlorvos affected the behavioural characteristics of C. gariepinus. The control specimens were not hyperactive and showed normal swimming patterns, skin colour, equilibrium status and fin movements throughout the exposure period. However, with increasing dichlorvos concentrations and exposure duration, hyperactivity, air gulping, erratic swimming and equilibrium instability increased. Besch [16] reported that there are contact (high excitability in a moment), exertion (fast swimming, leaping and attempts to jump out of the toxicant), equilibrium instability and death phases characterising behavioural responses of fish to toxicants. Prolonged exposure to dichlorvos imposed tiredness and stress on the fish suggesting that there is insufficient intake of oxygen accompanied with detrimental effect on energy production, body building mechanism and ultimately nervous breakdown of fish. Heath, Kormakik & Cameron, Kumar & Krishna Moorthy [17-19] reported deleterious limitations in the use of energy synthesising macromolecules in fish subjected to environmental stress. The quality of water for dichlorvos did not show any significant change in the mean value of pH, temperature and dissolved oxygen compared to the control. The values fall within the normal range of water quality for aquaculture [20]. In this research the 96 hours exposure has 17.21mg/L LC50 values with lower and upper confidence limits of 15.78 and 18.19 respectively. Ofojekwu et. al. [21] reported LC50 value of 15.85g/L for fingerlings of Tilapia zilli exposed to urea fertiliser. Toxicity of pesticides to organisms is affected by the strains of species, size, age, sex, temperature, water quality and formulation of the test chemicals [22]. Fishes are more susceptible to the environmental variations and respond more to pollutants than mammals. The fish liver has been shown as a model for studying the relationship between environmental factors with the hepatic structures and the functions [23]. Hepatosomatic Index (HSI) and Condition Factor (CF) gives an indication of the overall health condition of fish and have been widely used as stress indicator due to environmental pollutants [24]. In the present study, there was a significant reduction in HSI and CF on day 15 following exposure to 21 and 43mg/L compared to day 1 respectively. Jordan et. al. [25] reported decreased CF in fish exposed to various toxicants. The HSI values are generally elevated in vertebrates experiencing induction of hepatic microsomal P-450 for detoxification of the pollutants while decline in the HSI and CF may suggest a general detrimental effect of dichlorvos pesticide on the liver of fish [26].
References
- Calamari D, Naeve H (1994) Towards management of the aquatic environment. CIFA Technical paper (FAO) Technical papers 25: 7-22.
- Todd NE, Leuwen MV (2002) Effect of Cararyl insecticide on early life stages of zebra fish Danio rerio. Ecotoxicology Environmental Safety 53: 267-272.
- Pancetti F, Olmos C, Dagnino-Subiabre A, Rozas C, Morales B (2007) Noncholinesterase effects induced by organophosphates pesticides and their relationship to cognitive processes: Implication for the action of acylpeptide hydrolase. J Toxicol Environ Health B Crit Rev 10(8): 623-630.
- Suchismta D (2013) A review of dichlorvos toxicity to fish. Current World Environment 8(1): 143-149.
- WHO (2005) International programme on chemical safety. WHO recommended classification of pesticides by Hazard and Guidelines to classification.
- Baskaran P, Palanizhamy S, Visalakshi S, Balasubramanian MP (1989) Effects of mineral fertiliser on survival of the fish Oreochromis mossambicus. Environmental Ecology journal 7: 463-465.
- Kalavanthy K, Sivakumar AA, Chandran R (2001) Toxic effect of the pesticide Dimethoate on the fish Sarotherodon mossambicus. Journal of Ecological Research and Bioconservative 2(1-2): 189-195.
- Sambhu C (2004) African Catfish, Clarias gariepinus (Burchell, 1822): An ideal candidate for bio-waste management. Indian J Exp Biol 42(12): 1226-1229.
- (1985) Standard method for examination of water and waste waters. In: Arnold E Greenberg (ed). (21th edn). New York. 1193p.
- Finney DJ (1947) Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge University Press p256.
- Asuquo IE, Essien-Ibok MA and Abiabo NO (2016) The effects of some agricultural fertilizers on fingerlings of Heterobranchus bidorsalis. Journal of Aquaculture & Marine Biology 4(2): 1-7.
- Oti EE (2002) Acute toxicity of cassava mill effluent to the African Catfish fingerlings. Journal of Aquatic Sciences 17(1):31-35.
- Adakole JA (2005) Acute toxicity of metal finishing company waste water to Clarias gariepinus fingerlings. Journal of Aquatic. Sciences 20(2): 69-73.
- Pandey AK, Nagpure NS, Trivedi S (2011) Pronfenofos induced DNA damage in freshwater fish Channa punctatus using alkaline single cell gel electrophoresis. Mutat Res 726: 209-214.
- Adewum AA, Olakje VF (2011) Catfish culture in Nigeria: progress, prospect and problems. Africa journal of Agriculture Research 6(6): 1281-1285.
- Besch WK (1975) A biological monitoring system employing rheotaxis of fish. Proceedings of symposium on Biological Monitoring of water quality and waste water quality. Blacksbury USA p. 28-32.
- Heath GA (1995) Water pollution and fish physiology. In: (2nd edn). CRC Press, Florida p. 254.
- Kormakik GA, Cameron JN (1981) Ammonia excretion in animals that breathe water: a review. Marine Biology 2: 11-23.
- Kumar NJ, Krishnamoorthi KP (1983) Evaluation of toxicity of ammoniacal fertiliser effluents. Environmental pollution Service and Ecological Biology 30(1):77-86.
- Adeniji HA, Ovie SI (1989) A simple guide to water quality management in fish ponds. National Institute for Freshwater Fisheries Research 23.
- Ofojekwu PC, Nwani CD, Chinedu C (2008) Acute toxicity of NPK (15:15:15) fertilisers of Tilapia zilli fingerlings. Nigeria journal of Fisheries 5(1):31-37.
- QECD (1992) Guidelines for testing of chemicals, fish acute toxicity test. Organisation for economic cooperation and development Paris, France 203:1-9.
- Ensibi C, Perez-Lopez M, Soler Rodriguez F, Miguez-Santiyan MP, Daly Yahya MN, et al. (2015) Effects of semi-static exposure to carburant in liver phase I and phase II enzymes of common carp (Cyprinus carpio). Journal of Environmental and Analytical Toxicology 5(3): 1-5.
- Adams SM, Barton B, Mac Kinlay D (2002) Environmental stress and health in fish. Symposium proceedings in international congress on the biology of fish, American Fisheries society, University of British, Colombia, Vancouver, Canada.
- Jordan MS, Reineeke SA, Reinecke AJ (2013) Biomaker responses and morphological effects in juvenile Tilapia Oreochromis mossambicus following sequential exposure to the organophosphate azinphosemthyl. Aquat Toxicol 15(144-145):133-140.
- Adeyemi JA (2014) Oxidative stress and antioxidant enzymes activities in the African catfish Clarias gariepinus, experimentally challenged with Escericia coli and Vibrio fishcheri. Fish Physiology and Biochem 40: 347-354.






























