The Utility of Dietary Phosphorus Enrichment During Transition to Formulated Feeds of Juvenile Abalone Haliotis tuberculata
Colin Hannon1, Rick A Officer1 and Declan Hanniffy2
1 Marine & Freshwater Research Centre, Galway Mayo Institute of Technology, Ireland
2 Ocean Harvest Technology Ltd, Ireland
Submission: March 06, 2018; Published: October 24, 2018
*Correspondence author: Colin Hannon, Marine & Freshwater Research Centre, Galway Mayo Institute of Technology, Galway, Ireland.
How to cite this article: Colin H, Rick A O, Declan H. The Utility of Dietary Phosphorus Enrichment During Transition to Formulated Feeds of Juvenile Abalone Haliotis tuberculata. Oceanogr Fish Open Access J. 2018; 8(4): 555742. DOI: 10.19080/OFOAJ.2018.08.555742
Abstract
Sustaining the growth of abalone aquaculture globally requires farm operators to satisfy the specific diet requirements of the particular abalone species grown. Components of commercial aquaculture feeds usually contain animal protein in the form of fishmeal. Rather than adding generic animal protein foodstuffs, formulated diets may be more effective when supplemented with particular substances of metabolic use to the abalone. The inclusion of animal proteins may have physiological demands on the growth and survival of different species of abalone as formulated feeds change the colour of the shell bands and the components of the feed may not exist in the natural diet of abalone. Inclusion and enrichment of phosphorus in mixed macroalgal-meal diets for juvenile abalone Haliotis tuberculata was investigated. Macroalgae is the natural diet for juvenile and adult abalone and can be limiting in bioavailable phosphorus. Increasing the available dietary phosphorus content in the diet of juvenile H. tuberculata to 0.9% and 1.5% increased growth rates in shell length compared to Palmaria palmata diet (P<0.05). 100% survival was achieved but weight loss was observed in all treatments. The development of diets containing components that exist in the natural diet of abalone has implications for farm operators, as there is increasing importance for traceability of dietary components in abalone feed.
Keywords: Abalone; Haliotis tuberculata; Feeding transitions; Phosphorous; Macroalgae; Diet
Introduction
Weaning of juvenile abalone from their initial diatom diet to macroalgae changes their feeding behaviour [1,2]. This feeding transition results from a lack of nourishment when the diatom diet can no-longer support growth [3]. Longer foraging trips begin the weaning process. Behavioural changes during the weaning process are thought to occur as abalone move on to larger food sources dominated by macroalgae [4].
At this stage in the abalone growth cycle, farm operators may introduce harvested naturally occurring macroalgae, and/or introduce formulated artificial feeds [5]. Due to seasonal changes in the nutrient content of macroalgae and the costs associated with its collection, some operators prefer to use artificial diets, provided that they can provide adequate growth coupled with reduced operating costs [6].
Previous research has established the basic nutrient requirements of juvenile abalone [7-10] and identified that the timing of a dietary switch is crucial to farm outcomes [1,12,13]. If the animal cannot dislodge, graze or digest the diet, implementation of a switch in dietary regime will negatively affect survival [2,14].
Components of extant formulated artificial diets may be non-palatable to abalone and are sometimes absent from their natural diets. In particular, the inclusion of animal proteins in formulated feeds may increase trypsin activity; a metabolic reaction to the indigestibility of animal proteins by juvenile abalone [3].
Other commercially grown invertebrate species such as the sea urchin Paracentrotus lividus are capable of utilising animal proteins in artificial feeds as they are omnivorous in nature, however sea urchins are predominantly algivorous and share similar predispositions to macroalgal diets as abalone [15,16]. It may be possible that urchins will also respond to feeds with components existent in their natural diet.
Abalone are algivorous by nature and animal protein is not a component of their natural diet [5,17,18]. However, inclusion of fishmeal in the diets of juvenile abalone can increase growth rates [8,19,20]. However, as fishmeal is a controlled substance within Europe (EC Regulation 999/2001 & EC Regulation 183/2005), the fish species that may be used are restricted [21]. As the majority of fishmeal and fish oil is derived from offal and offcuts of processed fish, determining the quality of this component and its uses in the abalone aquaculture feed industry is crucial. Components of formulated feeds used during the abalone production cycle may also have an effect on the marketing of abalone by commercial enterprises. Abalone fed some artificial formulated feeds display altered colours in new shell growth and shell band colour [22] for which there may be poor market acceptance.
Viana et al. [20] & Guzmán & Viana [23] used abalone viscera silage as the animal protein source, which poses other more ethical problems than solutions to the animal protein component in formulated feeds for abalone. Traceability and declaration of the derived animal protein is important to commercial growers. Commercial considerations are also important as fishmeal is the most expensive component of the feed [3,19,23,24]. Obviating this expense through the use of a diet containing only components existing in the natural diet of abalone therefore represents a commercial opportunity for farmers.
Naidoo et al. [25] showed that mixed macroalgal diets were an effective substitute for commercially available feeds and formulated feeds. However, Mahoney O et al. [21] reported the lack of information or research on mixed macroalgal-meal diets. Hannon et al. [5] also identified an opportunity for European feed producers to formulate a mixed macroalgal-meal diet for abalone species cultured in Europe as specific macroalgal derived feeds do not exist for H. tuberculata & H. discus hannai.
Phosphorus has an important role in cellular function and production of several key enzymes, and is directly involved in energy producing cellular reactions [26]. Supplementation of phosphorus may enhance growth as it is limiting in the diet of abalone [27-29].
Few studies have been conducted on the dietary requirement for phosphorus relative to calcium in the diet of abalone [27,28]. Calcium was found not to be of significant benefit as a dietary supplement; abalone can obtain sufficient calcium directly from the water [30]. Thus the addition of calcium in the form of CaCO3 can be controlled in the culture conditions (recirculation system) rather than through their diet. Flow-through systems do not require the addition of CaCO3 as calcium will not be depleted, as the water is not in a closed system.
The rearing of abalone in recirculation systems has intensified the culture of abalone in some countries. However, the lack, or depletion of calcium in such closed systems, can cause stress, behaviour changes and degradation of the animal’s shells, more commonly known as Shiny shell [30].
Investigations of the ratio of Phosphorous and Calcium in the diet of two abalone species, Haliotis laevigata and Haliotis discus hannai, [27,28] found an increase in growth resulting from the addition of phosphorus. Additional dietary calcium intake had no effect on growth.
This research investigates the effect of differing levels of phosphorus enrichment in macroalgal-meal diets on the growth of juvenile H. tuberculata. The focus of the trial is on the addition of phosphorous in the diet of juvenile H. tuberculata as phosphorus availability can be a growth-limiting factor.
Materials and Methods
The feeding trial was conducted on site at Abalone Chonamara Teoranta, Rossaveal, Co. Galway, Ireland.
Abalone collection & maintenance
Hatchery produced juvenile H. tuberculata were collected from the on-growing unit and graded. 120 juveniles between 30-40mm in shell length were selected randomly from the cohort. Each abalone’s total shell length (SL) was measured to the nearest 0.01mm using a digital callipers and weighed (Wt) using an electronic balance to the nearest 0.01g. The abalone, initially stocked at 38.66±0.162mm (n =120) shell length, and 8.58±0.157g (n = 120) live weight (Mean±SE), were randomly divided between twelve, 25L experimental tanks (Table 1).
Abalone were held in a flow through system supplied from the main farm reservoir (13 °C) and under an ambient light regime. Tan et al. [31] & Tan et al. [32] experienced poor growth due to temperature fluctuations (9.8 °C - 26.4 °C). This was rectified in this trial using temperature control.
Holding the juvenile abalone in a flow through system mitigated against depletion of calcium in the form of CaCO3. Maintaining CaCO3 concentration is recommended by Cenni et al. [31], as depletion of calcium in a culture system can cause changes in behaviour and feeding.
The flow through system delivered 2L of seawater per minute into each 25L tank. The incoming water was filtered to 5μm and treated with ultraviolet light. Each 25L tank received aeration via a centrally placed air-stone. Filtered treated water entered from the opposite corner away from the outflow standpipe, which controlled the height of water in the tank.
Feed was withheld from the abalone spat during an acclimatisation period of 7 days prior to the introduction of the treatments. This was conducted to ensure any mortality due to handling and stress occurred prior to introduction of the experimental diets. Abalone feed was replenished three times per week ad libitum, following methods used in Mahoney O et al. [21]. Uneaten food and faeces were removed before feeding. A full exchange of water was conducted once per week to coincide with the collection of biometric data from the juvenile abalone, less mechanical damage is caused to the abalone when the water is drained from the tanks.
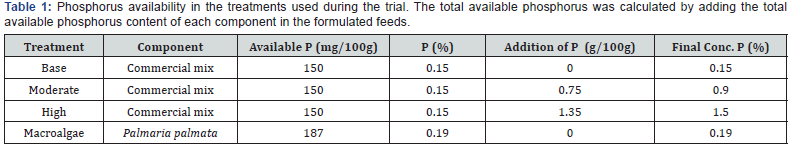
The trial consisted with 4 treatments. The un-enriched basal diet had a total of 0.15% (Base) available phosphorus. Juvenile abalone were given feeds with 2 levels of enrichment 0.9 (Moderate) and 1.5 % (High) total available dietary phosphorus. The P. palmata diet had a total available phosphorus of 0.187% (Table 1).
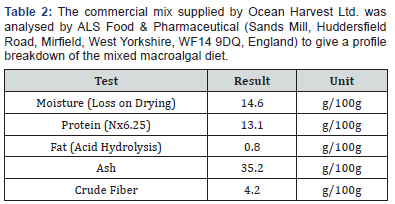
The trial utilised a commercial mixed-macroalgal meal produced by Ocean Harvest Ltd. (Galway, Ireland) and sodium alginate, to act as a binder (Oxoid Ltd. Basingstoke, Hampshire, England). Whilst the recipe of the feed used in the trial provided by Ocean Harvest cannot be disclosed due to commercial constraints (commercial mix), experimental feeds only contained ingredients that exist in the juvenile abalone natural diet. Palmaria palmata was used as a comparison diet for the trial, as the abalone were reared on it prior to the to the trial beginning. The breakdown of the analysed commercial mix is given in Table 2, which was analysed by ALS Food & Pharmaceutical, (West Yorkshire, England).
Monobasic potassium phosphate (KH2PO4) (Fisher Scientific U.K Ltd, Loughborough, England) was used for phosphorus enrichment as it is readily soluble and has a high bioavailability [29,31].
Experimental design
There were four treatments (diets), with three replicates (Tanks) per treatment. 10 Abalone were stocked per tank with a shell length of 30-40mm. These abalone were distributed amongst the rearing system, which consisted of 12 independent tanks using a completely randomised design.
Sampling & Data Collection
Collection of biometric data (Length & Weight) was conducted on a weekly basis; all abalone from each replicate pertreatment were measured and weighed. Survival (% survival) was assessed via dead shell counts on a weekly basis during tank cleaning and feeding. Daily increment in shell length (DISL) for each treatment was calculated at the end of the trial.
DISL (μm) = [Shell Length final (mm) – Shell Length Initial (mm)]/ (No. Days) X 1000
Data analysi
Biometric data of abalone growth were analysed using MINITAB 16 statistical software package (Minitab Ltd. Coventry, England). Normal distribution of the data and sphericity were confirmed. Repeat measures ANOVA was carried out to assess differences in growth rates due to each treatment. Univariate analysis of variance and post hoc (Tukeys HSD) tests were applied to test the mean abalone interval sizes per treatment.
Results
The short scale feeding trial lasted 4 weeks in total. 100% of the juvenile H. tuberculata survived. Each treatment had a different level of available dietary phosphorus (Table 1), which produced different growth rates in shell length, with moderate enrichment producing the highest increase in shell length (Figure 1). The results of the commercial mix provided by Ocean Harvest Ltd. gives a breakdown of the profile of the mixed macroalgal diet provided prior to enrichment with additional phosphorous (Table 2).
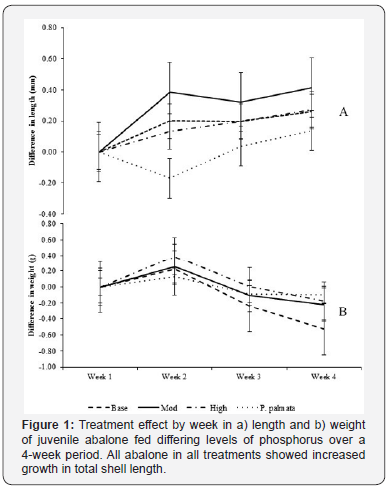
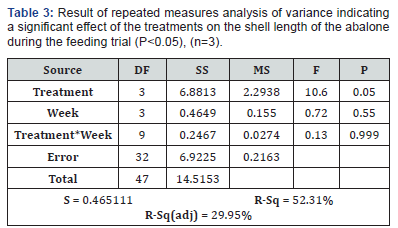
There was no significant difference in shell length in any of the abalone over the duration of the trial (ANOVA: df = 3, S = 1.428 R-Sq = 0.48%, R-Sq(adj) = 0.00% P>0.05), however the difference in increased shell length between the treatments was highly significant (P<0.05) (Table 3). All treatments exhibited weight loss in the feeding trial, the base diet proved to have the highest weight loss with P. palmata showing the least loss of weight (Figure 1).
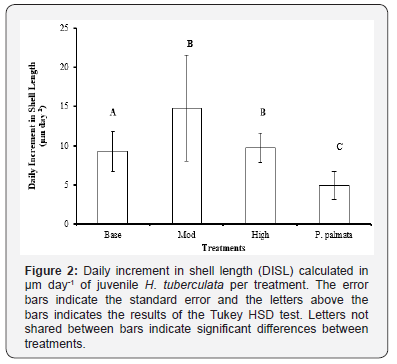
Daily incremental shell length (DISL) was used to measure the difference in growth rates between the treatments, as this measurement is not affected by the amount of water each abalone retains. The moderate (0.9%) phosphorus enrichment achieved the greatest increase in shell length per day (Figure 2). The high level enrichment 1.5% and the base treatment 0.15% phosphorus also had a greater increase in shell length when compared to the P. palmata diet (Figure 2).
There was no significant difference in shell length between the weeks, however there were significant differences between the treatments over the feeding trial (P>0.05) (Table 3). Weight loss was measured and observed in all treatments over the duration of the 4 week feeding trial. After week 3 weigh loss was beginning to slow in all treatments other than the base treatment. Both the moderate and high showed similar weight loss patterns to P. palmata between week 3 and week 4 (Figure 1).
Discussion
This research investigated the effects of addition of supplementary phosphorus on the total shell length and weight of juvenile H. tuberculata. All juvenile abalone in the trial grew in shell length (Figure 1). There was also significant difference in measured shell length between the treatments (Table 3).
Although all treatments exhibited weight loss, this did not affect survival. The P. palmata treatment measured the least amount of weight loss, however this weight loss followed a similar profile to the experimental diets (Figure 1). The 7-day starvation and acclimatisation period may have had an effect on the weight loss of the abalone in the trial. Abalone are sensitive to changes in dietary regime changes and in some cases up to 50% mortality can result if dietary change is implemented when the animals are not ready for a change [12].
Due to the nature and behaviour of abalone, shell length and volume needs to increase as the whole foot and soft body is enclosed inside the shell as a means of protection from predation and wave action [26]. This increase in shell length and width therefore precedes the increase in foot size or weight gain. An increase of foot size prior to shell growth would increase the likelihood of mortality due to predation or environmental conditions. Therefore, if the trial had run for a longer period of time, weight increase would also be expected.
Coote et al. [28] used an average of 0.68 % total phosphorus to enhance growth of H. laevigata. Tan et al. [29] recommended a total phosphorus level of 0.64-0.65 % for H. discus hannai. Tan et al. [32] recommended that the total level of available dietary phosphorus for H. discus hannai should be 0.9 – 1.1%, which is slightly above recommendations previously made by Tan et al. [29].
The addition of 0.9% (Moderate) and 1.5% (High) phosphorus in this trial was consistent with, and slightly above the dietary recommendations made by Tan et al. [32]. Our results suggest that H. discus hannai and H. tuberculata share similar preferences for phosphorus availability.
The highest growth rate in shell length was measured at the moderately enriched level of 0.9% available phosphorus, consistent with the recommendations made by Tan et al. [33] (Figure 2). This indicated that there is an optimal enrichment range for H. tuberculata as there is for H. discus hannai, which requires further investigation.
The diets used in experiments by Coote et al. [28] may have been deficient in available phosphorus because of the soybean component. Phosphorus is bound in the form of phytate in soybeans, thus the dietary addition of supplementary phosphorus by Coote et al. [28] diets may not have overcome this mild phosphorus deficiency.
Coote et al. [28] suggested further investigation into phosphorus bound in the form of Phytate. However, phytate (phytin phosphorus) is unavailable to animals with simple stomachs because they lack the enzyme phytase in the gastrointestinal tract. This suggests that abalone will be unable to absorb phosphorus bound in phytate [26], making phytate enrichment futile.
Coote et al. [28] findings were considered when the components of the experimental diets were selected for this study. Components with high levels of unavailable phosphorus such as soybeans were not included. As a result, our research focused on differing levels of available phosphorus in components naturally occurring in the animals diet (macroalgae), predicated on the assumption that abalone respond better to components actually found in their natural diet.
The experimental diets in this research were observed to loose stability in water after 8-12 hours post feeding, thus not allowing the juvenile abalone to efficiently feed on them for a longer period of time due to this solubility in water. However, weight loss cannot be solely attributed to the solubility of the experimental feeds in water, as a similar weight loss profile was measured on the P. palmata diet (Figure 1). Further investigation is required to identify affordable and palatable binders to increase stability in water. This short scale feeding trial observed differing increases in shell length, it also observed differing decrease in wet weight. Weighing mobile live animals that can retain and loose water may give differing results if weighed consecutively. Further investigation is required along with a longer trial however the abalone did grow in shell length in the different treatments.
It is common practice to use inorganic phosphorus as a supplement in abalone feeds [26]. Our use of Monobasic Potassium Phosphate increased the growth of juvenile H. tuberculata (Figure 3). Investigating phosphorus content of feeds for abalone may allow feed manufacturers to increase the available phosphorus content using natural components rather than components that are non-existent in the natural diet of abalone. We observed that abalone fed the enriched treatments required more effort to remove from the experimental tanks during the collection of biometric data when compared to those fed with P. palmata. This observation suggests an effect of increased phosphorus strengthening the gripping behaviour of juvenile H. tuberculata.
Phosphorus can be limiting in some macroalgae [33]. By using macroalgal-meal, it would be possible to increase the available phosphorus content by mixing more than one species of macroalgae with higher concentrations of phosphorus to increase the total phosphorus availability.
Our findings suggest similar dietary requirements between H. discus hannai and H. tuberculata for available phosphorus, at levels similar to these recommended by Tan et al. [32]. Enriching the diet of H. tuberculata with supplementary phosphorus appears to increase growth. Using a mixed macroalgal-meal diet proved to be more beneficial to growth when compared to P. palmata.
Acknowledgements
We thank Mrs Cindy O’ Brien from Abalone Chonamara Teoranta for the use of the facilities and supply of juvenile H. tuberculata for the feeding trial. We also thank Michael Griffin for his help in the initial experimental set up.
References
- Courtois de Viçose G, Viera MP, Huchette S, Izquierdo MS (2012) Improving nursery performances of Haliotis tuberculata coccinea: Nutritional value of four species of benthic diatoms and green macroalgae germlings. Aquaculture 334–337: 124-131.
- Kawamura T, Saido T, Takami H, Yamashita Y (1995) Dietary value of benthic diatoms for the growth of post-larval abalone Haliotis discus hannai. Journal of Experimental Marine Biology and Ecology 194(2): 189-199.
- Johnston D, Moltschaniwskyj N, Wells J (2005) Development of the radula and digestive system of juvenile blacklip abalone (Haliotis rubra): potential factors responsible for variable weaning success on artificial diets. Aquaculture 250(1-2): 341–355.
- Takami H (2003) Feeding Ecology of an Abalone, Haliotis discus hannai, in Their Early Life Stages. In Aquaculture and pathobiology of crustacean and other species. Davis and Santa Barbara, California USA, p. 180.
- Hannon C, Officer RA, Dorven J (2012) Review of the technical challenges facing aquaculture of the European abalone Haliotis tuberculata in Ireland. Aquaculture International 21(2): 243–254.
- Daume S, Davidson M, Ryan S, Parker F (2007) Comparisons of rearing systems based on algae or formulated feed for juvenile greenlip abalone (Haliotis laevigata). Journal of Shellfish Research 26(3): 729-735.
- Bautista-Teruel MN, Koshio SS, Ishikawa M (2011) Diet development and evaluation for juvenile abalone, Haliotis asinina Linne: Lipid and essential fatty acid levels. Aquaculture 312(1-4): 172-179.
- Bautista-Teruel MN, Fermin AC, Koshio SS (2003) Diet development and evaluation for juvenile abalone, Haliotis asinina: animal and plant protein sources. Aquaculture 219(1-4): 645-653.
- Gómez-Montes L, Garcı́a-Esquivel Z, D’Abramo LR, Shimada A, Vásquez- Peláez C, et al. (2003) Effect of dietary protein: energy ratio on intake, growth and metabolism of juvenile green abalone Haliotis fulgens. Aquaculture 220(1-4): 769–780.
- Mai K, Mercer JP, Donlon J (1995) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. III. Response of abalone to various levels of dietary lipid. Aquaculture 134(1-2): 65-80.
- Mgaya YD (1995) Synopsis of biological data on the European abalone (Ormer), Haliotis tuberculata Linnaeus, 1758 (Gastropoda: Haliotidae). Food & Agriculture Org. No. 156.
- Pang S, Zhang Z, Bao Y, Gao S (2006) Settling abalone veliger larvae in a free-swimming microalgal culture. Aquaculture 258(1-4): 327–336
- Huchette SMH, Koh C, Day RW (2003) The effects of density on the behaviour and growth of juvenile blacklip abalone (Haliotis rubra). Aquaculture International 11(5): 411-428.
- Kawamura T, Roberts RD, Yamashita Y (2001) Radula development in abalone Haliotis discus hannai from larva to adult in relation to feeding transitions. Fisheries Science 67(4): 596-605.
- Fernandez C, Boudouresque CF (1998) Evaluating artificial diets for small Paracentrotus lividus (Echinodermata: Echinoidea). Echinoderms: San Francisco. Balkema, Rotterdam, pp. 651–656.
- Lawrence JM (2007) Edible Sea Urchins: Biology And Ecology, Elsevier, Amsterdam, Netherlands.
- Leighton P (2008) Aquaculture explained: Abalone Hatchery Manual, Irish Seafisheries Board, No. 25, p. 95.
- Mai K, Wu G, Zhu W (2001) Abalone, Haliotis discus hannai Ino, Can Synthesize Myo-Inositol De Novo to Meet Physiological Needs. J Nutr 131(11): 2898-2903.
- Chao WR, Huang CY, Sheen SS (2010) Development of formulated diet for post-larval abalone, Haliotis diversicolor supertexta. Aquaculture 307(1-2): 89-94.
- Viana MT, López LM, García-Esquivel Z, Mendez E (1996) The use of silage made from fish and abalone viscera as an ingredient in abalone feed. Aquaculture 140(1-2): 87-98.
- Mahoney O, Rice M, Mouzakitis O, Burnell G (2014) Towards sustainable feeds for abalone culture: Evaluating the use of mixed species seaweed meal in formulated feeds for the Japanese abalone, Haliotis discus hannai. Aquaculture 430: 9-16.
- Gallardo WG, Bautista-Teruel MN, Fermin AC, Marte CL (2003) Shell marking by artificial feeding of the tropical abalone Haliotis asinina Linne juveniles for sea ranching and stock enhancement. Aquaculture Research 34(10): 839–842.
- Guzmán JM, Viana MT (1998) Growth of abalone Haliotis fulgens fed diets with and without fishmeal, compared to a commercial diet. Aquaculture 165(3-4): 321–331.
- Fleming AE, Hone PW (1996) Abalone aquaculture. Aquaculture 140(1-2): 1–4.
- Naidoo K, Maneveldt G, Ruck K, Bolton JJ (2006) A Comparison of Various Seaweed-Based Diets and Formulated Feed on Growth Rate of Abalone in a Land-Based Aquaculture System. Journal of Applied Phycology 18(3-5): 437-443.
- Sales J, Britz PJ, Viljoen J (2003) Dietary phosphorus leaching and apparent phosphorus digestibility from different inorganic phosphorus sources for South African abalone (Haliotis midae L.). Aquaculture Nutrition 9(3): 169-174.
- Mgaya YD, Mercer JP (1995) The effects of size grading and stocking density on growth performance of juvenile abalone, Haliotis tuberculata Linnaeus. Aquaculture 136(3-4): 297-312.
- Coote TA, Hone PW, Kenyon R, Maguire GB (1996) The effect of different combinations of dietary calcium and phosphorus on the growth of juvenile Haliotis laevigata. Aquaculture 145(1–4): 267–279.
- Tan B, Mai K, Liufu Z (2001) Response of juvenile abalone, Haliotis discus hannai, to dietary calcium, phosphorus and calcium/ phosphorus ratio. Aquaculture 198(1–2): 141-158.
- Chaitanawisuti N, Sungsirin T, Piyatiratitivorakul S (2010) Effects of dietary calcium and phosphorus supplementation on the growth performance of juvenile spotted babylon & Babylonia areolata; culture in a recirculating culture system. Aquaculture International 18(3): 303–313.
- Cenni, F, Parisi G, Scapini F, Gherardi F (2010) Sheltering behavior of the abalone, Haliotis tuberculata L., in artificial and natural seawater: The role of calcium. Aquaculture 299(1-4): 67–72.
- Tan B, Mai K, Zhi-guo L (2002) Dietary phosphorus requirement of young abalone Haliotis discus hannai Ino. Chinese Journal of Oceanology and Limnology 20(1): 22-31.
- Tan, Bei-ping Mai, Kang-sen, Wei X (2002) Availability of phosphorus from selected inorganic phosphate to juvenile abalone, Haliotis discus hannai ino. Chinese Journal of Oceanology and Limnology 20(2): 118- 128.
- Lobban CS, Wynne MJ (1981) The Biology of Seaweeds, Blackwell Scientific publications, Oxford, London.






























