The Blueprint for Retarding and Reversing Cardiovascular Aging
Vinod Nikhra*
Senior Chief Medical Officer and Consultant, Department of Medicine, Hindu Rao Hospital and NDMC Medical College, India
Submission: August 10, 2018; Published: September 21, 2018
*Corresponding author:Vinod Nikhra, Senior Chief Medical Officer and Consultant, Department of Medicine, Hindu Rao Hospital and NDMC Medical College, New Delhi, India.
How to cite this article: Vinod Nikhra. The Blueprint for Retarding and Reversing Cardiovascular Aging. OAJ Gerontol & Geriatric Med. 2018; 4(4): 555644. DOI: 10.19080/OAJGGM.2018.04.555644
Abstract
Cardiovascular Aging:
Mechanisms and Pathways: The heart is made up of millions of myocardial cells that contract and relax to pump blood adequately to various body organs. In turn, it is itself dependent for oxygen and nutrients on vascular supply. There goes on the ongoing damage to the heart and vasculature with aging. Various adaptive and compensatory measures including lifestyle interventions and related treatment modalities contributing directly or indirectly to strengthening of heart and myocardial preservation.
Modalities for Retarding CV Aging: Age is the most important determinant of cardiovascular health. There occurs an exponential rise in both the CV risk and the incidence of CV events in older adults parallel with the advancing age. With advancing age, there is an increase in incidence and prevalence of atherosclerosis, hypertension, coronary artery disease and heart failure; cerebrovascular and peripheral vascular disease. Simultaneously, important changes are taking place at micro level; at cellular, subcellular and molecular levels. The macro- and micro level changes have impact on quality of life and affect the individual survival. The most promising data comes from experimental biology highlightening calorie restriction as a tool for reduction in oxidative stress and increasing longevity. The metabolic manipulations enhance longevity by retarding aging comparable to CR, are not as effective as repair strategies which can reverse aging process.
The Modalities for Reversing CV Aging: The genetic manipulations causing alterations work through a common pathway across many species endorsing that there is an evolutionary genetic program that controls aging. Further, it appears that the metabolic changes will not produce significant effects on longevity in humans as they do in mice for the simple reason that lifespan is much more plastic in short-lived species. There are important potential target genes for future gene therapies to retard and reverse CV aging or compensating for age-related damage.
Conclusion:
Futuristic Visions: It is projected that the nano-biotechnology will help in reversal of CV aging by providing gene and small molecule therapy. Further impetus will come from bioengineering and stem cell technology. The 3-D bioprinting may provide the bulk supply of organs and blood vessls somatic repair and reversal of CV aging. These interventions will form the basis of regenerative medicine for aging and age-related CV aging and Cure for CVDs.
Keywords: Cardiovascular disease; Caloric restriction; Metabolic syndrome; p53; Oxidative stress; Reactive oxygen species; SIRT gene; Nanobiotechnology; Gene therapy; Stem cell therapy; 3D bioprinting
The Cardiovascular Aging: Facts, Theories and Concepts
The Aging and CV Risk
Aging, per se, is the most important determinant of CV health. At individual level, it is the most important risk factor for CVD, ae well. There occur progressive structural and functional changes in the heart and vasculature throughout life and include diffuse intimal and medial thickening, and increased stiffening and reduced distensibility of central arteries. Concurrently, an exponential rise occurs in both the CV risk and the incidence of CV events in older adults parallel with the advancing age. Statiscally, the older adults (>60 years old) account for more than 80% of coronary artery disease (CAD), more than 75% of heart failure (HF), and more than 70% of atrial fibrillation patients [1].
The increased age-dependent exposure time to various risk factors including hypertension, diabetes, hypercholesterolemia and smoking is accompanied by age-associated interactions of the intrinsic aging of heart and vasculature with progressive structural changes and functional decline. The aging, thus, has an impact on the risk and severity of the clinical manifestations for CVD, which is true for clinically overt disease as well as subclinical or occult diseases, such as, silent coronary atherosclerosis.
The DNA-Echosystem Theory
The intracellular DNA environment has nuclear, organelle and cytosolic components. Tissues constitute environments for cells and compose organs that define the somatic organism and the innate brain function, cognition and personality form the abstract part of a life-form. Communication between the CV system and other organ systems is crucial to preservation of health, which differ in the development of their cognitive and stress coping mechanisms, in part, due to differences in personality characteristics, which give rise to the development of distinct behavior and lifestyle influenced by the society. Further beyond, the organism is surrounded by geographical realities of climate, bio-spora, radiation, pollution and gravity. There is a continuous bidirectional signaling between the organism and echosystem to sustain the existence [2].
The phenotypic aging can be regarded, in a way, the manifestation of time-dependent failure of signaling in the DNA and Echosystem. Age-associated CVS failure and CVD play major roles in the time-dependent disorder that accrues within the system, contributing to stochastic epigenetic drift within the system. Further, with age, signals in the DNA-echosystem change, as does sensing of the signals, transmission of signals and responses to signals. Aging is, thus, characterized by impaired cell renewal in the proteome due to alterations in genomic transcription, mRNA translation and the proteostasis. The altered and disordered molecular interactions may lead to altered bio-chemical processes like, oxidation, nitration, phosphorylation, etc. and the age-associated molecular disorders lead to loss of robustness and flexibility of the system. The increasing molecular and cellular disorders with age lead to loss of tissue organ and system reserve functions and derangred compensatory mechanisms, which results in age-associated increasing frailty and vulnerability to diseases.
Altered Nutritional Needs with Age
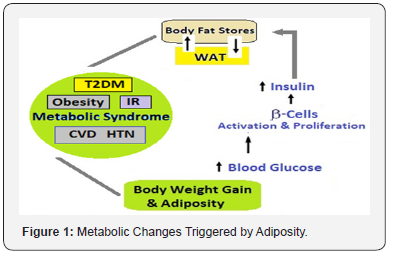
The diet-gene interaction is a major determinant of health and illness [3]. The amount and type of food ingestion and caloric intake influence the general health and life span [4]. On the other hand, the energy needs of individuals are determined by their body composition, especially the lean mass and level of physical activity. Most older adults lose fat free mass as they age, with skeletal muscle being lost at a rate of approximately 1% per year in the over 70s1 and many are less physically active [5]. There is a change in nutritional needs during the middle age and later, when no actual growth is taking place, though there is a continued additional need of nutrients to take care of increased wear and tear with age, but overall calorie requirements are less because of a sedentary nature of ADL. The availability of palatable food and increased consumption causes overnutrition and nutritional overload, giving rise to adiposity, which in turn lead to increased potentially damaging exposure to reactive oxygen species (ROS) at subcellular and cellular levels. Visceral fat increasing with age, induces inflammation which accelerates aging process. The adiposity, with ROS injury and inflammation leads to IR, MetS, T2DM and hypertension, and affects adversely the CV health (Figure 1).
CV Aging and CVDs Interrelationship
The age-associated changes in CVS alter the substrates on which CVD is superimposed [6]. First, they lower the extent of disease severity required to cross the threshold that results in clinically significant signs and symptoms. Secondly, ageassociated changes may also alter the manifestations and presentation of common cardiac diseases. Thirdly, the chronic inflammation degrades the landscape of CV morphology and physiology by promoting a markedly increased risk for CVD in older adults. The age-associated phenotypic changes in major vessels result from complex progressive aterations, ranging from molecules to cells to arterial tissue, the blood they transport and hormonal and neural factors that modulate the molecular and cellular processes.
The age-associated changes in CVS alter the substrates on which CVD is superimposed [6]. First, they lower the extent of disease severity required to cross the threshold that results in clinically significant signs and symptoms. Secondly, ageassociated changes may also alter the manifestations and presentation of common cardiac diseases. Thirdly, the chronic inflammation degrades the landscape of CV morphology and physiology by promoting a markedly increased risk for CVD in older adults. The age-associated phenotypic changes in major vessels result from complex progressive aterations, ranging from molecules to cells to arterial tissue, the blood they transport and hormonal and neural factors that modulate the molecular and cellular processes.
The initial physiological response is increased adrenergic signaling, increased activation of renin-angiotensin-aldosterone and endothelial dysfunction. The endothelial and VSM cells are phenotypically altered to produce inflammatory cytokines [11,12]. The excess caloric intake also mimics Ang II signaling and reduces expression of sirtuin and PPARs signaling, affecting metabolic function and resulting in mitochondrial damage.
Altered CV Reserve with Aging
CV output at rest accounts for about a third of the overall peak capacity that declines gradually age. The peak cardiac output decline is due to chronotropic insufficiency as peak stroke volume is preserved. The impaired heart rate acceleration and impaired augmentation of blood ejection from the left ventricle are the important changes with age in cardiac reserve capacity. There are multifactorial mechanisms underlying the age-associated decline in peak heart rate and ejection fraction are and include a reduction in intrinsic sinoatrial pacemaker cell function and ventricular myocyte intrinsic contractility and increased arterial-ventricular load mismatch. The ageassociated deficit in CV reserve is due to an altered of brainheart signaling, which includes diminished effectiveness of the autonomic modulation of heart rate, LV contractility, and arterial afterload. The components of autonomic cardiovascular regulation become compromised with advancing age [13].
There occur deficits in sympathetic modulation of cardiac and arterial functions with aging occur despite exaggerated neurotransmitter levels [14]. Plasma levels of norepinephrine and epinephrine response to stress increase to a greater extent in older compared with younger healthy humans, due to an increased spillover into the circulation and, to a lesser extent, reduced plasma clearance. Thus, there is a reduced response to acute stress and increased levels of neurotransmitters present in case of chronic stress [15].
Role of Wnts in Cardiac Aging
The Wnt signaling pathways are a group of signal transduction pathways made of proteins that pass signals into a cell through cell surface receptors. The Wnt signaling pathways are activated by the binding of a Wnt-protein ligand to a Frizzled family receptor, which passes the biological signal to the dishevelled protein inside the cell.
The Wnt signaling system accelerates aging and induces aberrant differentiation of skeletal muscle stem cells to fibroblasts, leading to increased fibrosis and decreased regeneration of aging skeletal muscle. The role of Wnts and their antagonists in preserving cardiac progenitors, maintaining cardiomyocyte renewal and retarding cardiac aging is a central question addressed in this proposal.
We hypothesize that (i) Wnt signaling increases in the heart with aging or hypertrophy and drives cardiac progenitor cells to differentiate into a fibrogenic lineage and (ii) interruption of Wnt signaling will preserve cardiac progenitor cell fate and ameliorate fibrosis in an aging and hypertrophied heart.
Oxidative stress and low-grade inflammation are important factors in accelerating the vascular aging process by affecting molecules that lead to cellular and matrix structural and functional changes [16].
Aging and Vascular Smooth Muscle Cells
The VSMCs possess phenotypic and functional plasticity for responding to vascular injury, when the activated VSMCs proliferate and migrate to contribute to vascular wall repair [14]. However, in atherosclerotic arteries the arterial VSMCs become aberrantly regulated and this leads to increased VSMC dedifferentiation and extracellular matrix formation in plaque areas [15]. In the atherosclerotic plaque VSMCs show increased DNA damage, including double-stranded breaks (DSBs). The DSBs promote cell senescence, apoptosis, and inflammation (Figure 2).
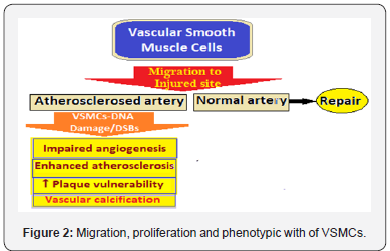
The VSMC DNA damage alters plaque phenotype inhibiting fibrous cap areas in advanced lesions (16), and VSMC apoptosis occuring during the progression of atherosclerosis, in advanced lesions promotes plaque necrosis leading to vulnerable atherosclerotic plaques. Akt1, a serine/threonine protein kinase, regulates several key endothelial cells and VSMC functions and its deficiency results in ↑including fibrous cap thinning and extensive necrotic core areas [17].
Age-associated changes encompass the activation of the intravascular renin-angiotensin-aldosterone system and alterations of functional properties of VSMCs, i.e., ECM synthesis, contractility, switch to an inflammatory phenotype, apoptosis and senescence in response to changes in signalling mechanisms and gene expression patterns. The age-associated pro-inflammatory phenotype is orchestrated by the concerted effects of aldosterone and Ang II via mineralocorticoid receptor signalling and aldosterone-mediated ERK1/2 activation. The intimal proliferation of VSMCs is induced by the release of platelet growth factors, such as PDGF. The VSMCs partially loose their contractile phenotype and acquire a synthetic one, leading to arterial inflammation, accumulation of fibronectin, collagen, and fibrosis [18].
Miscellaneous Factors in CV Aging
The mTOR Pathway and Nutrient Signaling: The target of rapamycin (TOR) kinase, serine-threonine protein kinase, is a gatekeeper that integrates nutrient and hormonal signals for modulation of growth and longevity. It is inhibited by the bacterial product rapamycin, hence the name. Evolutionary TOR is conserved from yeast to humans and integrates signaling from insulin and growth factors as well as senses intracellular amino acid levels to regulate cell size and growth, proliferation and survival, and protein synthesis and transcription [19]. TOR activity is high when nutrients are abundant to favor cell division and growth, whereas when nutrients are low, TOR activity is decreased, there is reduced growth and increased impetus to survival and resistance to stress. The PI3K/AKT/mTOR pathway lies at the intersections of numerous signaling pathways. Its inhibition activates autophagy and destruction of defective molecules and organelles and repression of cardiac hypertrophy due to pressure overload and promotion of CV health. In studies rapamycin is protective in models of cardiac hypertrophy and heart failure [20].
The AMP-Activated Protein Kinase: The AMPK is involved in glucose and lipid metabolism, cell growth and autophagy, and gene expression and modulation of mitochondrial function [21]. Within the longevity network, AMPK regulates mTOR through direct phosphorylation of the TSC1/2 complex, modulates the IGF-1 pathway through the extracellular signal-regulated kinase (Erk) cascade, and controls sirtuin activity. AMPK is cardioprotective during ischemia and reperfusion. In mouse models, activation of AMPK by metformin reduces pressure overload–induced cardiac hypertrophy. Being involved in energy sensing, AMPK may be a mediator of the positive effects of CR and resveratrol on both longevity and CVD.
Other Pathways: There is involvement of SIRTs in nutrient signaling and aging implicates epigenetic regulation of DNA expression. Forkhead transcription factors (FOXOs) play a role in regulating expression of genes involved in cell growth, proliferation, differentiation, and longevity [22]. FOXOs are also downstream effectors of the IGF-1 signaling cascade. The mammalian CLK-1 is a hydroxylase localized in mitochondria and is necessary for the biosynthesis of ubiquinone (coenzyme Q), the essential electron transporter of the mitochondrial respiratory chain. Its partial inactivation in mice extends lifespan. and protects from cerebral ischemia and reperfusion. The Catalase converts hydrogen peroxide, a major ROS, into water and oxygen. Targeting peroxisomal catalase to the mitochondria, leads to reduction in age-dependent LV hypertrophy and diastolic dysfunction in mice, and protects from Angiotensin II–induced cardiac hypertrophy as well as pressure overload–induced heart failure emphasizing the role of ROS in CVD. Still another, the Mammalian p66shc functions as a redox enzyme to modulate mitochondrial ROS. The decreased p66shc decreases ROS in the CVS cardiovascular system, decreases DNA damage, necrosis, apoptosis and atherosclerosis and preserves LV volume and function. Loss of p66shc also prevents Angiotensin II–induced hypertrophy and apoptotic cell death.
The Cardiac Stem Cells: In addition, there occurs a progressive decrease of cardiomyocyte turnover from 1% per year at the age of 25 to 0.45% per year at the age of 75 in older adults.
The Modalities for Retarding Cardiovascular Aging
The Research-Therapeutics Lag
The opportunities for applying latest research, experimental and interventional both, to the concurrent Clinical Therapies may exist but are scaecely utilized. There are multiple barriers to applying and include the research interventions to the current therapeutic modalities. The barriers can be preclinical as well as clinical. From CVS point of view also, there are various barriers and lags to translating the experimentally effective cardioprotective interventions into clinically effective therapies, which exist at the preclinical and at the clinical levels [23].
There is a neglect for both the basic and clinical researchers to carefully consider issues related to the eventual clinical application and modalities suitable for the clinical practice. Often, there is a lack of effective communication and coordination between those performing preclinical evaluations, those designing clinical studies, those developing therapeutically available interventions, and the medical service providers. There is failure to disseminate negative results. The ‘positive’ results receive greater publicity than ‘negative’ results distorting the overall objectivity and efficacy of a potentially useful cardioprotective discovery.
Some of the recent research interventions, such as, thave Altered gene expression, altered levels and functions of proteins that regulate Ca2+ homeostasis, altered membrane lipid composition, increased threat of reactive O2 species and Increased cell death in the context of reduced cell replacement hold promise of therapeutic applications in near future. There are novel compounds which supposedly repair the myocardial damage and improve physiological function of the heart. They include Co-enzyme Q10, resveratrol, flavonoids and other plant derived products, and certain peptides which have been proved to be cardioprotective in animal and human research studies. These have not found wider prescriptions by clinicians for one reason or other.
Cardioprotectives and Novel Drugs
All adaptive and compensatory measures including lifestyle interventions and related treatment modalities that directly or indirectly contribute to strengthening of heart and preserving the vascular integrity can be called cardioprotective [24]. This includes physiological adaptive measures like healthy lifestyle changes [25], treatment of hypertension, dyslipidemia, CVDs and HF with therapeutic agents such as ACE inhibitors, ARBs, CCBs, Beta blockers, statins, diuretics and anti-platelet agents like aspirin and clopidogrel.
The development program for novel Cardioprotective drugs aims is to develop new classes of drugs which can activate specific cardioprotective proteins and reduce damage to the heart muscle cells, have the potential to preserve the pumping ability of the heart and delay the onset of HF, and can improve QOL and reduce mortality. But, despite the increase in CVD prevalence worldwide, investment in CV drug development has stagnated over the past 3 decades. Still some new drugs are in pipeline and development phase. An example of a new drug recently introduced is PCSK9 to treat hypercholesterolemia [26].
Potential Interventions to retard CV Aging
It is now well-appreciated that aging can be modulated through various environmental, life style, genetic, and pharmacological interventions [27]. It is critical to understand the interactions of age-related molecular mechanisms in vascular cells with both CVD pathogenesis and systemic aging processes, and to develop interventions targeting these mechanisms to retard CV aging. There is reasonable consensus that oxidative stress and inflammation play a critical role in the pathogenesis of a range of age-related CVD. The concept that the same evolutionarily conserved pathways (such as sirtuins and Nrf2) controlling the aging process in mammals also determine CV health through changes in ROS production. These interventions include CR, exercise and the use of pharmaceuticals and nutraceuticals including metformin, CoQ10, resveratrol and rapamycin [28].
The CR and CRAN
CR and Adipose Tissue: There is increasing evidence that adiposity plays an important part in regulating aging process. The WAT stores fat as triglycerides when food is abundant and sheds it when in time of scarcity, and also with CR (Figure 3). The WAT senses nutritional status and sends appropriate signals to various organs. In addition, WAT is an endocrine organ and secretes hormones such as leptin and adiponectin. The idea that WAT regulates aging is strengthened by the findings that the FIRKO mice and C/EBP knockin mice stay lean and live long [29]. FIRKO mice have increased median and maximum lifespan compared with phenotypically normal littermate controls, having increased insulin sensitivity [30]. The WAT also mediates many age-associated metabolic disorders such as IR, MetS, T2DM and dyslipidemia which negatively affect the lifespan.

The Sirt1 regulates WAT, by repressing p53. It exerts effects on hormones, including growth factors, which influence cellautonomous effects. The combined effect of changes in hormones and a higher threshold for apoptosis in responsive cells is an advantage for the survival. Activating SIRT1 pathway through CR or CRMs like resveratrol is, thus, beneficial in preventing some manifestations of aging (Figure 3). The most striking features of CR is that it appears to forestall or prevent many late-onset disorders and diseases.
Impact of CR on Health and Disease: CR has an established ability to extend life span and to mitigate aging and disease processes in various tissues. The CRAN seems to possess many beneficial effects in retarding numerous disease states [31]. The CR represents an important intervention to extend both mean and maximum life span in various organisms. This is due to numerous metabolic alterations following CR. The CR preserves muscle tissue in nonhuman primates and rodents. Mechanisms include reduced muscle cell apoptosis and inflammation, protection against age-related mitochondrial abnormalities and preserved muscle stem cell function. The two bio-metabolic pathways, sirtuin pathway and Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor erythroid 2-related factor 2 (Nrf2)/ antioxidant response element (ARE) pathway, also referred to as the Keap1/Nrf2/ARE pathway are concerned with an organism’s longevity and upregulation of cytoprotective genes essential for cell survival respectively [32].
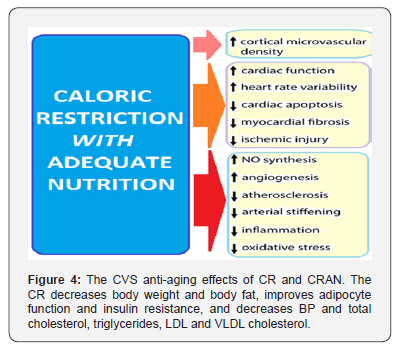
The CVS Anti-Aging Effects: To date, CR is the most robust intervention (Figure 4) that has been reproducibly shown to prolong lifespan and delay the onset of age-associated diseases in both invertebrates and vertebrates, including mammals [33]. The studies of the effects of CR in rhesus monkeys have shown a reduction in body weight, body fat, BP and triglyceride levels that was accompanied by improvement in glucoregulation and lipoprotein profile [34]. There is, thus, increasing epidemiological and experimental evidence from animal models that CR confers multifaceted CV protective effects [35].
The Pharmacological Strategies: It is critical to understand the interactions of age-related molecular mechanisms in vascular cells with both CVD pathogenesis and systemic aging processes, and to develop interventions targeting these mechanisms to retard CV aging. Several examples of such potential therapies include CR mimetics, mitochondrial protective agents and mTOR inhibitors. There is reasonable consensus that oxidative stress and inflammation play a critical role in the pathogenesis of a range of age-related CV and cerebrovascular diseases. The concept that the same evolutionarily conserved pathways (such as sirtuins and Nrf2) controlling the aging process in mammals also determine CV health through changes in ROS production. The age-related cardiac, arterial and microvascular, and ischemic heart disease and stroke increase exponentially with advanced age. The mechanism underlying cardiac and vascular effects of aging is the mechanistic effects of aging per se on the CV system. The possible benefits of therapeutic strategies that have the potential to improve CV function in the elderly and delay the onset of age-related CVDs are the therapeutic targets. The ageassociated alterations in cardiac and vascular system can be delayed by targeting the related pathways with small molecules. Some of the pharmacological strategies have been shown to improve longevity in healthy mammals.
Metformin: Increase in AMPK activity in yeast, flies, worms, mice, diabetic human subjects. There occurs improvement in endothelial function, increase in blood flow, reduction in systolic BP and improvement in vasodilation. The regulation of endothelial progenitor cell differentiation, stimulation of ischemia-induced revascularization, improvement in vascular anti-inflammatory properties and decrease of serum levels of high-sensitivity C-reactive protein
ACE Inhibitors and ARBs: Angiotensin- converting enzyme inhibition increases of NO production and causes improvement in cardiac function and metabolism and endothelial function. Improvement in cardiac function and metabolism and enhanced endothelial function. Increase in eNOS expression in the heart and carotid artery and marked reduction in tissue ACE expression/activities.
Aspirin: Irreversible inactivation of cyclooxygenases AMPK activator. Decreased expression of inducible nitric oxide synthase (iNOS) and Cox-2. Hypertensive mammals.
β-Blockers: β-adrenergic receptor antagonists. Use in treatment of hypertension and ischemic heart disease, prevention of the transition to heart failure via NO-dependent mechanisms (celiprolol) Flies, only mean lifespan in mice
Statins: Inhibition of HMG-CoA reductase Flies Reduction in ROS levels in cardiac muscle. Increased NO synthesis and neoangiogenesis in endothelial cells and the central nervous system
Resveratrol: Sirt1 activator Yeast, worms, Controversial results in flies, mice and humans Lowering of blood pressure, increase in flow-mediated dilatation of the brachial artery, improvement of endothelial function, decrease in plasma inflammatory biomarkers.
PUFAs: Peroxidation Membrane modification Formation of lipid mediators. Low amounts increase lifespan in worms. High amounts decrease Lowering of triglyceride levels in the blood.
SRT1720/SRT2104: Sirtuin activation confers diverse antiaging cardiovascular protective effects Members of the sirtuin family of protein deacetylases are among the best-studied mediators of CR and the NAD+-dependent deacetylase SIRT1 is involved in several key cellular functions, including chromatin remodeling - through histone deacetylation – and gene expression, and also in cellular energy metabolism. The deletion of SIRT1 interferes with CR-mediated lifespan extension in yeast, worms and flies. These findings have led to the search of small molecule activators of SIRT1 as therapeutics to improve CV health. Earlier studies have established that the natural polyphenol resveratrol was able to activate Sir2 in yeast and SIRT1 in humans and increasing cell survival through acetylation of p53. In rodents, resveratrol promotes transcriptional responses comparable to CR-mediated SIRT1 activation, improves health and survival of mice on a high-calorie diet, and confers multifaceted anti-aging vascular effects (including potent mitochondrial protective and anti-inflammatory effects) and protection against atherosclerosis, hypertension, ischemia/reperfusion injury, and heart failure. Resveratrol improves cerebromicrovascular function, increases cerebromicrovascular density and prevents cerebral microhemorrhages, all of which likely contribute to resveratrol-mediated improvement of cognitive function in aged mice. Pre-clinical studies also indicate that resveratrol supplementation reduces platelet aggregation and improves lipid metabolism, while inhibiting atherosclerotic plaque formation and markers of oxidative stress and inflammation. It is within this context that resveratrol improves arterial stiffness in nonhuman primates fed high-fat, high sugar diet through decreased levels of caspase 3 and lipid peroxidation. However, resveratrol also elicits off-target cellular effects, whereby AMPK is activated in a SIRT1-independent fashion and phosphodiesterases inhibited nonselectively, causing a rise in intracellular cAMP levels with concomitant, sirtuin activation and improvement in age-related phenotypes. The redox-sensitive transcription factor Nrf2 is potently activated by resveratrol. Sirt1 activators Mice and rats Under clinical trial for humans Improvement in endothelial function and attenuation in vascular oxidative stress and inflammation Anti-atherogenic activity Preservation of cardiac stem cell pool.
Cardioprotective Herbal Derivatives: The current research holds promise about therapeutic use of herbs and herbal-extracts for retarding CV aging. There are two groups of constituents found in herbs employed for CVD and cardioprotection. These are, flavonoids and triterpenes, which appear to be safe for longterm administration and are present in various plants and herbs. The herb therapies have certain potential pitfalls, though. The herbal ingredients may interact with the drugs, might do little, or might even worsen certain outcomes.
Flavonoids: Studies indicate that the flavonoids inhibit platelet aggregation, thrombus formation and coagulation. They reduce oxidative stress, atherosclerosis and arterial blood pressure. They may favorably modify vascular inflammatory and endothelial and capillary function. They may improve lipid profile values and regulate carbohydrate metabolism.
There is a research support for a regular dietary intake of polyphenols - particularly of flavonoids and the specific class of flavonoids named flavanols exert beneficial vascular effects and reduce the risk for CV morbidity and mortality. The flavonoids, particularly in cocoa and tea flavanols, are helpful in protecting CV diseases [36]. Apart from these, flavonoids are the active components of Gingko leaf, crataegus (hawthorn fruit) and Pueraria mirifica.
The improvement in endothelial function have been observed in CAD patients on daily consumption of flavonoids from purple grape juice (which includes flavan-3-ols, flavonols, proanthocyanidin, and anthocyanins), black tea and flavonolrich cocoa. The flavonoids in red wine (e.g., flavan-3-ols, flavonols, proanthocyanidin, and anthocyanins) improve endothelial function by up-regulating endothelial nitric oxide synthase (eNOS) expression and increasing endothelial cell NO production [37]. and thus, improving endothelium-mediated vasodilation [38]. The European Food Safety Authority has approved the claim that consumption of 200 mg flavanols from cocoa daily can improve blood flow. There is growing evidence that regular ingestion of flavonoids can reduce the incidence of cardiovascular disease by reducing blood pressure, blood coagulation and blood fats.
Phytosterols: The phytosterols also promote general health and well-being and reduce CV risk. The potential action mechanisms of polysulfides in cardioprotection is through hydrogen sulfide releasing activity, radical scavenging activity and in enzymatic activity inhibition and intervention of gene regulation pathways [39].
Cruciferous vegetables and the Allium family include garlic (Allium sativum) and onion (Allium cepa) and are rich in organopolysulfide as natural donor of H2S. The organosulfides in Alliums are well known for their broad spectrum of health promoting benefits. However, human clinical trials in garlic found that allicin has no effect on reducing cholesterol level [40].
Triterpenes: Triterpenes are the main active components of ginseng and other herbs like gynostemma, rhodiola and ganoderma. The saponins in ginseng (triterpene glycosides) are believed to be useful for recovery from ACS/MI. The triterpenes lower blood lipids and enhance oxygen utilization and reduce ROS. They have been shown to have cardiac protective effects.
Other Herbs: Salvia divinorum (active constituent: a diterpenoid called Salvinorin A) can effectively improve myocardial ischemia and abnormal parameters in patients with coronary heart disease. Astragalus (active constituent: cycloastragenol a possible telomerase activator) has ionotropic effect and enhance cardiac systolic function in patients with HF. It also has protective effects against myocardial ischemia and reperfusion injury, free radical injury and on platelet aggregation [41]. Astragalus and salvia exhibit a synergistic effect for improvement of cardiac function. Aconite (Aconitum) having the alkaloid psuedaconitine, can effectively improve sinus-node function and cardiac performance in patients with sick sinus syndrome. There are several other natural polyphenols with antioxidant, anti-inflammatory, anti-apoptotic and/or antisenescence properties, including quercetin, kaempferol and epicatechin, which may also potentially exert beneficial effects in CV aging either alone or in combination with existing drugs.
Potential Interventions to Retard CV Aging
a) Novel Cardioprotective Drugs: The recent research is focussed on identifying novel strategies to recover the damaged and the dysfunctional yet viable myocardium, improve symptomatology and QOL, and prolong survival. These drugs can be able to activate specific protective biomolecules and reduce ongoing damage to the heart and vasculature. These drugs will potentially preserve the myocardium and its functional ability of the heart, and delay the onset of heart failure, and reduce CV mortality.
b) Carnitine and L-Carnitine: In experiments, the administration of carnitine increases glucose oxidation in the isolated perfused rat heart by increasing the acetyl-carnitine concentration and decreasing the acetyl-CoA concentration, and thus relieving acetyl-CoA inhibition on PDH [42]. A mediumsize randomized double-blind trial in 472 myocardial infarction patients showed that oral carnitine therapy (6 g/day) initiated within 24 h after the onset of chest pain failed to affect clinical outcome or LV injection fraction over the course of 1 year of treatment; however, it did significantly reduce the rate of increase in the LV end-diastolic volume [43]. Some small studies suggest that people who take L-carnitine supplements soon after a myocardial infarction may be less likely to have another episode, die of heart disease, or develop heart failure [44]. However, few other studies show no benefit. Controlled trials in CAD patients relating to carnitine are needed [45].
c) Coenzyme Q10 (CoQ10): The CoQ10, endogenously synthesised and diet-supplied lipid-soluble cofactor, is a key component in the mitochondrial ETC for ATP generation, and exists in abundance in the normal myocardium (Figure 5). It functions in the mitochondrial inner membrane to transfer electrons from complexes I and II to complex III. By virtue of its redox activity, also acts as a membrane antioxidant. The myocardial CoQ10 content tends to decline with age and myocardial dysfunction. A number of controlled trials with supplemental CoQ10 have shown improvements in functional parameters such as ejection fraction, stroke volume and cardiac output. Recently, long-term therapy with CoQ10 has been shown to reduce major adverse cardiovascular events (MACE) and improve HF symptoms and found CoQ10 safe and well tolerated [46]. Subsequent meta-analyses have also confirmed these findings [47].
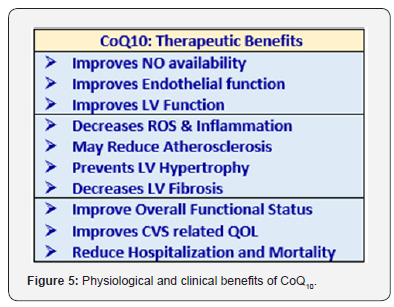
Additionally, CoQ10, through its antioxidant effects, may reduce oxidative stress, which is known to adversely affect left ventricular ejection fraction and alter disease outcomes. Lastly, CoQ10 may stabilise calcium-dependent channels in the myocardium, enhancing effective ATP synthesis. There is a potential role CoQ10 plays in the protection of heart, prevention of CVD and HF. CoQ10 supplementation improves heart and vascular function, reduces atherosclerosis, improves endothelial function, and protects against myocardial damage. For HF patients with either preserved (HfpEF) or reduced (HfrEF) ejection fraction CoQ10 presents as a safe therapeutic option. The recent Q-SYMBIO randomized controlled trial has demonstrated a reduction in major adverse cardiovascular events with CoQ10 supplementation in HF patients [48]. A recent randomized controlled trial has suggested that there may be a mortality benefit in patients with HFrEF with CoQ10 supplementation [49].
CoQ10 formulations are available as either ubiquinol (reduced form) or ubiquinone (oxidized form). Regardless of whether the formulation contains ubiquinol or ubiquinone, after ingestion CoQ10 appears in the plasma circulation as ubiquinol. Nausea is the most common symptom, followed by allergic maculopapular rash. Caution should be taken in the patients who are on oral anticoagulant therapy, given the similarities of CoQ10 with vitamin K. There may occur a reduction in blood pressure and heart rate, without any significant ECG changes. Theophylline is also affected by cytochrome p450 enzymes and animal studies have demonstrated altered pharmacokinetics of theophylline with co-administration of CoQ10 [50] Statin therapy appears to deplete CoQ10 levels [51]. Here, CoQ10 supplementation will help.
d) Resveratrol: Resveratrol (3,5,4,trihydroxystilbene), is a polyphenol, found predominantly in grapes and berries, and a major component of red wine. Resveratrol has multiple beneficial cardiovascular effects and its use as a nutraceutical for CVD and HF has been highlighted. Current research has suggested its potential in preventing or regressing defects in cardiac structure and function in experimental models of heart disease. There are strong indications about its potential in preventing or retarding the development of HF [52] and it has efficacy of in humans with CVD and HF [53]. The administration of resveratrol has been shown to improve outcomes of in animal models of HF induced by myocardial infarction, pressure overload, myocarditis, and chemotherapy-induced cardiotoxicity in animal studies [54]. In addition, experiments in animal have shown that resveratrol improves cardiac function and survival when co-administered with the treatment for established HF. Resveratrol acts on the peripheral tissues to improve skeletal muscle and endothelial/ vascular function. With resveratrol treatment in mice, there is lessening of cardiac fibrosis and improvement in molecular and structural remodeling of the heart, cardiac diastolic function, vascular function, and energy metabolism [55].
e) HSP20: The myocardium makes certain peptides including various heat-shock proteins (HSPs) to counteract apoptosis following physical and oxidative stress. In the heart, transient ischemia followed by reperfusion (ischemia/ reperfusion, I/R) induces necrosis and apoptosis, leading to myocardial dysfunction. Preservation of myocardial function after I/R depends on critical adaptive responses, some of which are believed to involve the heat-shock proteins (HSPs). The HSPs synthesis arises transiently as a tool to protect cellular homeostasis after exposure to stressful and potentially deleterious stimuli. Thus, HSPs are mediators of myocardial protection, particularly in ischemia and reperfusion injury [56].
The heart makes certain proteins to counteract heart cell apoptosis. One such protein, HSP20 is known to protect heart muscle cells following physical stress. It also improves the pumping ability of the heart. As the protective functions of HSP20 is required during stress, HSP20 lies dormant in heart cells until when required. It is then switch on by a process called phosphorylation. The HSP20 regulates activities of vasodilation and platelet aggregation. The increased expression of HSP20 in cardiomyocytes is associated with improved contraction and protection against β-agonist–induced apoptosis.
The cardioprotective effects of HSP70 have been shown in isolated animal hearts after global or regional ischemia. Recently, protection during myocardial ischemia has also been shown for the small heat-shock proteins HSP27 and αB-crystallin. The HSP20 shares considerable homology with HSP27 and αBcrystallin, which appear important in cardioprotection against ischemic injury. In studies, the increased HSP20 expression in the heart protects against IR injury, resulting in functional recovery and reduced infarcted area. Thus, outlining the significance of HSP20 in contractile function of heart and cardioprotection [57].
The idea is to develop innovative medicines that would ‘switch on’ HSP20 in individuals having a high risk of developing coronary heart disease (CHD) or to prevent the disease progression. Certain peptides do the cardio-protective ‘switch on’ and effectively boost HSP20. Experimentally, these peptides protected against some of the features of high pressure and improved cardiac function. The data from animal studies, support the hypothesis that this new class of drugs ca provide protection against CHD, cardiac hypertrophy and HF.
f) Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors: There are extraordinary CV effects of SGLT2i and GLP-1 agonists [58]. when DECLARE study is out by 2019, which assesses both primary and secondary prevention effect of dapagliflozin. However, the primary subset of CANVA cohort is a preliminary answer to that question. The American Diabetes Association clinical guidelines suggested the use of the SGLT2i (empagliflozin and canagliflozin) and the GLP-1 agonists (liraglutide) as the second line in case there is a history of CV insult, secondary prevention. Moreover, adverse effects of SGLT2i are mostly minor and treatable, with exception of amputation in case of canagliflozin [59].
g) Rapamycin: mTOR signaling is an important modulator of the cardiovascular aging phenotype. A leading target for antiaging interventions is the nutrient response pathway controlled by mTOR signaling. Inhibition of this pathway by CR extends lifespan and confers healthspan increase in various animal models [60]. The mTOR is a serine/threonine kinase that activates cell anabolism, especially increasing protein synthesis and cell growth, while inhibiting catabolic mechanisms, notably autophagy. mTOR inhibitor Yeast, flies, worms, mice Attenuation of load-induced cardiac hypertrophy, restraint in the increase in myocyte cell size Reduction of ischemic injury after myocardial infarction Decrease in inflammation and hypertrophy Higher metabolism The fundamental role of mTOR signaling in metabolic regulation contributes to the biogenesis and proper functioning of the CV system. There is a progressive incidence of cardiac hypertrophy and diastolic dysfunction with advancing age as well as accumulation of protein damage mediated by oxidation and ubiquitination. Of significance, these age-associated conditions are hampered by short term CR and rapamycin treatment [61]. Inhibition of mTORC1 by rapamycin confers protection against these age-related CVD, especially in the presence of metabolic disorders.
h) Other Drugs: Berberine is an AMPK activator Flies Decrease in the expression of iNOS and Cox-2 as well as increase the (AMP+ADP)/ATP ratio by impeding the efficiency of mitochondrial electron transport. Another drug, Nrf2 (Nfe2l2) activators - Activate NRF2-antioxidant response Flies and worms Regulation of cellular antioxidant defenses and maintenance of redox homeostasis Regulation of the proteasome and removal of oxidized proteins Maintenance of the functional integrity of the heart and vasculature [62]. The GH deficiency and low circulating levels of IGF-1 significantly increase the risk for CV and cerebrovascular diseases in humans [63]. Microvascular dysfunction due to age-related IGF-1 deficiency has been causally linked to the pathogenesis of vascular cognitive impairment and has also implications for the pathophysiology of cardiac failure. Increase in nutrient and growth factors availability stimulates Akt-mediated activation of mTOR but suppresses AMPK function. Activation of AMPK occurs during stress or energy deprivation, thereby inhibiting mTOR.
i) The PARP-1 Inhibitors: Pharmacological inhibition of the PARP pathway has emerged as a potentially important therapeutic target for aging and age-associated diseases. PARP- 1 is a member of the DNA damage surveillance network. The catalytic activity of PARP-1 was reported to increase in old age due to the age-related increases in peroxynitrite-mediated DNA strand interruptions. An increase in PARP-1 activity results in SIRT1 inhibition due to lower substrate availability. This antagonistic crosstalk between PARP-1 and SIRT1 represents a potentially important mechanism by which PARP- 1 over-activation promotes age-related cardiac and vascular dysfunction. Indeed, there is evidence suggesting that inhibition of PARP-1 may confer protection against CV aging.
The Modalities for Reversing CV Aging
The Emerging Concepts and Strategies
The Concept of Genome Stability and Aging: The agedependent changes occur in both the heart and vasculature, evidenced by numerous alterations at cellular and subcellular levels as a result of deregulation of various molecular longevity pathways [64]. A number of diverse stimuli induce senescence. But, they appear to converge on certain pathways that influence cell cycle regulation, DNA repair and apoptosis, and the process of cellular senescence (Figure 6).
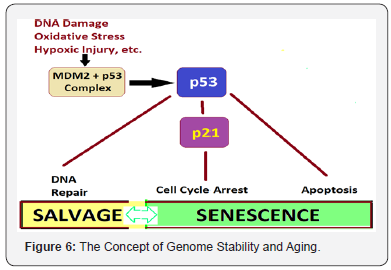
These pathways are mainly regulated by the tumor suppressor proteins p53 and pRb. The p53 is a crucial mediator of the cellular response to damaged DNA and dysfunctional telomeres, and in turn activates the cyclin-dependent inhibitor p21. It is considered that senescence occurs via the p53 pathway in response to DNA damage and telomere dysfunction [65], whereas the p16/pRb pathway mediates senescence caused by oncogenic stimuli, chromatin disruption, and other cellular stresses. The chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. Many of these genes and their related pathways have been extensively researched in model organisms like, Saccharomyces cerevisiae (yeast), the Caenorhabditis elegans (nematode), Drosophila melanogaster (fruit fly), and Mus musculus and other mice.
Angiogenesis, another critical mechanism, is responsible for repairing tissues after damage caused by daily wear and tear and events such as myocardial ischemia and infarction and cerebrovascular stroke [66]. Processes and pathways contributing to impairment of angiogenesis, include cellular senescence, telomere attrition, oxidative damage, NO, hypoxia, and vascular growth factors, and the age-related impaired angiogenesis contributes to increased end-organ damage and affects cardiovascular health.
The ROS and Aging Process: The modalities to prevent, delay or reverse CV changes that accompany aging will reduce the prevalence of CVDs. But, retarding the rate of progression of CVDs at subclinical level need to be considered before the clinical disease becomes manifest. There are strategies aiming to reduce the oxidative stress which exacerbates aging process and leads to debility to human body in general and CVS in particular. The most promising data comes from experimental biology highlightening CR as a tool for reduction in oxidative stress and increasing longevity. The life-style interventions in form of regular physical exercise, smoking cessation and intervention in sleep disorders, are known to mitigate oxidative damage and exert a beneficial effect on CVS. Certain medications or humoral factors are known to reduce CVDs are practical ways to prevent and treat atherosclerosis and CVD. Further, a multitude of therapeutic interventions relating to various pathways involved in cellular aging hold promise.
.The Genetic Alterations and Mutations
The Mitochondrial Genetic Mutations: The genetic mutations in mitochondria, appear to trigger apoptosis and accelerate the aging process. By altering the gene called polymerase-ƴ, which functions as a spellchecker during the copying of mitochondrial DNA in mice led them to age fast. Because the mitochondria also control the natural process of cell death, called apoptosis, mistakes by the spell-checker gene enhance apoptosis. As mitochondrial mutations accumulate, there are increased cell deaths and appearance of the aging characteristics.
The Single Gene Mutations in Humans: There have been discovered a variety of longevity mutations in species ranging from C. elegans to Drosophila to mammals. These mutations have been shown to extend life by about 50% in mammals and more in nematodes. In human longevity studies, single nucleotide polymorphism (SNP) analysis identified a large number of genetic variants with metabolic effects.
The Modalities and Manipulations
Calorie Restriction and CR Mimetics: The CRAN diet combined with exercise appears to increase the health span by stimulating the expression of ‘longevity genes’ that promote cellular defence against aging and age-related diseases. There are some fundamental molecular processes involved in CRmediated protection of CVS. CR modulates various factors in the longevity network and alters cellular responses to oxidative stress. It reduces oxidative stress and inflammation in the vasculature by suppressing the activity of vascular adhesion molecules, prostanoids, and inflammatory cytokines. It improves endothelial function and reduces atherosclerosis and arterial stiffness [67]. CR also delays the age-related decline in diastolic function accompanied by reductions in inflammation, myocardial degeneration, cardiac fibrosis, and cardiomyopathy
The recent research highlights that the CR not only reduces metabolic rate and damage caused by ROS; it also triggers an active defence response that promotes a network of redundant pathways involving the longevity genes lying dormant through the evolutionary process. These so-called ‘longevity regulatory pathways’, include insulin/insulin-like growth factor 1 (IGF-1), the mammalian target of rapamycin (mTOR), AMP-activated kinase (AMPK), and nicotinamide adenine dinucleotide (NAD)+- dependent deacetylases (sirtuins). The CR is extensively-studied longevity intervention and has been shown to increase lifespan in various model organisms, from yeast and the nematode, C. elegans to mice, rats, and rhesus monkeys [68,69].
The CR involving a moderate dietary restriction (35% reduction in calorie intake) attenuates a variety of age-related pathologies and is protective against CVDs in mice and other nonhuman primates [70]. In addition to its protective effects of long-term, the CR for a brief period of 10 wk was able to reverse the preexisting cardiac hypertrophy and diastolic dysfunction in old mice, and that this was accompanied by proteomic and metabolomic remodeling to a more youthful state [71].
A study in human volunteers undertaking CR for a mean of 6.5 yr showed reduced blood pressure and systemic oxidative stress and improved diastolic function [72]. Similar improvements in diastolic function have been reproduced in individuals maintained on 1-yr CR and in human trials of alternate-day fasting, demonstrating that even transient activation of the pathways involved in the CR response has beneficial effects. The individuals on CR diets for 0.5–8 years, have reduced triglycerides, lower blood pressure, reduced inflammatory markers and decreased oxidative stress, and a relative protection from CVDs. Multiple mechanisms have been implicated in the beneficial effects of CR including inhibition of mTOR signaling, normalization of mitochondrial biogenesis, attenuation of mitochondrial ROS production and the subsequent ROS-induced signaling, and increased SIRT1 signaling [73].
Studies suggest several synergistic mechanisms that work with CRAN. There occurs an increase in NO with a combination of reduced ROS is both neuro and cardioprotective, due to activation of the Nrf2 antioxidant pathway. The CR reduces oxidative stress-induced induction of proinflammatory markers, like NF-κB-mediated cytokine synthesis, protection from endothelial damage and reversal of the progression to atherosclerosis. Further, a reduction in myocyte size, and cell death via apoptosis is observed in calorie-restricted aged hearts, a mechanism attributed to the protection of mitochondria from membrane collapse. The changes seen due to CR can be attributed to SIRT1 and PPAR. Further, RAS inhibition and CR seem to have converging effects in their mechanism, both mediated by PPAR upregulation.
The use of CR mimetics such as resveratrol and metformin, which activate the SIRT1-AMPK system, and rapamycin, which inhibits mTOR, show that it is possible for a rodent to be obese and sedentary while maintaining the physiology of a lean animal. Further, the recent research has identified a hormone, irisin [74], which when increased, induces energy expenditure in the absence of exercise, positively influencing obesity and glucose homeostasis.
The Gene Therapy
The metabolic manipulations enhance longevity by retarding aging comparable to CR, are not as effective as repair strategies which can reverse aging process. The genetic manipulations causing these alterations work through a common pathway across many species endorsing that there is an evolutionary genetic program that controls aging. Further, it appears that the metabolic changes will not produce significant effects on longevity in humans as they do in mice for the simple reason that lifespan is much more plastic in short-lived species.
Some of the important potential target genes for future gene therapies to retard and reverse CV aging or compensating for age-related damage, are:
Angiotensin-Converting Enzyme (ACE): Lowered levels of ACE have been shown to extend mean life span in C Elegans.
Adenylyl Cyclase Type 5 (AC5): Knockout of AC5 extends life in mice possibly due to increased resilience of the cardiovascular system. Some aspects of AC5 knockout mice resemble those of CR mice.
AMPK: Targeted overexpression of AMPK in Drosophila increases life span. This has been related to CR response leading to improved cell maintenance and modestly slowed aging.
Angiopoietin-like 4 (ANGPTL4): The rare variant of this gene, present in less than 1% of the European population, reduces the risk of heart attack by half possibly due to alternations in cholesterol metabolism.
Angiotensin II Receptor Type 1 (Agtr1a): Lowering Agtr1a levels protects mitochondrial function and modestly extends life in mice.
Apolipoprotein A-1: Increased amounts of Apo A-1 improve cholesterol metabolism and slows progression of atherosclerosis by transporting away the damaged lipids build up in blood vessel walls.
β2 microglobulin (B2M): B2M levels rise with age, and in mice reducing levels of B2M in older mice restores some of the loss of cognitive decline due to aging. The role of B2M also relates to adaptive immune system.
C-Myc: Lowered levels of c-Myc retard aging and extend life in mice through its effects on insulin metabolism.
C1Q: The C1Q gene plays a role in the immune system. Its levels rise in brain with aging and removing it improves the state of cognitive function in older mice.
Catalase: Gene therapy to increase levels of the antioxidant catalase in the mitochondria reduces damage to mitochondria as a result of ROS.
CLK1: Reduced CLK1 activity extends life in mice due to altered mitochondrial function and consequently lowered generation of ROS.
Cyclin A2: The increased levels of cyclin A2 increase the regenerative capacity of heart tissue. It may also be beneficial in retarding degeneration in the heart.
FGF21: Overexpression of FGF21 occurs as CR response, and when induced using gene therapy can extend life in mice. The aging retarding effect is connected to growth hormone/insulinlike growth factor-1 signaling pathway.
Follistatin: Its increased level leads to muscle growth, a potentially useful compensation for the loss of muscle mass and strength that occurs with aging
FOXO3: A variant of FOXO3 is associated with a modest reduction in CVD and mortality in human-beings.
GDF11: Higher levels of GDF11 improve various factors related to aging in mice, such as heart function and exercise capacity, possibly due to increased stem cell activity
Growth Hormone/Growth Hormone Receptor/Insulin- Like Growth Factor/Insulin Receptor: Disrupting the growth hormone-insulin metabolism extend life in mice.
Interleukin-21 (IL-21): The higher levels of IL-21 improve the state of the immune system by increasing the pace at which new immune cells are generated. May correct the loss of immune function with age.
KLF4: Selectively lowering levels of klf4 in improves smooth muscle cells function in blood vessel walls. Their overreaction to damaged lipids arriving in the bloodstream is muted, which slows the progression of damage and reaction to towards atherosclerosis.
miR-195: The microRNA miR-195 interacts with telomerase, and inhibiting it has much the same beneficial effect on stem activity as increasing levels of telomerase.
Mechanistic Target of Rapamycin (mTOR): Alterations to the mTOR gene have been shown to modestly extend life span in several species. The mTOR protein is involved in many fundamental cellular processes and cellular metabolism.
Myostatin: Reduced myostatin increases muscle growth, may be a useful for the loss of muscle mass with aging.
NF-κB: Its inhibition extends life modestly in a number of lower species, given its involvement in immunity, inflammation, apoptosis, and other fundamental processes.
P21: Both MRL mice and P21 knockout mice can regenerate small injuries with no scarring and reduced levels of the p21 protein seems to be the common factor in these engineered mouse lineages. P21 is closely related to the tumour suppressor gene P53.
P53: The protein p53 plays the role of tumour suppressor. An increase in p53 levels accelerate aging by reducing tissue maintenance through the creation of new cells.
PCSK9: Loss of function mutations in PCSK9 reduce the risk of cardiovascular disease, most likely through lowered blood cholesterol levels.
Plasminogen Activator Inhibitor-1 (PAI-1): Reducing levels of PAI-1 appears to modestly slow aging, possibly by removing one aspect of the harmful impact of senescent cells.
Pregnancy-Associated Plasma Protein-A (PAPP-A): Knockout of the PAPP-A gene interferes with insulin metabolism and produces a similar extension of health and life in mice when compared with other methods of achieving this end.
Phosphatase and Tensin Homolog (PTEN): Adding an extra copy of the tumour suppressor gene PTEN to mice produces lower rates of cancer, much as expected, but also increased life span.
Rpd3: A reduction in Rpd3 level produces improved cardiac function and modestly increased longevity in flies.
TGF-β1: TGF-β1 expression rises with age and is implicated in loss of stem cell function.
Troponin C: Researchers have shown that delivering a modified version of the calcium receptor troponin C into the mammalian heart can improve heart function and the performance of the cardiovascular system.
Uncoupling Proteins (UCP): Uncoupling proteins manipulate mitochondrial function. The altered levels or genetic variants can improve health and longevity
Urokinase (uPA): The αMUPA mouse lineage has the addition of a urokinase gene and has a longer life span. The uPA gene is related to PAI-1 and is argued to achieve life extension in mice through behavioural change - these mice eat less, and thus the CR response comes into play.
Telomerase: Increased levels of telomerase have been shown to extend life in mice, as well as reducing cancer incidence. The most possible mechanism appears to be increased stem cell activity, while effects on cancer may involve a more active immune system.
Conclusion
Regenerative Medicine
For Cardiovascular Health and Well-Being: The phenomenon of aging is universal in the kingdom of living. With time, all living beings age. There have been immense developments in the scientific understanding of biology of aging and the changes that take place with aging at cellular and molecular levels. At the same time, the progress in healthcare and technology has made possible to delay aging process and bring about healthy aging. The longer life and aging slowly are mutually related. A healthy food, adequate physical activity and wholesome lifestyle keep the daily attrition-related damage at minimum and can retard aging. With the progress of science and technology, there are futuristic visions of achieving significant longevity [75].
The Life extension program (LEP) aims to increase the maximum lifespan beyond the current maximum for humans. It can be visualised to go through three steps - taking advantage of the existing knowledge for slowing aging like CR and CRAN; utilizing the advances in genetics and biotechnology; and using the future nanotechnology and artificial intelligence revolution to repair the mutations and other defects due to aging at molecular and cellular levels. The futuristic goal is, thus, to achieve rejuvenation and state of non-aging.
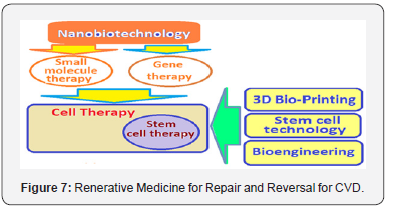
Apart from CR, the regenerative medicine is the next concrete step for treating CVD and afflictions due to CV aging [76]. The most promising in regenerative medicine are nanobiotechnology giving access to gene therapy and small molecule therapy, stem cell technology, bioengineering and 3D bioprinting for organ production in bulk, and perhaps, the therapeutic cloning (Figure 7). The regenerative medicine does not retard or slow aging but corrects the organ failure and diseases that accompany aging, in general, and CVD and CV aging in particular. It is an advanced form of future medicine.
References
- Incidence of CVD by age and sex, Incidence and prevalence 2006. Chart Book on Cardiovascular and Lung Diseases - Nat. Heat, Lung and Blood Inst.
- Lakatta LE (2015) So! What’s aging? Is cardiovascular aging a disease? J Mol Cell Cardiol 83: 1-13
- McKay JA, Mathers JC (2011) Diet induced epigenetic changes and their implications for health. Acta Physiol 202: 103-118.
- Koubova J, Guarente L (2003) How does calorie restriction work? Genes & Dev 17: 313-321.
- Tylor A, Cable N, Faulkner G (2004) Physical activity and older adults: a review of health benefits and the effectiveness of interventions. Journal of Sports Sciences 22(8): 703-725.
- Lakatta EG, Levy D (2003) Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A “Set Up” for Vascular Disease. Circulation: New Frontiers 107(1): 139-146.
- Lakatta EG, Levy D (2003) Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises Part II: The Aging Heart in Health: Links to Heart Disease. Circulation: New Frontiers 107(2): 346-354.
- Lakatta EG (2003) Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises Part III: Cellular and Molecular Clues to Heart and Arterial Aging. Circulation: New Frontiers 107(3): 490-497.
- AlGhatrif M, Lakatta EG (2014) The Reality of Aging Viewed from the Arterial Wall. In Safar ME, O Rourke MF, Frohlich ED Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases. London: Springer pp. 137-154.
- Wang M, Lakatta EG (2006) Central Arterial Aging: Humans to Molecules. In Safar M Handbook of Hypertension: Arterial Stiffness in Hypertension. Elsevier 9: 137-160.
- Wang M, Shah A (2015) Age-associated pro-inflammatory remodeling in the heart and large arteries. J Mol Cell Cardiol 83: 101-111
- Wang M, Jiang L, Monticone RE, Lakatta EG (2014) Proinflammation: the key to arterial aging. Trends Endocrinol Metabol 25(2): 72-79.
- Harvey A, Montezano AC, Touyz RM (2015) Vascular biology of aging - implications in hypertension. J Mol Cell Cardiol 83: 112-121.
- Donato AJ, Morgan RG, Walker AE, Lesniewski LA (2015) Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol 89: 122- 135.
- Chistiakov DA, Orekhov AN, Bobryshev YV (2015) Vascular smooth muscle cell in atherosclerosis. Acta Physiol 214(1): 33-50.
- Gray K, Kumar S, Figg N (2015) Effects of DNA damage in smooth muscle cells in atherosclerosis. Circ Res 116(5): 816-826.
- Fernández-Hernando C, Jó Zzsef L, Jenkins D (2009) Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol 29(12): 2033- 2040.
- Bennett MR, Sinha S, Owens GK (2016) Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 118(4): 692-702.
- Jewell JL, Guan K (2013) Nutrient Signaling to mTOR and Cell Growth. Trends Biochem Sci 38(5): 233-242.
- Sciarretta S, Volpe M, Sadoshima J (2014) mTOR Signaling in Cardiac Physiology and Disease. Circ Res 114(3): 549-564.
- Long YC, Juleen YCR, Zierath J (2006) AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 116(7): 1776-1783.
- Xinab Z, Mac Z, Jiang S (2017) FOXOs in the impaired heart: New therapeutic targets for cardiac diseases. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1863(2): 486-498.
- Peretti CS (1999) Therapeutic action lag time and resistance to treatment. Encephale Spec 25(2): 49-54.
- Kosteva K, David Wood D, Backer GD (2009) Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet 373: 929-940.
- Kosteva K, David Wood D, Backer GD (2009) EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil 16(2): 121-137.
- Fordyce CB, Roe MT, Ahmed T (2015) Cardiovascular Drug Development: Is it Dead or Just Hibernating? Journal of the American College of Cardiology 65(15): 1567-1582.
- de Cabo R, Carmona-Gutierrez D, Bernier M (2014) The search for antiaging interventions: From elixirs to fasting regimens. Cell 157: 1515-1526.
- Longo VD, Antebi A, Bartke A (2015) Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 14(4): 497-510.
- Fahy GM, West MD, Coles LS, Harris SB (2010) The Future of Aging Pathways to Human Life Extension.
- Blüher M, Kahn BB, Kahn CR (2003) Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299(5606): 572-574.
- Omodei D, Fontana L (2011) Calorie restriction and prevention of ageassociated chronic disease. FEBS Lett 585(11): 1537-1542.
- Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89-116.
- Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI (2011) Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol 51(2): 263-271.
- D Costa AP, Lenham JE, Ingram RL, Sonntag WE (1993) Moderate caloric restriction increases type 1 IGF receptors and protein synthesis in aging rats. Mech Ageing Dev 71: 59-71.
- Mattison JA, Roth GS, Beasley TM (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489: 318-321.
- Grassi D, Desideri G, Croce G (2009) Current Pharmaceutical Design 15(10): 1072-1084.
- Erdman Jr JW, Balentine D (2005) Flavonoids and Heart Health: Proceedings of the ILSI North America Flavonoids Workshop, Washington, DC, USA.
- Davison K, Coates AM, Buckley JD, Howe PRC (2008) Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes 32(8): 1289-1296.
- Bradley JM, Organ CL, David J Lefer DJ (2016) Garlic-Derived Organic Polysulfides and Myocardial Protection. J Nutr 146(2): 403S-409S.
- Shouk R, Abdou A, Shetty K (2014) Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr Res 34(2): 106-115.
- Tocmo R, Liang D, Yi Lin, Huang D (2015) Chemical and biochemical mechanisms underlying the cardioprotective roles of dietary organopolysulfides. Front Nutr 2: 1.
- Vaduganathan M, Butler J, Pitt B, Gheorghiade M (2015) Contemporary drug development in heart failure: Call for hemodynamically neutral therapies. Circ Heart Fail 8(4): 826-831.
- Iliceto S, Scrutinio D, Bruzzi P (1995) Effects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: The L-Carnitine Ecocardiografia Digitalizzata Infarto Miocardico (CEDIM) Trial. J Am Coll Cardiol 26(2): 380-387.
- Kobayashi A, Masumura Y, Yamazaki N (1992) L-carnitine treatment of congestive heart failure - experimental and clinical study. Jpn Circ J 56(1): 86-94.
- Brown DA, Perry JB, Allen ME (2017) Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nature Reviews Cardiology 14(4): 238-250.
- Mortensen SA, Rosenfeldt F, Kumar A (2014) The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail 2(6): 641- 649.
- James J, Di Nicolantonio, Jaikrit Bhutani (2015) Coenzyme Q10 for the treatment of heart failure: A review of the literature. Open Heart 2(1): e000326.
- Belch JJ, Bridges AB, Scott N (1991) Oxygen free radicals and congestive heart failure. Br Heart J 65(5): 245-248.
- Mortensen SA, Rosenfeldt F, Kumar A (2014) The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail 2(6): 641- 649.
- Baskaran R, Shanmugam S, Nagayya-Sriraman S (2008) The effect of coenzyme Q10 on the pharmacokinetic parameters of theophylline. Arch Pharm Res 31(7): 938-944.
- Berthold HK, Naini A, Di Mauro S (2006) Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma: A randomised trial. Drug Saf 29(8): 703-712.
- Wojciechowski P, Louis XL, Thandapilly SJ (2010) Potential of Resveratrol in Preventing the Development of Heart Failure. Current Chemical Biology 4(1): 84-88.
- P Raj, XL Louis, SJ Thandapilly (2014) Potential of resveratrol in the treatment of heart failure. Life Sciences 95(2): 63-71.
- Sung MM, Dyck JR (2015) Therapeutic potential of resveratrol in heart failure. Ann N Y Acad Sci 1348(1): 32-45.
- Sung MM, Das SK, Levasseur J (2015) Resveratrol Treatment of Mice With Pressure-Overload-Induced Heart Failure Improves Diastolic Function and Cardiac Energy Metabolism. Circulation: Heart Failure 8(1): 128-137.
- Fan GC, Ren X, Qian J (2005) Heart Failure: Novel Cardioprotective Role of a Small Heat-Shock Protein, Hsp20, Against Ischemia/Reperfusion Injury; Circulation. 111: 1792-1799.
- Fan GC, Kranias EG (2011) Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. Journal of Molecular and Cellular Cardiology 51(4): 574-577.
- Han Ling, Chen Keji (2001) Advances in experimental pharmacological studies of effects of astragalus on the cardiovascular system. Chinese Journal of Integrated Traditional and Western Medicine 7(2): 146-151.
- Abdelgadir E, Rashid F, BashierA, Ali R (2018) SGLT-2 Inhibitors and Cardiovascular Protection: Lessons and Gaps in Understanding the Current Outcome Trials and Possible Benefits of Combining SGLT-2 Inhibitors With GLP-1 Agonists. J Clin Med Res 10(8): 615-625.
- Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3): 471-484.
- Flynn JM, O’Leary MN, Zambataro CA, Academia EC (2013) Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12(5): 851-862.
- Ballard VL, Edelberg JM (2007) Stem cells and the regeneration of the aging cardiovascular system. Circ Res 100(8): 1116-1127.
- Ungvari Z, Csiszar A (2012) The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci 67(6): 599-610.
- Kurz DJ, Decary S, Hong Y (2004) Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117: 2417-2426.
- Moslehi J, DePinho RA, Sahin E (2012) Telomeres and Mitochondria in the Aging Heart. Circ Res 110(9): 1226-1237.
- Lahteenvuo J, Rosenzweig A (2012) Effects of Aging on Angiogenesis. Circulation Research 110: 1252-1264.
- Riordan MM, Weiss EP, Meyer TE (2008) The effects of caloric restriction- and exercise-induced weight loss on left ventricular diastolic function. Am J Physiol Heart Circ Physiol 294: 1174-1182.
- Niemann B, Chen Y, Issa H (2010) Caloric restriction delays cardiac ageing in rats: Role of mitochondria. Cardiovasc Res 88(2): 267-276.
- Mattison JA, Roth GS, Beasley TM (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489(7415): 318-321.
- Shinmura K, Tamaki K, Sano M (2011a) Impact of long-term caloric restriction on cardiac senescence: Caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50(1): 117-127.
- Dai DF, Karunadharma PP, Chiao YA (2014b) Altered proteome turnover and remodelling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13(3): 529-39.
- Meyer TE, Kovacs SJ, Ehsani AA (2006) Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 47(2): 398-402.
- Lopez-Lluch G, Hunt N, Jones B (2006) Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci 103(6): 1768-1773.
- Erickson HP (2013) Irisin and FNDC5 in retrospect: An exercise hormone or a transmembrane receptor? Adipocyte 2(4): 289-293.
- Nikhra V (2006) Ageing slowly, Living longer. Spandan Innovative in association with Sahni Publications.
- de Grey A, Rae M (2008) Ending Aging: The Rejuvenation Breakthroughs That Could Reverse Human Aging in Our Lifetime.






























