What Ages First: Pulp or Dentin?
Michel Goldberg*
INSERM UMR-S1124, University Paris Cité, Faculté des Sciences Fondamentales et Biomédicales, Cellules souches, Signalisation et Prions, Paris, France
Submission: May 05, 2018; Published: May 18, 2018
*Corresponding author:Goldberg Michel, Cellules souches, Signalisation et Prions, INSERM UMR-S1124, University Paris Cité / Faculté des Sciences Fondamentales et Biomédicales, 45 rue des Saints Pères, Paris 6, 75006, France.
How to cite this article: Michel G. What Ages First: Pulp or Dentin?. OAJ Gerontol & Geriatric Med. 2018; 4(2): 555635. DOI: 10.19080/OAJGGM.2018.04.555635
Abstract
Dental pulps stem cells have regeneration potentials. Young pulp cells convert when they mature into cell-producing dentin. In the pulp, the targeted cells are specifically pulpoblasts, fibroblasts, immune and inflammatory cells. In the coronal part of the teeth, capillaries irrigate 100-150 m round or oval domains, allowing the cleaning of continuous zones. In the root, an uninterrupted fish net-like arrangement is located at the periphery of the dental pulp. Thrombus leads to degenerative processes, or to pulp degradation. Pulp necrosis, apoptosis, or nemosis guide pulp impairment. They may influence pulp renewal. Stem cells include Dental Pulp Stem Cells (DPSCs), Exfoliated Deciduous Teeth Stem Cells (SHEDs), Platelet Derived Growth Factors (PDLSCs), Dental Follicle Precursors (DFSCs) and Apical Papilla Stem Cells (SCAPs). An ascending layer of cells issued from the apical papilla mesenchyme contributes to pulp regeneration. Initially, apical cell-rich zones are undifferentiated, and cell sliding involves the transfer from the apical part of the root to the crown, moving from the sub-odontoblastic layer to the radicular dental pulp. Linked by intercellular junctional complexes, pulp cells are interconnected by gap- and tight- junctions. They are transported toward the crown, tightly associated by intercellular junctions. In addition, lateral sliding occurs between the mesial cavities and the central pulp. Later, translocation takes place between the central pulp and the distal horn. This is obvious after an injection with Bio (a Glycogen Synthase Kinase-3 specific inhibitor implicated in regenerative medicine). After a single injection, labeled cells become scarce and in the apical papilla mesenchyme, cells slide laterally from the mesial to the distal pulp horn, where they become undetectable. As pulp cells become older, VEGF promotes blood vessel formation. The activation of the ERK pathway leads to the expression of osteogenesis-related genes, such as Cbfa1, Col I, ALP, and OCN, responsible for dentin formation and mineralization of extracellular matrix components (Tables 1 & 2). TNF-α, Notch, p38 MAPK, TGF-β, Msx1, Msx2, and JNK signaling pathways are implicated in osteogenic differentiation. Dental pulp cells, young and/or old odontoblasts/osteoblasts contribute to bone and dental tissues regeneration. Adipose tissue is another source of mesenchyme stem cells. Young pulp cells become older, producing a dentin layer that contribute efficently to geriatric odontology.

Keywords: Pulp; Dentin; Stem Cells; A Mini-Review.
Abbrevations: DPSCs: Dental Pulp Stem Cells; SHEDs: Exfoliated Deciduous Teeth Stem Cells; PDLSCs: Platelet Derived Growth Factors; DFSCs: Dental Follicle Precursors; SCAPs: Apical Papilla Stem Cells; ASCs: Adipose-Derived Stromal/Stem Cells; OPN: Osteopontin; OCL: Osteocalcin; ECM: Extracellular Matrix Components; ALP: Alkaline Phosphatases
Introduction
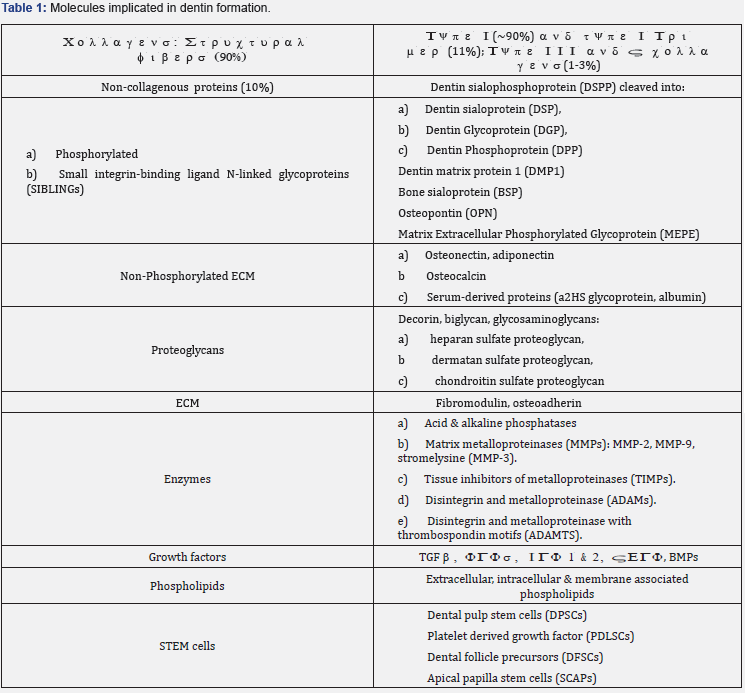
Deep carious lesions lead to irreversible pulp damage. Stem cells located in dental pulps replicate and retain potentials for regeneration [1-12]. They are implicated in the repair of defective cell types, carious lesions and genetic therapies. After the formation of a thin outer mantle dentin, a thick circumpulpal dentin is created, including the primary and secondary dentins orthodentin (tubular dentin) and osteodentin, which are developed long after the pulp formation. Reparative (or tertiary) dentin is formed after a pulp horn exposure (Figure 1).
In the pulp, the targeted cells are specifically pulpoblasts, fibroblasts, immune and inflammatory cells. Different groups of cells are concerned by pulp regeneration. They include odontoblast-like cells, a whole collection of immune cells, central and peripheral nerves, ending at close vicinity of odontoblast cell bodies. Odontoblasts involve the Raschkow’s sub-odontoblastic network. Vascular and lymphatic vessels recolonize and irrigate the wounded pulp tissue [13,14]. Altogether, these cells play a role in pulp physiology, including functionality within the central pulp (Figure 2).


In the coronal part of the teeth, capillaries irrigate 100-150 m broad domains, round or oval areas, allowing the cleaning of continuous adjacent zones [15,16]. Along the periphery of the pulp, capillaries allow peripheral vascularization and this distribution favours pulp regeneration. In the middle of the pulp, arterioles and venules are in continuity and contribute to stimulate pulp regeneration. In the root part, a fish net–like arrangement is continuous at the periphery of the dental pulp. Thrombus leads to degenerative processes, and ultimately to pulp degradation. Pulp necrosis, apoptosis, or nemosis leads either to the totality of pulp degradation, or specifically allows pulp renewal [17,18] (Figure 3).
In contrast, the formation of dentin implicates a series of molecules (Table 2). Mineralizing molecules are including adiponectin, type I collagen, alkaline phosphatase, DMP- 1, 1-dentin sialoprotein, dentin sialoprotein and dentin sialophosphoprotein, MEPE, dentin matrix metalloprotease MMP-3, MMP-9, PGs (decorine, biglycan, osteoadherin, fibromodulin) and osteopontin [19-22]. To conclude with the construction of dental tissues, first a dental pulp is formed, and later a dentin layer is deposited along the initial layer of mantle dentin (orthodentin and osteodentin). At the periphery of the pulp, odontoblasts polarize and differentiate (Figure 4).
The prevalence of caries is rather high (about 85% in the 65-74 year-old patient) and significant in the aging population. In younger patients, the 35-45 year-old group of patients, the carious prevalence is limited to 80.2%. Pulp inflammation is lower in young patients and higher in the older patient group. In this clinical context, a significant impact is related to the aging pulp.
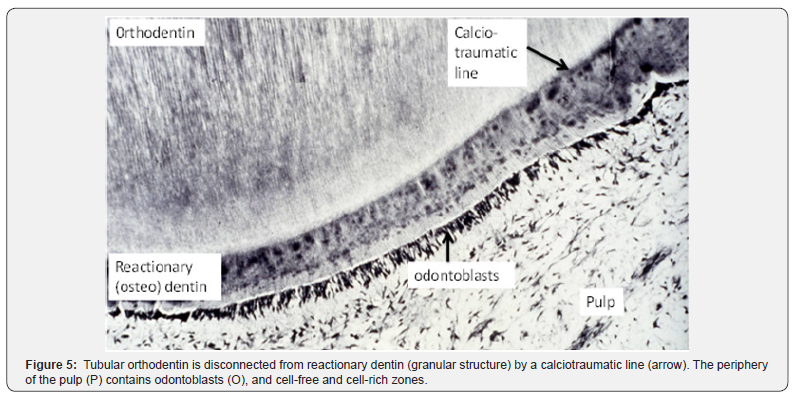
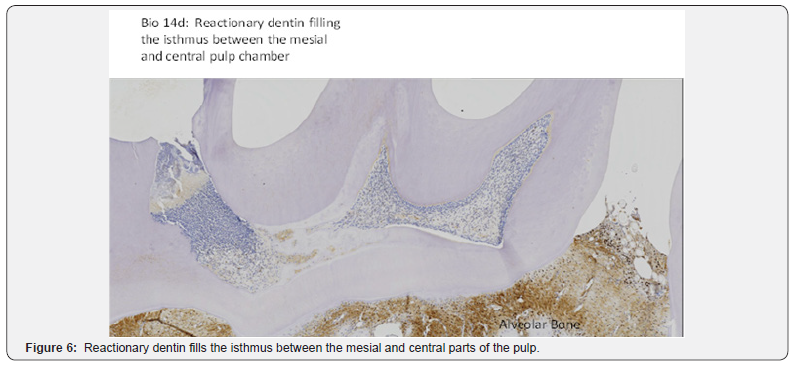
Pulp stem cells constitute a heterogeneous population. In dental tissues, stem cells include 1) dental pulp stem cells (DPSCs), 2) exfoliated deciduous teeth stem cells (SHEDs), 3) platelet derived growth factor (PDLSCs), 4) dental follicle precursors stem cells (DFSCs) and 5) apical papilla stem cells (SCAPs). Adipose-Derived Stromal/Stem Cells (ASCs) play crucial role in the treatment of craniomaxillofacial defects [23]. ASCs are committed toward an osteogenic phenotype. Angiogenesis and osteogenesis support bone regeneration. Plasma membrane-derived vesicles are important mediators in cell-to-cell communication. Growth factors, cytokines, RNAs and microRNA perform biological activities on target cells. They activate regenerative or reparative processes [18]. Bioengineering teeth may be obtained from cultured tooth bud cells [24,25] (Figures 5 & 6).
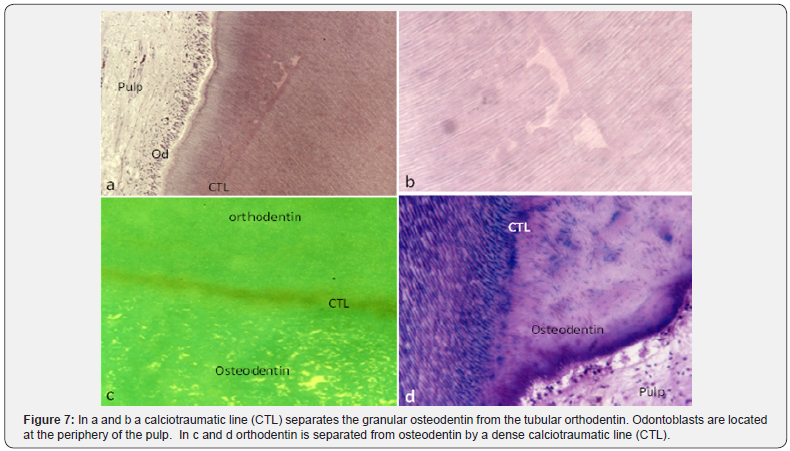
ASCs derived from pulp donors showed a high expression of osteogenic markers. This is the case for Osteopontin (OPN), Osteocalcin (OCL), and BMP-2. A high mineral content is found in the pulp and dentin of old patients [1,9,21,26] (Figure 7).
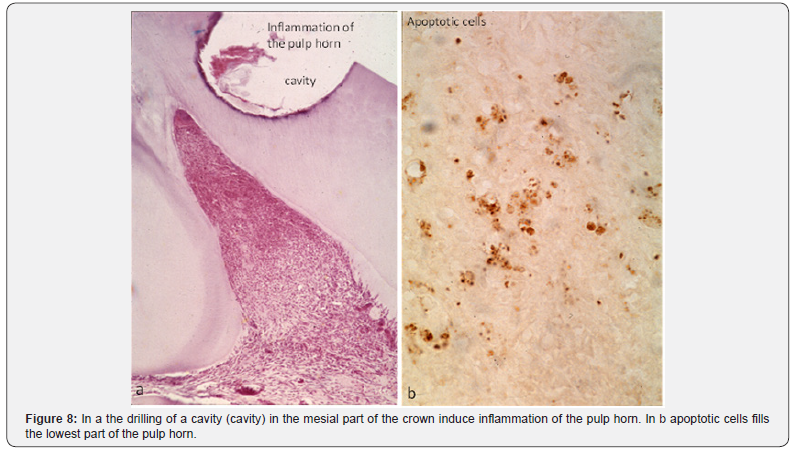
Pulp regeneration implies a cascade of cells, sliding from the apex toward the upper part of the crown. In the apical part, undifferentiated cells contribute to colonize the root. The ascending cells move beneath the odontoblast layer, and form a continuous layer that will further colonize the sub-odontoblastic layer. They proliferate, multiply and concentrate in the apical cell-rich zone. In the root, cell sliding starts near the apical part. The ascending layers of cells contribute to pulp regeneration [24,25] (Figure 8).
Initially, pulp cells are undifferentiated, and move from the sub-odontoblastic layer to the collar of the tooth. Presumably, cell sliding involves an ascending transfer from the apical part of the root toward the crown [27,28].
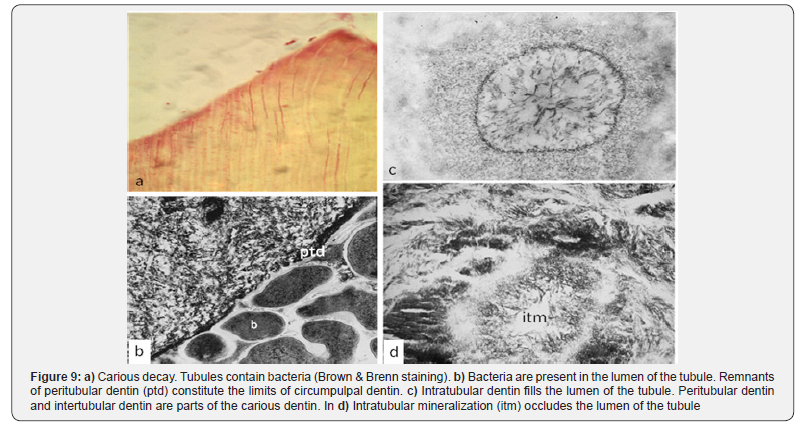
Connected by intercellular junctional complexes, namely desmosome-like junctions, pulp cells are linked by gap- and tightjunctions and they move simultaneously. They are transported along an ascending way, tightly connected by intercellular junctions. They move from the central part of the root to the periphery of the crown where they fan out [29-32] (Figure 9).
In addition, lateral sliding is occurring between mesial cavities prepared after drilling, and the central pulp horn. Afterward, translocation occurs between the central horn and the distal pulp. This is noticeable mostly for rats injected with Bio (a Glycogen Synthase Kinase-3 specific inhibitor implicated in regenerative medicine [32]). After a single injection, labeled cells become scarce in the mesial part of the pulp and they are grouped in the central pulp area. Bio-labeled cells located beneath the odontoblast layer are less numerous in the distal pulp. It comes out that cells slide laterally from the mesial pulp to the distal pulp horn whereas sliding becomes undetectable in the distal part of the pulp.
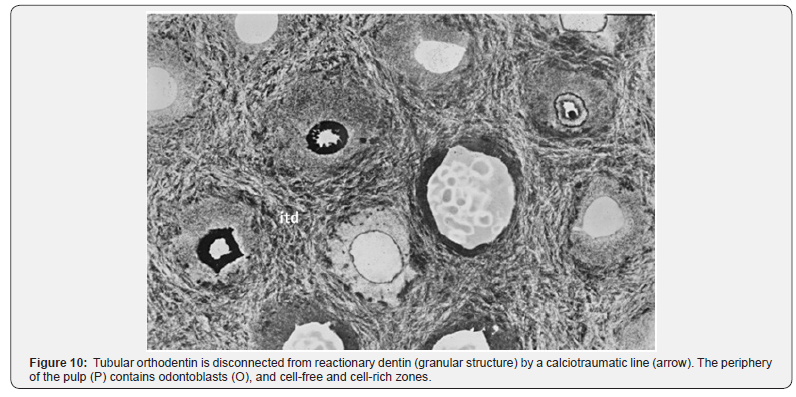
The conclusions that arise from these experimental approaches are 1) that cells slide in an ascending way from the apex toward the crown, 2) afterward, lateral sliding occurs between the mesial horn and the central/distal pulp. This evolution takes place mostly in the coronal pulp, leading to the terminal differentiation of odontoblasts. In addition, terminal differentiation was strongly linked to the strategic mesenchymal stem cells that are implicated in dentinogenesis, and angiogenesis. Pulp cells are implicated in the implantation of bioactive molecules located in the root, within the dental pulp (Figure 10).
Pulp cells are implicated in geriatric odontology. Angiogenesis shows vascular endothelial growth factor, as well as platelet-derived growth factor, and hepatocyte growth factor. IGF-1, VEGF-D and interleukine-8 improve the recruitment of undifferentiated and/or hematopoietic stem cells associated to different tooth compartments [31]. Combined with biomaterials, such as -tricalcium phosphate, bioactive glass and plateletrich plasma, the dental pulp or bone tissue display potential in pulp regeneration. Pulp renewal is also dependent of adiposederived stromal /stem cells (ASCs).
As cells become older, VEGF promotes new blood vessel formation, and they are also able to recruit hematopoietic stem cells. The activation of the ERK pathway in ASCs leads to the expression of osteogenesis-related genes, such as Cbfa1, Col I, ALP, and OCN, which appears to be responsible for pulp mineralization of Extracellular Matrix Components (ECM) [21,23,26,29,30,31].
As a conclusion, TNF-α may enhance the osteogenic differentiation of ASCs by increasing specific gene expression, such as osteopontin (OPN), runx-related transcription factor 2 (RUNX-2), and Alkaline Phosphatases (ALP) (Tables 1 & 2) [11]. Molecular investigations clearly confirmed that ERK, TNF-α, Notch, p38 MAPK, TGF-β, Msx1, Msx2, and JNK signaling pathways are strongly implicated in the odontogenic/osteogenic differentiation of ASCs [31-36].
Conclusion
Altogether, young and old dental pulp cells, young and old odontoblasts and osteoblasts contribute to bone and dental tissues differentiation/regeneration. Adipose tissue is an active source of mesenchyme stem cells. Noticeably, aging tissues contribute efficiently to geriatric odontology.
References
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411): 143-147.
- Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, et al. (2000) Odontoblast differentiation of dental human pulp cells in explant cultures. Calcif Tissue Int 66(2): 129-138.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97(25): 13625-13630
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, et al. (2001) SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100(10): 5807-5812.
- Prockop D (2007) “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 82(3): 241-243.
- Sloan A, Waddington RJ (2009) Dental pulp stem cells: what, where, how? Int J Paediatric Dentistry 19(1): 61-70.
- Goldberg M, Six N, Chaussain C, Den Besten P, Veis A, et al. (2009) Dentin extracellular matrix molecules implanted into exposed pulps generate reparative dentin: a novel strategy in regenerative dentistry. J Dent Res 88(5): 396-399.
- Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, et al. (2011) Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A 17(15-16): 1911-1920.
- Prockop D (2007) “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 82(3): 241-243.
- Goldberg M, Kulkarni AB, Young M, Boskey A (2011) Dentin: Structure, Composition and Mineralization - The role of dentin ECM in dentin formation and mineralization. Front BioSci (Elite Ed) 3: 711-735.
- Fouad SAA (2015) Dental stem cells: a perspective aera in dentistry. Int J Dental Sciences and research 3(2A): 15-25.
- Goldberg M (2017) Young vs. old dental pulp treatment: repair vs. regeneration. J Dent & Oral Disorders 3(1): 1052
- Pashley DH, Walton RE, Slavkin HC (2008) Histology and physiology of dental pulp. In Ingle JI, Balkind LK, Walton RE, Slavkin HC., Ingle’s endodontics. Endodontics (6th edn.); Hamilton, Ontario: BC Deckerm, p. 25-62.
- Volponi AA, Pang Y, Sharpe PT (2010) Stem cell-based biological tooth repair and regeneration. Trends in Cell Biology 20: 715-722.
- Takahashi K (1985) Vascular architecture of dog pulp using corrosion resin cast examined under a scanning electron microscope. J Dent Res 64: 579-589.
- Kim S (1985) Regulation of pulpal blood flow. J Dent Res 64: 590-596.
- Goldberg M, Njeh A, Uzunoglu E (2015) Is Pulp Inflammation a Prerequisite for Pulp Healing and Regeneration? Mediators Inflamm 2015: 347649.
- Goldberg M, Hirata A (2017) The dental pulp: composition, properties and functions. JSM Dentistry 1079: 1-10.
- Qin C, Brunn JC, Cadena E, Ridall A, Butler WT (2003) Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res 44(1): 179-183.
- Yasuda Y, Koiko T, Kawamorita T, Saito T (2008) Adiponectin induces dentin sialophospho protein in rat dental pulp cells: an in vitro study. J Endod 34(6): 679-683.
- Goldberg M, Lacerda-Pinheiro S, Jegat N, Six N, Septier D, et al. (2006) The impact of bioactive molecules to stimulate tooth repair and regeneration as part of restorative dentistry. Dent Clin N Am 50: 277- 298.
- Ogbureke KUE, Weinberger PM, Looney SW, Li L, Fisher LW (2012) Expressions of matrix metalloproteinase-9 (MMP-9), dentin sialophosphoprotein (DSPP), and osteopontin (OPN) at histologically negative surgical margins may predict recurrence of oral squamous cell carcinoma. Oncotarget 3(3): 286-298.
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, et al. (2016) Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review Gerontology 62(2): 216-225.
- Duailibi SE, Duailibi MT, Young CS, Vacanti JP, Bartlett JD, et al. (2004) Bioengineered dental tissue grown in the rat jaw. J Dent Res 83: 523- 528.
- Ohazama A, Modiano SA, Miletich I, Sharpe PT (2004) Stem-cell-based tissue engineering of murine teeth. J Dent Res 83(7): 518-522.
- Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons, Gaëlle C, et al. (2006) Isolation and Characterization of a Population of Immature Dental Pulp Stem Cells Expressing OCT-4 and Other Embryonic Stem Cell Markers. Cells Tissues Organs 184(3-4): 105-116.
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, et al. (2009) Characterization of apical papilla and its residing stem cells from human immature permanent teeth- a pilot study. J Endod 34(2): 166- 171.
- Wigler R, Kaufman AY, Steinbock N, Hazan-Molina H, Torneck CD (2013) Revascularization is a treatment for permanent teeth with necrotic pulp and incomplete root development. J Endod 39: 319-326.
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, et al. (2008) Dental Pulp Tissue Engineering with Stem Cells from Exfoliated Deciduous Teeth. Journal of Endodontics 34(8): 962-969.
- Atari M, Gil-Recio C, Fabregat M, Garcia-Fernandez D, Barajas M, et al. (2012) Dental pulp of the third molar: a new source of pluripotent-like stem cells. J of Cell Science. 125: 3343-3356.
- Paduano F, Marrelli M, Amantea M, Rengo C, Rengo S, et al. (2017) Adipose tissue as a strategic source of mesenchymal stem cells in bone regeneration: a topical review on the most promising craniomaxillofacial applications. Int J Molecular Sciences 18: 2140- 2152.
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3- specific inhibitor. Nature Med 10: 55-63.
- Cao Y, Song M, Kim E, Shon W, Chugal N, et al. (2015) Pulp-dentin regeneration: current state and future prospects. J Dent Res 94: 1544- 1551.
- Kenmotsu M, Matsuzaka K, Kokubu E, Azuma T, Inoue T (2010) Analysis of side population cells derived from dental pulp tissue. Int Endo J 43(12): 1132-1142.
- Albuquerque MTP, Valera MC, Nakashima M, Nör JE, Bottino MC (2014) Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 93(12): 1222-1231.
- Liu Q, Cen L, Zhou H, Yin S, Liu G, et al. (2009) The role of the extracellular signal-related kinase signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng Part A 15(11): 3487-3497.






























