Antihistamine Drugs Possess some Antiacetylcholine Properties
Krishnasarma Pathy*
IPL Research Centre, India
Submission: February 13, 2018; Published: March 08, 2018
*Corresponding author: Krishna Sarma Pathy, Head QC/QA-IPL Research Centre, Lucknow, India, Tel: 9919188895; Email: drkrishnasarmapathy@yahoo.in
How to cite this article: Krishnasarma P. Antihistamine Drugs Possess some Antiacetylcholine Properties. Nutri Food Sci Int J. 2018; 5(5): 555674. DOI: 10.19080/NFSIJ.2018.05.555674
Keywords
Keywords: Diphenhydramine hydrochloride (DPH); Parkinsonism; Sedative drugs histamine; Anti allergic, Antiemetic; Antitussive; Acetylcholine receptor antibodies; Acetylcholine
Introduction
The first antihistamine was mepyramine maleate ("neo- antergan"), and it has been sold in Great Britain under the trade name "anthisan". The aim of other manufacturers was therefore either to introduce a compound of a quite different formula with the same properties or to introduce a similar compound with a modified formula. As examples of a similar compound with a modified formula we may take tripelennamine ("pyribenzamine"), methapyrilene ("histadyl"), halopyramine (synopen), and also chlorpheniramine maleate ("piriton"), all of which are very close chemically to anthisan. Diphenhydramine, or "benadryl," differs considerably from anthisan, though both contain the grouping dimethylaminoethyl. Substances chemically close to benadryl are bromazine ("ambodryl") and diphenylpyraline ("histryl"); while chlorcyclizine ("histantin", "diparalene"), meclozine ("ancolan"), and buclizine ("vibazine") form a third group. The substances which have been steadily added to the original antihistamines mepyramine (anthisan) and diphenhydramine (benadryl) have not greatly extended the scope of therapy. They differ from the original drugs mainly in two ways-first, in their rates of absorption and excretion, and secondly in the relative strength of other properties besides antihistamine action which all compounds of this type possess. It is thus possible to choose a drug with a brief or prolonged action, a drug which is a sedative or one which is not, according to the needs of the patient. It is also often found empirically that a particular patient will respond better to one drug than to another, even though statistics show the first drug to be less potent or to produce a higher incidence of toxic side-effects than the second [1-5].
Sedative Drugs
The most strongly sedative drugs are diphenhydramine and promethazine ("phenergan"). Sometimes this sedative action is useful, but it is better not to give these drugs to patients who work during the day, and they may be dangerous when taken by drivers or people working with machinery. Moderate sedation is produced by mepyramine, tripelennamine, and bromazine, and the other drugs, though usually free from sedative effects, may induce drowsiness in some individuals. The patients should be warned of this possibility. Diphenhydramine and promethazine are also highly effective in preventing travel sickness. They are usually given for this purpose in the form of- their chlorotheophyllinate salts ("dramamine" and "avomine'). Meclozine is also effective in preventing sickness. Other antihistamine drugs have weaker anti-emetic properties; this action is not related to the antihistaminic activity. The local anaesthetic action of the drugs is a useful additional property when they are applied to the skin to relieve the itching of urticaria or insect bites. Sensitization reactions, however, are rather frequent, and antihistamine creams are best not used indiscriminately for skin diseases (British Medical Journal, 1954). The antihistamine drugs do not cause addiction. A few cases of agranulocytosis have occurred, but these have been rare considering how frequently the drugs are prescribed. They are dangerous poisons if swallowed by children, producing ataxia and convulsions which may be followed by fatal respiratory depression. The treatment (Craig, 1955) depends chiefly on gastriclavage and artificial respiration (Figure 1).
Antiacetylcholine Properties
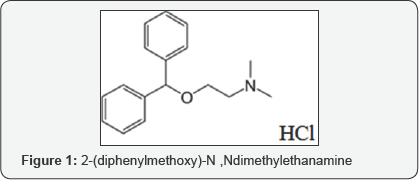
Diphenhydramine chloride is an ethanolamine antihistamine with the chemical formula C17H21NO^HCl, the IUPAC designation 2-(diphenylmethoxy)-N,Ndimethylethanamine, and the following structure: Figure 1-DPH As one ofthe most common antihistamines in production worldwide, diphenhydramine is found in particularly large quantities in municipal effluents, sediment, and in the tissues of fish (Berninger 2011). 4 Diphenhydramine hydrochloride is classified as a first-generation antihistamine and is marketed as an over-the-counter allergy medication under the brand name Benadryl®, though it is present in combination with other compounds in many medications, including Tylenol PM, Advil PM, Excedrin PM, Sudafed PM, and Robitussin. It is used to treat the symptoms associated with allergies, hay fever, and the common cold, including sneezing, runny nose, and itchy, red, and watery eyes. It also possesses a sedative effect, which makes it useful in treating insomnia or functioning as a sleep aid. Diphenhydramine has been shown to control tremors in people with early stage Parkinson's disease as well as to reduce motion sickness (Haas 2008). The primary target receptor of diphenhydramine is the histamine H1 receptor, which functions in regulating the allergic response. In an allergic response, the presence of an allergen causes mast cells to release histamine, a powerful vasodilator. Histamine binds to H1 receptors present in smooth muscle throughout the body, increasing the permeability of blood vessels to cells of the immune system and resulting in vasodilation. In the skin, these affected areas appear red and swollen, are warm to the touch, and painful-the classic symptoms of histamine-mediated inflammation. These actions also occur through the same mode of action in the GI tract and the uterus. In the respiratory tract, inflammation can result in bronchoconstriction and hinder ventilation (Haas 2008) [5-10].
Diphenhydramine is ingested orally and easily absorbed through the gastrointestinal tract and travels freely to the rest of the body, including its crossing of the blood-brain barrier. Its oral availability is 61%, and 78% is bound to plasma.
It is metabolized in the liver by cytochrome P450 2D6 is enzyme to the active metabolite nordiphenhydramine, (+dinordiphenhydramine ) and diphenylmethoxyacetic acid. 5 It reaches its peak effect after 2-3 hours, and has a total duration of 4-6 hours. 1.9% of the unmetabolized compound is excreted in the urine. Diphenhydramine has several target receptors, but it primarily acts as an H1 competitive antagonist. By binding to H1 receptors, it prevents histamine from binding and causing an allergic response (Estelle 2004). In addition to its antihistaminergic properties, diphenhydramine inhibits the reuptake of serotonin by the 5-HT reuptake transporter (SERT) at the presynaptic cleft. This discovery led to the synthesis of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, which are marketed as antidepressants in medications including Prozac (Estelle 2004). Diphenhydramine also functions as an anticholinergic agent in the central nervous system where binds to muscarinic cholinergic receptors is. This contributes to its effectiveness in treating Parkinson’s disease as well as Alzheimer’s disease. It also functions as an anticholinergic agent in the parasympathetic division of the autonomic nervous system. In a cholinergic neuron, acetylcholine (ACh), a neurotransmitter, is released from the presynaptic neuron as a result of depolarization. It travels through the synaptic cleft and binds to muscarinic cholinergic receptors in the postsynaptic neuron, resulting in depolarization. Diphenhydramine competitively binds to the muscarinic cholinergic receptors, inhibiting ACh from binding and causing an increase of ACh in the synaptic cleft (Estelle 2004). Though diphenhydramine is a relatively safe medication, is does have several side effects (Koppel 1987). It can cause depressed alertness, drowsiness, impaired psychomotor performance, agitation, confusion, and irritability. When taken in excess, it 6 may cause tachycardia, convulsions, seizures, and serious cardiac complications, likely caused by its anticholinergic properties [10-15].

Diphenhydramine hydrochloride is the active pharmaceutical ingredient in several widely used medications (e.g., Benadryl, Zzzquil, Tylenol PM, Unisom), and its worldwide demand ishigher than 100tons/year. In 2013, Jamison and co-workers developed a continuous flow process for the synthesis of 3 minimizing waste and reducing purification steps and production time with respect to existing batch synthetic routes (Scheme 1). In the optimized process, chlorodiphenylmethane 1 and dimethylethanolamine 2 were mixed neat and pumped into a 720|iL PFA tube reactor (i.d. = 0.5mm) at 175 °C with a residence time of 16 min. Running the reaction above the boiling point of 2 and without any solvent resulted in high reaction rate. Product 3, obtained in the form of molten salt (i.e., above the melting point of the salt), could be easily transported in the flow system, a procedure not feasible on the same scale under batch conditions. The reactor outcome was then combined with preheated NaOH 3M to neutralize ammonium salts. After quenching, neutralized tertiary amine was extracted with hexanes into an inline membrane separator. The organic layer was then treatedwith HCl (5M solution in iPrOH) in order to precipitate diphenhydramine hydrochloride 3 with an overall yield of 90% and an output of 2.4g/h (Figure 2).
Another process first monobrominate a benzene ring using Br2 and FeBr3, a catalyst. Then added lithium and form an organolithium then react 2 equivalents of the organolithium with methanoic acid. The first organolithium will abstract a proton from the acid, and the second equiv. will attack the carbonyl carbon. The sp3 intermediate will be frozen in time, until we perform the work-up step that will produce an aldehyde. Work-up - H30+/H 20 At this point, add another equivalent of our phenyl organolithium. Then after adding SOCl2 to turn into an acyl chloride. To mix the acyl chloride with our product from then O- (that was coordinating with Li+) will attack the acyl chloride (hard goes w/ hard) and undergo an addition-substitution type reaction. Next, simply remove the C=O group using hydrazine and base [15-20].
Conclusion
Some of the drugs have a more prolonged action, and adequate treatment is possible with a single daily dose. The non-sedative drugs chlorcyclizine and diphenylpyraline may be given once daily. Chlorpheniramine is made in double tablets, in which half the dose is in the outer shell and half in a coated inner core for delayed action; they are effective for 8-10 hours. Promethazine also has a long-lasting action. Because of its sedative properties it should be given at night, and on the following day it continues to exert antihistaminic activity after the sedative effect has worn off. Meclozine is also given at night. When avomine and ancolan are taken to prevent travel sickness they should be taken on the night before the journey; dramamine should be taken every four hours . amount needed by the same patient may also vary at different times, depending on such factors as the weather (for hay-fever subjects) and emotional stress. When a rapid action is required an antihistaminic drug may be injected, and those which are suitable for injection are supplied in injectable form. Triprolidine ("actidil") is reported to act more quickly than the other drugs when given orally. Several of the drugs are supplied as creams and ointments, but they should not be applied too freely to the skin. Antazoline ("antistin"), which is the weakest and also the least toxic, is supplied as the phosphate for application to the eye and the nasal mucous membrane.
References
- Blaschko H, Richter D, Schlossman H (1937) The oxidation of adrenaline and other amines. Biochem J 31(12): 2187-2196.
- Bliss CI (1935) Quantitative Ecotoxicology. Ann appl Biol 22: 134-307.
- Chapman NB, James JW, Graham JDP, Lewis GP (1951) British Journal of Pharmacology and Chemotherapy. Chem Ind (Rev.), pp. 633.
- Chen G, Nash VL, Russell D (1950) An evaluation of adrenergic blocking agents. Arch Int Pharmacodyn Ther 84(2-3): 269-275.
- Gaddum JH (1943) Introductory address Part I. Biological aspects: the antagonism of drugs. Trans Faraday Soc 39: 323-332.
- Graham JDP (1949) The Inhibition of Adrenaline By Spasmolytic Agents. JPharm Pharmacol 1(1): 17-27.
- Graham JDP, Lewis GP (1952) Relationship between anti-adrenaline and antihistamine activity in a series of beta-haloethylamines. JPhysiol 116(2): 37p-8p.
- Heymans C, Regniers P (1929) The Antihistamine and Antiadrenaline Properties of a series of n-naphthylmethyl-2-haloethylamine derivatives. Arch int Pharmacodyn 36: 116.
- Kapeller-Adler R (1949) Studies on histaminase. Biochem J 44(1): 7077.
- Loew ER, Micetich A (1948a) J Pharmacol 94: 339.
- Nickerson M (1949) The Pharmacology adrenergic. J Pharmacol (Rev) 95: 27.
- Goodman LS (1945) Proc Amer Fed ciin Res 2: 109.
- Nickerson M, Gump WS (1949) The chemical basis for adrenergic blocking activity in compounds related to dibenamine. J Pharmacol Exp Ther 97(1): 25-47.
- Nickerson M, Harris FB (1949) Antihistaminic properties of the P-haloalkylamine (dibenamine) series of adrenergic blocking agents. Fed Proc 8: 321-322.
- Nickerson M, Nomaguchi GM (1958) Adrenergic Blocking Action of Phenoxyethyl Analogues of Dibenzamine. J Pharmacol exp Ther 101: 379-396.
- Ickerson M, Smith SM, Goodman LS (1946) The prevention of epinephrine-cyclopropane cardiac irregularities in dogs with dibenzyl- Beta-chloroethyl amine. Fed Proc 5(1 Pt 2): 195.
- Tickner A (1951) Inhibition of Amine Oxidize by Antihistamine Compounds and Related Drugs. Brit J Pharmacol 6(4): 606-610.
- Rankin J (2010) What drugs are known to cause memory loss? Livestrong Accessibility verified: April 20, 2012.
- Campbell NL, Boustani MA, Land KA, Gao S, Hendrie H, et al. (2010) Use of anticholinergics and the risk of cognitive impairment in an african american population. Neurology 75(2): 152-159.
- Indiana University School of Medicine. Medications found to cause long term cognitive impairment of aging brain, study finds. ScienceDaily (2010). Accessibility verified: April 20, 2012.






























