- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Paleolithic Diets and Metabolic Risk Factors
Philipe Chauveau1,2*, Laetitia Koppe3, Denis Fouque3, Christian Combe1,2,3 and Michel Aparicio2
1Aurad-Aquitaine, Gradignan, France
2Service de Néphrologie Transplantation Dialyse, Centre Hospitalier Universitaire de Bordeaux, France
3Department of Nephrology, Université de Lyon, France
4Unité INSERM 1026, Univ Bordeaux, France
Submission: January 16, 2018; Published: February 28, 2018
*Corresponding author: Philipe Chauveau, Aurad-Aquitaine, Gradignan, France, Service de Nephrologie Transplantation Dialyse, Centre Hospitalier Universitaire de Bordeaux, Hopital Pellegrin, Bordeaux, France, Tel: +33-557350242; Fax: +33-557350243; Email: ph.chauveau@gmail.com
How to cite this article: Chauveau P., Koppe L. Fouque D., Combe C. and Aparicio M, et al. Paleolithic Diets and Metabolic Risk Factors. Nutri Food Sci Int J. 2018; 5(4): 555669. DOI: 10.19080/NFSIJ.2018.05.555670.
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Abstract
Eaton and Konner, more than thirty years ago, proposed that the evolutionary discordance between our genome, which hardly changed since the paleolithic era, and our contemporary diet was one of the main causes of some chronic metabolic diseases in western societies. It has been suggested that reversion to the paleolithic diet (PD) could be proposed to correct or prevent the development of diseases of civilization which is observed in contemporary hunter-gatherer (HG) populations after westernization (reduced physical activity and consumption of an energy-dense diet). There is no universal paleolithic dietary pattern, but a wide variety of possibilities according to the climate and geographical latitude. Notwithstanding the detailed type of diets, PDs have in common the complete exclusion of refined cereal grains, dairy products, refined fats and sugars.
Data from 3 uncontrolled trials and 6 randomized controlled trials (RCT), comparing PD with several reference diets were reported in the medical literature. PD resulted in improvement of anthropometry, blood pressure control, and lipid profile and insulin sensitivity.
Among the nutritional characteristics of PD, lower total energy, changes in fatty acids composition, consumption of complex carbohydrates resulting in a lower glycemic load and lower serum insulin concentration, high fiber content, strong increase in potassium intake and reduced sodium intake, lastly high vitamin and phytochemical content, all contribute to these favorable outcomes.
Before recommending such a diet for preventing the epidemic development of diseases of civilization, further larger trials adequately designed will be required to confirm the encouraging results of early studies.
Keywords: Paleolithic diet; Metabolic disease; Diabetes diet; Metabolic syndrome; Waist circumference
Abbreviations: PD: Paleolithic Diet; PD: Paleolithic Diet; MD: Mediterranean Diet; D-Diet: Diabetes Diet; MetS: Metabolic Syndrome; NNR: Nordic Nutrition Recommendation; ADA: American Diabetes Association recommendations; AGHE: Australian Guides to Healthy Eating; BW: Body Weight; BMI: Body Mass Index; WC: Waist Circumference
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Introduction
Contemporary western type-diet and modern lifestyle are considered as mainly responsible for the rapidly increasing rates of the epidemic « diseases of civilization »: insulin resistance and type II diabetes, cardiovascular diseases, hypertension, stroke and obesity. On the contrary, hunter- gatherer (HG) societies were largely free of these diseases [1]. When HG societies adopted western-type foods and lifestyle, their health worsened and diseases of civilization became commonplace [2]. On the other hand, when HGs were temporarily reversed to their traditional diet and lifestyle, they showed marked improvement of metabolic syndrome and blood pressure [3]. According to these results, it was suggested that a return to the PD would be able to correct or prevent these civilization diseases.
Since the seminal paper by Eaton & Konner [4], PDs have gained considerable attention and become a trendy health topic: « Paleo » was the most searched diet-related term on Google in 2013 and 2014 [5]. Sometimes called the « Caveman Diet » or the « Old Stone Age Diet », PD has received wide media coverage and is currently praised by celebrity proponents [6].
PDs were based on estimation of food eaten during the Paleolithic era, before the advent of agriculture and animal husbandry i.e., 2 million - 10000 years ago. Such types of diet included lean meat from animals that grazed, fish and shellfish, uncultivated fruits and vegetables, roots, eggs and nuts while they required the complete exclusion of refined cereal grains, dairy products, legumes, refined fats and sugars which make up nearly 75% of the dietary energy in western countries today. Lastly, heating was the only food-processing procedure [7].
There was no universal PD but a wide range of possibilities according to local availability of food depending on the climate and geographical latitude in which HG populations lived. We do not actually know with certainty what Paleolithic humans ate, but it is estimated that, except in circumpolar environment, the average macronutrient energy distribution was 22-40% carbohydrates, 19-35% protein and 28-58% fat.
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
Several studies on the health of HG populations have shown that prevalence of cardiovascular and metabolic risk factors was much lower in contemporary HG populations than in western populations. In a study of the inhabitants of Kitava, Papua New Guinea, it was reported that this population was free of overweight, hypertension, ischemic heart disease and stroke despite a non-negligible smoking rate and a lipid profile which was not more favorable than westerner's [8]. Fasting serum glucose was two standard deviations below the westerner, mean insulin concentration in older adults was 50% lower than in their westerner counterparts. It was concluded to a virtual absence of insulin resistance and of type II diabetes in this population. HG are lean, fit and free from signs and symptoms of chronic diseases, these results have been attributed to their lifestyle: diet (food supply and energy intake) and physical activity [8].
Because of their putative health benefits, Paleolithic-style diets (PDs) that attempt to approximate the characteristics of ancestral diets have been proposed in western populations to correct metabolic risk factors and their consequences. However, there is a paucity of research data that showed clinical benefits. A systematic review and meta-analysis of the effects of PDs on metabolic risk factors only mentioned 3 uncontrolled trials, in which subjects were studied as their own controls, and no more than 5 randomized controlled trials (RCT). These latter compared the short-term effects of PD with those of other dietary intervention reported to have health benefits on component variables of the metabolic syndrome, i.e. a cluster of 5 risk factors: waist circumference, blood pressure, serum concentrations of glucose, triglycerides and HDL cholesterol in fasting conditions [5].
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Uncontrolled Trials (Table 1)
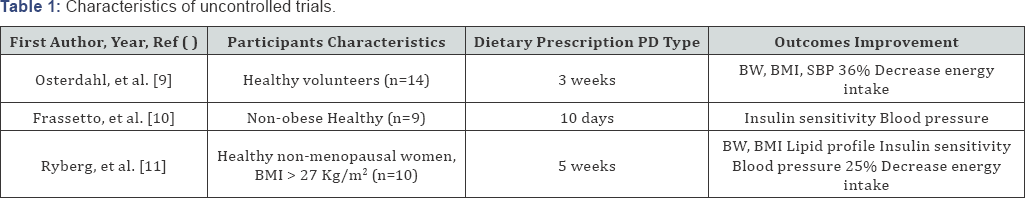
Three uncontrolled trials ranging from 10 days to 5 weeks were quoted; they all addressed a small number of healthy volunteers. In a pilot study, 14 healthy volunteers were proposed ad libitum consumption of a PD-type diet over 3 weeks. Compared to baseline values, energy intake was decreased by 36% as intake of fat, especially saturated fat, and carbohydrates decreased significantly. At the end of the study, there was a mean decrease of 2.3kg, 0.8kg/m2 and 1.5cm in body weight, BMI and waist circumference respectively, systolic blood pressure was slightly reduced by 3mm Hg. It was concluded that PD resulted in favorable effects on metabolic risk factors [9].
Another metabolically controlled study performed in 9 non-obese sedentary healthy volunteers, compared a PD to their usual diet. After only 10 days on a paleo-type diet, total cholesterol, LDL cholesterol and triglyceride levels were reduced by 16%, 22% and 35% respectively. Fasting insulin was decreased by 68% and insulin area under curve during a 2h oral glucose tolerance test was reduced by 39%. Significant reductions in systolic (-2.6mmHg) and diastolic blood pressure (-3.4mmHg) were observed associated with improved arterial distensibility, these changes being unrelated to body weight. It was concluded that even short-term consumption of paleo- type diet improved metabolic risk factors [10].
The last uncontrolled study reported on 10 healthy postmenopausal women with a BMI >27kg/m2 who were placed on a paleo-type diet. Five weeks later, mean energy intake decreased by 25% with a weight loss of 4.5kg, waist and hips circumferences were significantly reduced as diastolic pressure: - 7mmHg. Fasting glucose (-6%), fasting insulin (-19%), total cholesterol (-19%), LDL cholesterol (-23%), triglyceride levels (-37%) and HOMA indices were reduced but whole-body insulin sensitivity (euglycemic hyperinsulinaemic clamp technique) remained unchanged. Liver triglyceride levels, assessed by proton magnetic resonance spectroscopy, decreased by 49%. Conclusions of this study were akin to those of the two previous trials [11].
The loss of weight and the improvement of insulin resistance and lipid metabolism reported in 10 Australian aborigines after a 7-week reversion to Paleolithic lifestyle could also be considered as the results of another uncontrolled study [3].
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Randomized Controlled Trials (Table 2)
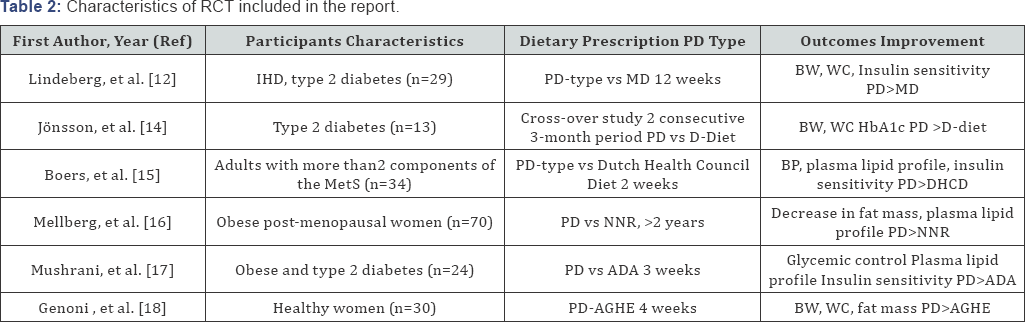
PD: Paleolitic diet; MD: Medittereanean diet; D-Diet: diabetes diet; MetS: Metabolic syndrome; NNR: Nordic Nutrition Recommendation; ADA: American Diabetes Association Recommendations; AGHE: Australian Guides to Healthy Eating; BW: Body Weight, BMI: Body Mass Index, WC: Waist Circumference
Seven RCTs are included in this review. Six of them were included in a recent meta-analysis [5]. The first RCT compared, in 29 non-hypertensive patients with ischemic heart disease plus either glucose intolerance or type II diabetes, the effects of PD and Mediterranean-like diets (MD). Over 12 weeks, weight loss was on average 4.4kg with no significant group difference. Glucose tolerance was significantly improved in the PD group, independently of energy intake and macronutrient composition [12].
The second RCT was a randomized cross-over pilot study which compared the effects of PD and a diabetic-like diet in 13 type II diabetes over two consecutive three-month periods. When consuming a PD, patients demonstrated a 33% decrease in triglycerol levels and 5% reduction of diastolic blood pressure levels compared with baseline [13].
The third included RCT studied 34 adults with 2 or more components of the Met S who were randomized for two weeks to a paleo-type diet or an iso-energetic reference diet based on the Dutch Health Council guidelines. Over a two-week period, the paleo-type diet resulted in lower blood pressure and improved plasma lipid profile, compared to diet reference [14].
The fourth RCT studied the long-term effects on anthropometric measurements and metabolic balance of a paleo-type diet and of a reference diet based on the Nordic Nutrition Recommendations. Seventy obese post-menopausal women (mean BMI of 33kg/m2) were assigned to an ad libitum diet over a 2-year period. Despite a poor adherence to the target protein intake, both groups decreased total fat mass, measured by DEXA During this long-lasting study, a total of 30% of the participants were lost to follow-up, the percentage of drop-outs being higher in the controlled group [15].
The fifth RCT was a small randomized metabolically controlled diet which compared for 3 weeks in 24 obese patients with type 2 diabetes, the metabolic effects of a PD with an isocaloric standard diet based on the recommendations by the American Diabetes Association (ADA). Average changes in weight and blood pressure were similar in the two groups. Both groups showed improvements in metabolic measures but benefits on glucose control (HbA1c, fructosamine) and lipid profile were greater with PD [16].
A most recent RCT compared 39 healthy women, randomized to either an ad libitum PD (n=22) or to the Australian Guide to Healthy Eatings diet (AGHE) (n=17) and measured the effects of the two diets on anthropometric, cardiovascular and metabolic risk factors. After the four-week dietary intervention, a significantly greater decrease of body weight (-4.3%), fat mass and waist circumference (-3.8%) was observed in the PD group compared to the AGHE group [17].
To summarize, the results of these RCTs showed that, most often, PD resulted in greater improvement on anthropometry, blood pressure and on most of the components of the metabolic syndrome than did reference diets: i.e. Mediterranean diet, diabetic-like diet, ADA diet or other currently recommended guideline-based control diets. PDs appear to be more satiating per calorie than other diets in relation with a high protein content, low carbohydrate and low energy-dense food [18]. These positive data nevertheless call for some restrictive considerations.
All the studies were heterogeneous in design and underpowered. The number of trials was limited. Half of the studies have been performed by the same group. In most of the studies the number of subjects was limited (9 to 70) and duration of the study was short (10 days to 3 months). However, it is noticeable that improvement of markers of the metabolic syndrome occurred after only a short period of time and lasted until the end of the single long-lasting study [15]. There was no information on the outcome of overall quality of life neither on adverse event; only a greater frequency of diarrhea has been reported in two studies, maybe due to changes in fibre consumption. Despite recommendations of ad libitum consumption of diets, target intakes were not fully achieved.
Another limitation could be the compliance and adherence to the diet. Patients often complained that PD was somewhat monotonous and difficult to adhere to, absence of milk, legumes and cereal products can contribute to this difficulty and may represent a significant hurdle for long-term compliance. There was a poor adherence to the protein intake target and a profound decrease in energy intake (- 20% to -36%) which resulted in a weight loss (-1.3kg to -8kg) and reduced BMI even when measures were taken to keep body weight stable (additional snacks provided to participants who lost > 2kg BW without being hungry) [14].
However, despite these reservations, there were less dropouts in PD groups than in control groups. During the 2-yr study of Mellberg et al. [11], 21 out of 70 participants were lots of follow-up, 8 in the PD and 13 in the Nordic Nutrition Recommendations, respectively.
Another caveat of a PD is its increased cost which may limit adherence. PD is approximately 10% more expensive than a usual diet of similar nutritional value, and it has been accurately estimated that a 9.3% increase in yearly income would be required to adopt such diet [19].
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
Since the paleolithic era, changes in food staples and the advent of food processing resulted in modifications of some fundamental nutritional characteristics concerning macronutrients and some selected micronutrients [20]. Many of the components of PD absent or strongly modified in Western diets would contribute to the reduction of cardiovascular disease risks when applied to modern-day humans:
Protein
Hunter-gatherer (HG) protein consumption varied with latitude but provided usually at least 30% of energy intake in PD, exceeding RDA recommendations. Although there was no correlation between protein intake and satiety quotients [21], high-protein diets have been reported to produce a sustained decrease of appetite and ad libitum calorie intake, facilitating weight loss which is desirable in the treatment of type II diabetic patients [22].
Fat
Present percentage of energy intake, cholesterol intake was similar or even higher than that of modern-day humans, but it is well known that dietary cholesterol does not greatly contribute to serum cholesterol or its fractions. By contrast, LDL cholesterol-raising saturated fats did not exceed 6% of total energy intake [7]. Compared with Western-type diets, amounts of monounsaturated fatty acids (MUFAs) were 1.5 to 2.O times higher in PD and contributed to more than 30% of total energy in replacement of carbohydrates [23]. Levels of long-chain omega-3 fatty acid, which came from grass- fed animals and from wild plants, were several times higher resulting in an omega-3/omega-6 ratio of about 1/1, which contrasts with a ratio close to 1/15 currently observed in the United States and in Western and Northern Europe [24]. Such an omega-3/omega-6 ratio was associated with a strong improvement in inflammatory markers and lipid profile providing protection from CVD. In Inuit diets, this ratio, which did never exceed 1.0, was associated with very low triglyceride levels and prevention of CVD. Compared with the Greenland Inuits, CVD mortality has been estimated to be 7 times greater among US and European populations [25]. Lastly, high fat content of PD is also a potent stimulator of satiety and prevents high energy intake and weight gain.
Carbohydrate
Consumption which represents more than 50% of energy intake in modern-style diet was lower in PD, ranging from 22% to 40% of total energy intake. Nearly all carbohydrates came from fruits, vegetables and nuts, not from grains and had low glycemic index which attenuates post prandial glucose and insulin responses which are strong independent predictors of CV events [26]. There was no refined sugar except for seasonally available honey to be compared with the 15% from added sugars in the western diets. Moreover, a reduction in carbohydrate, per se has beneficial effects on lipid profile. A recent meta-analysis of 23 RCT from multiple countries which compared the effects of low-carbohydrate diets (<45% of energy) and low-fat diets on metabolic risk factors showed that, when carbohydrates are replaced by monounsaturated fatty acids (MUFAs), healthy adults receiving low-carbohydrate diets experienced a similar reduction in body weight and waist circumference [27].
Fibre
Uncultivated vegetables and fruits contain more than 3 times fibres than their commercial counterparts. PDs provided total fibres from 80g/d to 150g/d, with a 1:1 ratio of soluble to insoluble fibres, whereas current recommendations suggest 25g/d to 34g/d. Besides their advantages on GI tract, dietary fibres have favourable effects on CV risk factors. Results of the recent INTERMAP Study have shown that higher intake of fibres, especially insoluble ones, may contribute to lower blood pressure [28]. This study confirms previous meta-analyses indicating that increased intake of dietary fibres may contribute to the prevention of hypertension or to the reduction in blood pressure in patients with hypertension [29]. Meta-analyses also showed that moderate doses of soluble fibres resulted in a sustained LDL-cholesterol and triglycerides lowering effect [30]. As phytic acid is almost absent from uncultivated fruits and vegetables, the high fiber content of PD most probably had a limited impact on minerals availability.
Sodium-potassium ratio
In PDs, sodium content was 4 to 5 times lower and potassium 3 to 4 times higher than in modern-style diet, Na/K ratio which was 0.073, was reversed to be approximately 1.28 in the western diet because of a strong increase in Na and a reduction in K intakes [31]. Most of Na intake in our food comes from manufactured salt (90% of salt consumption), while only 10% is intrinsic to the eaten food. The reduction of K intake is explained by the displacement of food rich in K+, which are also usually rich in CO3H- yielding precursors (fruits and vegetables), by potassium-poor whole grains, dairy products, vegetable oils and refined sugars. In these circumstances, less than 10% of the US adults meet recommendations for Na intake (<2300mg/d) and only 5% of US men meet recommendations for K intake (>4700mg/d) [32]. Supplementing dietary potassium attenuates the pressor effect of NaCl through diuretic and natriuretic effects. Patients with high blood pressure would benefit from a reduction of salt intake and a simultaneous increase of potassium intake as obtained during PD [33].
Acid-base balance
PDs were net base yielding, because many plant-based foods are a key source of K+ and Mg++, known as major contributors to the dietary alkali load. The mean net endogenous acid production for 159 retrojected ancestral pre-agricultural diets was estimated to be -88mEq/d, 87% of them were net- base producing [34]. On the other hand, modern diets which are rich in acid-producing foods and poor in K+ alkali salts, and the accompanying modern processing and preparation of foods which lead to losses of base-forming nutrients, result in a mean net acid load of about 1mEq/kg/d [35]. PDs may prevent the negative effects of the low-grade systemic metabolic acidosis frequently observed in healthy adults consuming western diets. This may result in various deleterious effects, among which renal calcium wasting [36]. The calcium content in PD is approximately 50% lower compared to the reference diets but the large intake of K+ and HCO3- precursors reduce bone calcium efflux and renal excretion of calcium [37]. Reduced calcium intake is also counterbalanced by the higher dietary Mg++ intake. Studies of bone size and structure of ancient skeletons found in archaeological sites around the world showed that bones were « stronger » in hunting and gathering diets and « weaker » in agricultural ones [38].
PD is also rich in antioxidants
High amounts of vitamin C, vitamin E, beta-carotene, selenium, folate, phenolic compounds and other phytochemicals from green leafy vegetables, all associated with a reduced risk of CV disease. A recent pooled cross-sectional study confirmed that PD pattern scores were negatively associated with biomarkers of oxidative stress [39]. Similarly, to Mediterranean and Okinawan healthful diets, PDs are rich in nuclear factor erythroid 2-related factor 2 (Nrf2) raising nutrients, particularly plants with low calorie densities which provide more phytochemicals, unlike Western diets which are currently deficient in these nutrients. The deficiency of this transcription factor may contribute to the high predominance of diseases afflicting modern populations. Indeed, activation of Nrf2 raises the transcription of over 500 genes, most of which having cytoprotective properties with respect to oxidative stress, inflammation and mitochondrial dysfunction which all characterize the current diseases of civilization. It is interesting to note that exercise and caloric restriction which are part of paleolithic lifestyle also promote Nrf2 activity [40].
Gut microbial
Lastly, gut microbiota, whose richness and diversity are associated with better state of health and metabolic profile, is another potential target of PD. Indeed, it has been recently shown that CKD patients present profound alterations of the gut microbiota composition which may contribute to inflammation, oxidative stress, uremic toxicity, malnutrition and other morbidities [41]. During human evolution, due to social and demographic changes, human microbiomes lost ancestral microbial diversity including organisms able to ferment fibre-rich dietary components and became specialized for animal-based diets leading to intense transformation of gut commensal flora [42]. Whether related to food intake or to chronic disease, loss of bacterial diversity has made the human gut microbiota a pro-inflammatory, pro-atherogenic or insulinoresistant-inducing « inner organ » [43]. As gut microbiota rapidly alters its cellular composition in response to dietary shifts, [44], PD-induced changes in the microbiota should be further studied in westerner and chronic disease patients, in large clinical trials, to ascertain whether PD may positively influence microbiological diversity with health consequences [45].
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
Conclusion
Clinical trials have suggested that, in some instances, PDs, for which we were genetically best programmed, reduce some risk factors for metabolic syndrome and cardiovascular consequences. However, PD may not the dietary Holy Grail and it is not discussed here to reproduce a whole Paleolithic dietary model. However certain characteristics of that life style, diet and physical activity, must be taken into account before initiating some health program to prevent or correct the deleterious effect of some diseases of civilization. Among the main concerns, compliance to PD which is found somewhat monotonous by patients could be improved by the addition of some food groups of the western-style diet such as grains, legumes and dairy products to make it more palatable.
- Review Article
- Abstract
- Introduction
- Effects of Paleodiet on the Components of the Metabolic Syndrome (Met S)
- Uncontrolled Trials (Table 1)
- Randomized Controlled Trials (Table 2)
- Paleo and Decrease of Risk Factors for Metabolic Syndrome and Cardiovascular Disease how does it Work?
- Conclusion
- References
References
- Cordain L, Miller JB, Eaton SB, Mann N, Holt SH, et al. (2000) Plant- animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets Am J Clin Nutr 71(3): 682-692.
- 0'Dea K (1991) Westernisation, insulin resistance and diabetes in Australian aborigines. Med J Aust 155(4): 258-264.
- O'Dea K (1984) Marked improvement in carbohydrate and lipid metabolism in diabetic Australian aborigines after temporary reversion to traditional lifestyle. Diabetes 33(6): 596-603.
- Eaton SB, Konner M (1985) Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 312(5): 283-289.
- Manheimer EW, van Zuuren EJ, Fedorswicz Z, Pijl H (2015) Paleolithic nutrition for metabolic syndrome : systematic review and metaanalysis. Am J Clin Nutr 102(4): 922-932.
- Chang ML, Nowel A (2016) How to make stone soup: is the « Paleo diet » a missed opportunity for anthropologists?. Evol Anthropol 25(5): 228-231.
- Konner M, Eaton SB (2010) Paleolithic nutrition: twenty-five years later. Nutr Clin Pract 25(6): 594-602.
- Lindeberg S, Cordain L, Eaton SB (2003) Biological and clinical potential of a paleolithic diet. J Nutr Environ Med 133(3): 149-160.
- Osterdahl M, Kocturk T, Koochek A, Wandell PE (2008) Effects of a short-term intervention with a paleolithic diet in healthy volunteers. Eur J Clin Nutr 62(5): 682-685.
- Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC, Sebastian A (2009) Metabolic and physiologic improvements from consuming a paleolithic hunter-gatherer type diet. Eur J Clin Nutr 63(8): 947-955.
- Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, et al. (2013) A paleolithic-type diet causes strong tissue-specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med 274(1): 67-76.
- Lindeberg S, Jonsson T, Granfeldt Y, Borgstrand E, Soffman J, et al. (2007) A Paleolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 50(9): 1795-1807.
- Jonsson T, Granfeldt Y, Ahren B, Branell UC, Palsson G, et al. (2009) Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over. Cardiovasc Diabetol 8: 35.
- Boers I, Muskiet FA, Berkelaar E, Schut E, Penders R, et al. (2014) Favourable effects of consuming a Paleolithic-type diet on characteristics of the metabolic syndrome : a randomized controlled pilot-study. Lipids Health Dis 13: 160.
- Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, et al. (2014) Long-term effects of a Paleolitic-type diet in obese post-menopausal women : a two-year randomized trial. Eur J Clin Nutr 68(3): 350-357.
- Masharani U, Sherchan P, Schloetter M, Stratford S, Xiao A, et al. (2015) Metabolic and physiologic effects from consuming a hunter-gatherer (Paleolithic)- type diet in type 2 diabetes. Eur J Clin Nutr 69(8): 944948.
- Genoni A, Lyons-Wall P, Lo J, Devine A (2016) Cardiovascular, metabolic effects and dietary composition of ad libitum Paleolithic vs Australian Guide to Healthy Eating Diets: a 4-week randomized trial. Nutrients 8(5): 314.
- Jonsson T, Granfeldt Y, Erlanson-Albertsson C, Ahren B, Lindeberg S (2010) A Paleolithic diet is more satiating per calorie than a Mediterranean-like diet in individuals with ischaemic heart disease. Nutr Metab 7: 85.
- Genoni A, Lo J, Lyons-Wall P, Devine A (2016) Compliance, palatability and feasibility of Paleolithic and Australian Guide for Healthy Eating diets in healthy women: a 4-week dietary intervention. Nutrients 8(8): E 481.
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, et al. (2005) Origin and evolution of the Western diet : health implications. Am J Clin Nutr 81(2): 341-354.
- Jonsson T, Granfeldt Y, Lindeberg S, Halberg AC (2013) Subjective satiety and other experiences of a Paleolithic diet compared to a diabetes diet in patients with type 2 diabetes. Nutr J 12: 105-112.
- Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, et al. (2005) A high protein diet induces sustained reduction in appetite, ad libitum caloric intake and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 82(1): 41-48.
- Kuipers RS, Joordens JC, Muskiet FA (2012) A multidisciplinary reconstruction of Paleolithic nutrition that holds promise for the prevention and treatment of diseases of civilization. Nutr Res Rev 25(1): 96-129.
- Simopoulos AP (2001) The Mediterranean diets: what is so special about the diet of Greece? The scientific evidence. J Nutr 131(11 Suppl): 3065S-3073S.
- Simopoulos AP (2002) Omega-3 fatty acids and cardiovascular disease: The epidemiological evidence. Environ Health Prev Med 6(4): 203-209.
- Riccardi G, Rivellese AA, Giacco R (2008) Role of glycemic index and glycemic load in the healthy state, in prediabetes and in diabetes. Am J Clin Nutr 87(1): 269S-274S.
- Hu T1, Mills KT, Yao L, Demanelis K, Eloustaz M, et al. (2012) Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol 176(Suppl 7): S44 -S54.
- Aljuraiban GS, Griep LM, Chan Q, Daviglus ML, Stamler J, et al. (2015) Total insoluble and soluble dietary fiber intake in relation to blood pressure: the INTERMAP Study. Br J Nutr 114(9): 1480-1486.
- Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, et al. (2005) Effect of dietary fiber intake on blood pressure: a meta-analysis of radomized controlled clinical trials. J Hypertens 23(3): 475-481.
- Tiwari U, Cummins E (2011) Meta-analysis of the effect of beta-glucose intake on blood cholesterol and glucose levels. Nutrition 27(10): 10081016.
- Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, et al. (2012) Sodium and potassium Intakes among US adults: NHANES 2003-2008. Am J Clin Nutr 96(3): 647-657.
- Aaron KJ, Sanders PW (2013) Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc 88(9): 987-995.
- Morris RC Jr, Schmidlin O, Frassetto LA, Sebastian A (2006) Relationship and interaction between sodium and potassium. J Am Coll Nutr 25(3 Suppl): 262S-270S.
- Sebastian A, Frassetto LA, Sellmeyer DE, Merriam DL, Morris RC (2002) Estimation of the net acid load of the diet of the ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr 76(6): 1308-1316.
- Remer T, Manz F (2003) Paleolithic diet, sweet potato eaters, and potential renal acid load. Am J Clin Nutr 78(4): 802-803.
- Frassetto LA, Morris RC Jr, Sellmeyer DE, Todd K (2001) Diet, evolution and aging. Eur J Nutr 40(5): 200-213.
- Bushinsky DA (1996) Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol 271(1 Pt 2): F216-F222.
- Larsen CS (2003) Animal source foods and human health during evolution. J Nutr 133(11 Suppl 2): 3893S-3897S.
- Whalen KA, Mc Cullough ML, Flanders WD, Hartman TJ, Judd S, et al. (2016) Paleolithic and Mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J Nutr 146(6): 1217-1226.
- Tarantino G, Citro V, Finelli C (2015) Hype or reality: should patients with metabolic syndrome-related NAFLD be on the hunter-gatherer (paleo) diet to decrease morbidity. J Gatrointestin Liver Dis 24(3): 359-365.
- Mafra D, Lobo JC, Barros AF, Koppe L, Vaziri ND, et al. (2014) Role of altered microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol 9(3): 399-410.
- Moeller AH, Li Y, Mpoudi Ngole E, Ahuka-Mundeke S, Lonsdorf EV, et al. (2014) Rapid changes in the gut microbiome during human evolution. Proc Natl Acad Sci USA 111(46): 16431-16435.
- Barros AF, Borges NA, Ferreira DC, Carmo FL, Rosado AS, et al. (2015) Is there interaction between gut microbial profile and cardiovascular risk in chronic kidney disease. Future Microbiol 10(4): 517-526.
- Logan AC, Katzman MA, Balanza-Martinez V (2015) Natural environments, ancestral diets and microbial ecology, is there a modern "paleo-deficit disorder”? Part I. J Physiol Anthropol 34: 1.
- Koppe L, Mafra D, Fouque D (2015) Probiotics and chronic kidney disease Kidney. Int 88(5): 958-966.
- Metzgar M, Rideout TC, Fontes-Villalba M, Kuipers RS (2011) The feasibility of a Paleolithic diet for low-income consumers. Nutr Res 31(6): 444-451.






























