Naturally Occurring Nrf2 Activators in the Management of Diabetes
Rashmi Rajappa', Venugopalreddy Bovilla2 and SubbaRao V Madhunapantula2*
1Division of Water and Health, Jagadguru Sri Shivarathreeshwara University, India
2Department of Biochemistry, Jagadguru Sri Shivarathreeshwara University, India
Submission: February 07, 2017;Published: March 30, 2017
*Corresponding author: SubbaRao V Madhunapantula, Professor, Center of Excellence in Molecular Biology and Regenerative Medicine (CEMR), Leader, Special Interest Group on Cancer Biology and Cancer Stem Cells (SIG-CBCSC), Jagadguru Sri Shivarathreeshwara Medical College, Jagadguru Sri Shivarathreeshwara University (Accredited “A” Grade by NAAC and Ranked 35th by NIRF ranking system of MHRD, Government of India) Mysore, 570 015, Karnataka, India, Tel: 918105278621; Email: mvsstsubbarao@jssuni.edu.in; madhunapantulas@yahoo.com
How to cite this article: Rashmi R, Venugopal R B, SubbaRao V M. Naturally Occurring Nrf2 Activators in the Management of Diabetes. Nutri Food Sci Int J. 2017; 2(4): 555595. DOI: 10.19080/NFSIJ.2017.02.555595 DOI:10.19080/NFSIJ.2017.02.555595
Abstract
Nuclear factor erythroid-2 (NFE-2)-related factor-2 (Nrf2), a key leucine zipper transcription factor that regulate the expression of antioxidant enzymes, is an important target for mitigating the complications of diabetes. Several lines of evidences have concluded that targeted activation of Nrf2 using phytochemicals helps to protect insulin secreting pancreatic p-cells thereby reduce hyperglycemia induced changes such as retinopathy, neuropathy, nephropathy, cardiomyopathy, and many other complications. Mechanistically, Nrf2 activators promote the release of Nrf2 from Keapl - Nrf2 complex by disrupting the protein - protein interactions or by promoting the degradation of Keapl in cells.
Once released, the Nrf2 translocates in to nucleus thereby trigger the transcription of genes such as NQO1, GST, HO1, HIF1 α, and many more, which are involved in controlling oxidative stress. Therefore, identification of naturally occurring Nrf2 activators and the use of diets rich in these activators is a potential strategy to control diabetes. However, further studies are warranted to test the safety and efficacy of Nrf2 activators for treating diabetes in clinical trials. In this mini-review, we have summarized the recent findings of cell-based and preclinical studies evaluating the role of Nrf2 in preventing and treating diabetes. In addition, the details of preclinical studies that have tested the Nrf2 activators are tabulated. For additional and more detailed information the readers can refer the publications listed in the references section.
Keywords: Nrf2; Diabetes; Pancreatic β-cells; Nfr2 -activators; Sulforaphane; Curcumin
Abbreviations: AGEs: Advanced Glycation End-products; AR: Aldose Reductase; CREB: cAMP Response Element Binding Protein; CUR: Curcumin; DAG: Diacylglycerol; DM: Diabetes Mellitus; eNOS: Endothelial NOS; ET1: Endothelin-1; G6pc: Glucose-6-Phosphatase Catalytic- subunit; GCL: Glutamine Cysteine Ligase; GCS: Gamma-glutamine Cysteine Synthase; Glo-1: Glyoxalase-1; GSH: Glutathione; GST: γ- Glutamyl Cysteine Synthetase; Hk2: Hexokinase 2; HO-1: Heme Oxygenase 1; ICAM1: Intracellular Adhesion Molecule-1; IDF : International Diabetes Federation; LB: Magnesium Lithospermate B; MG: Methylglyoxal; NF- kB: Nuclear Factor Kappa B; NQO1: NAD(P)H:quinone Oxidoreductase 1; Nrf2: Nuclear factor E2-related factor 2; PKC: Protein kinase C; Pkm2: Pyruvate kinase; PLA2: Phospholipase A2; ROS: Reactive Oxygen Species; RSV: Resveratrol; SFN: Sulforaphane; Slc2a1: Solute carrier family 2 member 1; TGF-α: Transforming growth factor- α; TGF-β1: Transforming Growth Factor Beta 1; VCAM 1: Vascular Cell Adhesion Molecule-1; VEGF: Vascular Endothelial Growth Factor
Introduction
Despite various cost-effective treatment strategies and public health campaigns highlighting the key risk factors, the incidence and burden due to diabetes is increasing at an alarming rate globally with an estimated 422 million individuals currently suffering from this disease. Recent predictions by IDF projected that the number of diabetics is likely to increase to 642 million by 2040 [1]. Etiologically, Type-1 and Type-2 diabetes have originated due to a significant decrease in the number of insulin- producing β-cells [2]. As a result, diabetics experience chronic hyperglycemia [3,4] and several secondary abnormalities that include loss of vision, malfunction of kidneys and ultimately coma and death [5].
Recent studies have identified that oxidative stress, caused by excess reactive oxygen species, is one of the most important causing factors for diabetes complications [6]. Moreover, since pancreatic β-cells express very low antioxidant defence enzymes, they are more susceptible to the damage caused by
Therefore, coordinated up-regulation of genes coding for detoxifying and antioxidant enzymes has been shown to be a potential therapeutic strategy against oxidative stress-induced pancreatic β-cell damage [9,10]. Use of naturally occurring photochemical is one such strategy to mitigate the toxic effects, and protect pancreatic β-cells [11]. Several recent clinical trials have also confirmed the advantages of using bioactive compounds derived from natural sources for diabetes management [12-14].
Among various phytochemicals, polyphenolic compounds had shown potent anti-diabetic properties [15]. Polyphenols can
- Activate key transcription factors such as nuclear factor erythroid-2 (NFE-2)-related factor-2 (Nrf2), a known master regulator of the antioxidant response- and phase-II detoxifying enzymes; and
- Reduce toxic reactive oxygen species in to less toxic hydroxyl radical and hydrogen peroxide [16].
Hence, in this review, we have summarized the key findings of various research studies demonstrating the anti-diabetic properties of naturally occurring Nrf2 activators. Interested readers can refer recent review articles published for more detailed information.
Role of Nrf2 in Diabetes
Several studies have demonstrated the role of Nrf2 in mitigating the complication of diabetes using cell-based and animal model systems [17,18]. In vitro studies using human and animal cells indicated that activation of Nrf2 depends on cell type and glucose concentration [19,20]. Moreover, many studies have also demonstrated that mice lacking Nrf2 (Nrf2- knockout mice) or Keap1 (Keap1- knockout mice) failed to reduce the complications of insulin resistance as they could not activate Nrf2 and its target genes, indicating a key role of Nrf2 in preventing diabetic complications [21].
Additionally, recent clinical findings have also shown that Nrf2 function has significantly decreased in subjects with diabetes [22]. Supporting this, analysis of peripheral blood mononuclear cells (PBMC) from prediabetic and diabetic subjects showed decreased Nrf2 and HO-1 levels [23]. Likewise, a separate study reported that diabetic skin tissue showed down regulation of Nrf2 and its target genes NQO1 and HO-1, at both the mRNA and protein levels compared with non-diabetic tissue. Many other studies similarly have shown changes in the expression of Nrf2 in diabetes compared to non-diabetic individuals highlighting its role as a key regulator in diabetes.
Nrf2 Modulates Metabolic Pathways to Control Hyperglycemia-induced Aberration in Diabetes
Hyperglycemia arising from uncontrolled glucose regulation causes tissue damage through increased polyol pathway producing excessive sorbitol by aldose reductase activity [24,25]. Elevated sorbitol induces TGFβ1 and inhibits Nrf2 expression resulting in elevated ROS levels [26]. Excessive cellular ROS in turn activate the TGFβ1 pathway in feed-forward mechanisms, leading to increased AR expression.
Therefore, restoring Nrf2 activity using naturally occurring Nrf2 activators helps to down-regulate sorbitol-mediated ROS induction. In support of this, a separate study recently showed that activation of Nrf2 inhibits the function of TGFβ1 [27]. Formation of toxic advanced glycation end (AGE) products methylglyoxal (MG), glyoxal and carboxymethyl lysine is another characteristic feature of diabetes [28]. AGEs that are produced as a result of non-enzymatic binding of reducing sugars with the amino groups in proteins, lipids or nucleic acids damage target cells by directly disrupting matrix-matrix and matrix-cell interactions through excessive cross-linking of proteins [29]. One way to overcome AGEs-mediated toxic effects is to increase the expression of glyoxalase- 1 (Glo-1), a key enzyme which catalyzes the conversion of MG to lactic acid [30].
Interestingly, Glo-1 is a direct target of Nrf2 [31]. A study by Chang wc et al. [32] showed induction of hepatic glyoxalase mRNA and glutathione (GSH) by elevated Nrf2, which helped in reducing the serum and hepatic AGEs as well as inflammatory factors in MG-treated rats. In a separate study it has been demonstrated that Nrf2 activation prevented the AGEs-induced ROS formation in LX-2 and human stellate cells through up regulation of γ-glutamyl cysteine synthetase and glutathione synthesis [33,34].
Elevated diacylglycerol (DAG) levels are another characteristic feature of diabetes [35]. DAG is usually produced when the glycolytic intermediate dihydroxy acetone phosphate (DHAP) gets reduced in a series of reduction reactions first producing glycerol-3-phosphate followed by DAG [36]. DAG thus produced activates protein kinase C (PKC), a serine/threonine kinases responsible for various structural and functional changes that include
- Cellular permeability;
- Inflammation;
- Cell growth;
- Angiogenesis;
- Extracellular matrix expansion; and
- Apoptosis [37].
In addition, PKC regulate intracellular eNOS, NADPH oxidase, endothelin-1 (ET-1), phospholipase A2 (PLA2), VEGF, connective tissue growth factor (CTGF), vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), NF-kB, and TGF-α [38].
In summary, PKC is a key regulator of various processes that lead to the
- Overproduction of ROS;
- Development of insulin resistance;
- Impaired glucose tolerance and
- β-cell dysfunction [38].
Nrf2 plays an important role in modulating these metabolic aberrations during hyperglycemias by regulating PKC and the enzymes involved in glucose metabolism [39]. Mechanistically, Nrf2 up regulate Slc2a1, Hk2 and Pkm2 expression in liver, brown adipose tissue, brain and kidney, while down-modulating G6p expression, cAMP- CREB signalling pathways. Therefore, activation of Nrf2 using phytochemicals is a better strategy to inhibit diabetes-related complications.
Hence, several researchers have developed screening and validation methods to identify potent Nrf2 activators from plant sources. One widely used screening method for identifying Nrf2 activators is complementation assay. Complementation screening system works on the principle that natural products activating Nrf2 prevents the expression of luciferase by interfering with Nrf2 binding to Keapl protein [40]. Therefore, a decrease in luciferase signal is an indicator of potent activator of Nrf2. Table 1 shows various plant products known to activate Nrf2 [41-64].
Future Direction
Even though several studies have demonstrated the vital role played by Nrf2 in controlling diabetes related complications, and identified potential activators of Nrf2, not many studies have evaluated Nrf2 activators in clinical trials. Therefore, future studies should investigate the potential of naturally occurring Nrf2 activators for preventing / treating diabetes in clinical trials. In addition, strategies such as use of nano-formulations should be adopted to deliver poorly bioavailable Nrf2 activators.
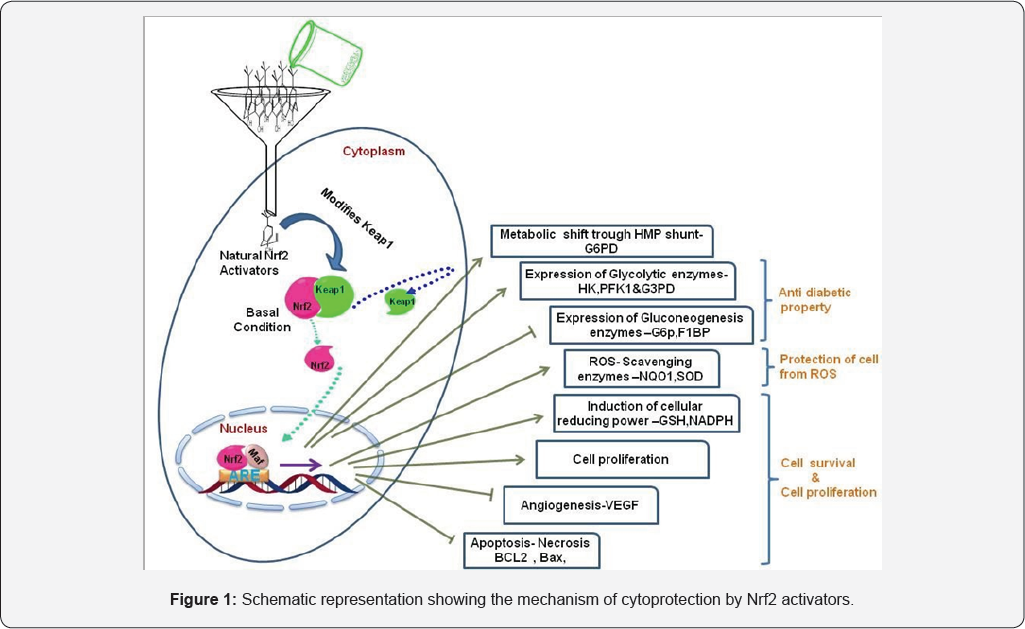
Schematic representation showing the mechanism of cytoprotection by Nrf2 activators: Schematic representation demonstrating various signaling cascades modulated through Nrf2 pathway in diabetes. Nrf2- activators reduce diabetes by upregulating glycolytic enzymes hexokinase (HK), phosphofructokinase-1 (PFK1) and glyceraldehyde 3-phoshate dehydrogenase (G3PD), and enzymes of hexose monophosphate shunt pathway in particular glucose-6-phosphate dehydrogenase (G6PD). In addition, Nrf2-activators inhibit the enzymes of gluconeogenesis Glucose 6 Phosphatase (G6P) and F1BPase. Furthermore, Nrf2 target genes protect pancreatic p-cells from ROS induced damage by up regulating NQO1 and SOD levels. Additionally, Nrf2 controls cell proliferation, apoptosis, autophagy and angiogenesis in diabetes (Figure 1).
References
- International Diabetes Federation. IDF Diabetes atlas (2015) International Diabetes Federation. (7th edn), Brussels.
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, et al. (2005) Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(2): 97-107.
- Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol Lausanne 4: 37.
- Meier JJ, Bonadonna RC (2013) Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes Care 36 (2): 113-119.
- Forbes JM, Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93(1): 137-188.
- Asmat U, Abad K, Ismail K (2016) Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J 24(5): 547-553.
- Hodish I, Liu M, Rajpal G, Larkin D, Holz RW, et al. (2010) Misfolded proinsulin affects bystander proinsulin in neonatal diabetes. J Biol Chem 285(1): 685-694.
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H (2003) Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52(3): 581-587.
- Qin S, Hou DX (2016) Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol Nutr Food Res 60(8): 1731-1755.
- Xu Y, Wang L, He J, Bi Y, Li M, et al. (2013) Prevalence and control of diabetes in Chinese adults. JAMA 310(9): 948-959.
- Islam MA, Alam F, Solayman M, Khalil MI, Kamal MA, et al. (2016) Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid Med Cell Longev 2016: 5137431, p. 25.
- Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, et al. (2012) Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabetes Res Clin Pract 96(3): 348-354.
- Jimenez-Osorio AS, Garcia-Nino WR, Gonzalez-Reyes S, Alvarez-Mejia AE, Guerra-Leon S, et al. (2016) The Effect of Dietary Supplementation With Curcumin on Redox Status and Nrf2 Activation in Patients With Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J Ren Nutr 26(4): 237-244.
- Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S (2009) Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition. 25(9): 964-972.
- Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5): 270-278.
- Lacher SE, Lee JS, Wang X, Campbell MR, Bell DA, et al. (2015) Beyond antioxidant genes in the ancient Nrf2 regulatory network. Free Radic Biol Med 88(Pt B): 452-465.
- Zhong Q, Mishra M, Kowluru RA (2013) Transcription factor Nrf2- mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 54(6): 3941-3948.
- Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, et al. (2008) Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 13(11): 1159- 1170.
- Li H, Wang F, Zhang L, Cao Y, Liu W, et al. (2011) Modulation of Nrf2 expression alters high glucose- induced oxidative stress and antioxidant gene expression in mouse mesangial cells. Cell Signal 23(10): 1625- 1632.
- Bitar MS, Al-Mulla F (2011) A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am J Physiol Endocrinol Metab 301(6): 1119-1129.
- Slocum SL, Skoko JJ, Wakabayashi N, Aja S, Yamamoto M, et al. (2016) Keap1/Nrf2 pathway activation leads to a repressed hepatic gluconeogenic and lipogenic program in mice on a high-fat diet. Arch Biochem Biophys 591: 57-65.
- Long M, Rojo de la VM, Wen Q, Bharara M, Jiang T, et al. (2016) An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 65(3): 780-793.
- Jimenez-Osorio AS, Picazo A, Gonzalez-Reyes S, Barrera-Oviedo D, Rodriguez-Arellano ME, et al. (2014) Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci 15(11): 20290-20305.
- Jiang T, Che Q, Lin Y, Li H, Zhang N (2006) Aldose reductase regulates TGF-beta1-induced production of fibronectin and type IV collagen in cultured rat mesangial cells. Nephrology Carlton 11(2): 105-112.
- Jiang T, Qu JJ, Nishinaka T, Zhang N (2008) Transcription factor AP-1 regulates TGF-beta (1)-induced expression of aldose reductase in cultured human mesangial cells. Nephrology Carlton 13(3): 212-217.
- Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, et al. (2005) Smad3-ATF3 signaling mediates TGF- beta suppression of genes encoding Phase II detoxifying proteins. Free Radic Biol Med 38(3): 375-387.
- Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, et al. (2010) The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59(4): 850-860.
- Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48(1): 1-9.
- Schmidt AM, Yan SD, Yan SF, Stern DM (2000) The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 1498(2-3): 99-111.
- Lee BH, Hsu WH, Hsu YW, Pan TM (2013) Dimerumic acid attenuates receptor for advanced glycation endproducts signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic Biol Med 60: 7-16.
- Mastrocola R (2016) AGEs and neurodegeneration: the Nrf2/ glyoxalase-1 interaction. Oncotarget 8(4): 5645-5646.
- Chang WC, Wu SC, Xu KD, Liao BC, Wu JF, et al. (2015) Scopoletin protects against methylglyoxal-induced hyperglycemia and insulin resistance mediated by suppression of advanced glycation endproducts (AGEs) generation and anti-glycation. Molecules 20(2): 2786-2801.
- Koo YC, Pyo MC, Nam MH, Hong CO, Yang SY, et al. (2016) Chebulic acid prevents hepatic fibrosis induced by advanced glycation end-products in LX-2 cell by modulating Nrf2 translocation via ERK pathway. Toxicol In Vitro 34: 8-15.
- Tang Y, Chen A (2014) Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling. Lab Invest 94(5): 503-516.
- Yan LJ (2014) Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res 2014: 137919.
- Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, et al. (1994) Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes 43(9): 1122-1129.
- Noh H, King GL (2007) The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl (106): 49-53.
- Geraldes P, King GL (2010) Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106(8): 1319-1331.
- Huang HC, Nguyen T, Pickett CB (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response elementmediated transcription. J Biol Chem 277(45): 42769-42774.
- Ramkumar KM, Sekar TV, Foygel K, Elango B, Paulmurugan R (2013) Reporter protein complementation imaging assay to screen and study Nrf2 activators in cells and living animals. Anal Chem 85(15): 75427549.
- Wu H, Kong L, Cheng Y, Zhang Z, Wang Y, et al. (2015) Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2. Free Radic Biol Med 89: 431442.
- Shang G, Tang X, Gao P, Guo F, Liu H, et al. (2015) Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2signaling pathway. J Nutr Biochem 26(6): 596-606.
- Zhang Z, Wang S, Zhou S, Yan X, Wang Y, et al. (2014) Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/ AMPK pathway. J Mol Cell Cardiol 77: 42-52.
- Cheng AS, Cheng YH, Lee CY, Chung CY, Chang WC (2015) Resveratrol protects against methylglyoxal- induced hyperglycemia and pancreatic damage in vivo. Nutrients 7(4): 2850-2865.
- Palsamy P, Subramanian S (2011) Resveratrol protects diabetic kidney by attenuating hyperglycemia- mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta 1812(7): 719-731.
- Cheng AS, Cheng YH, Chiou CH, Chang TL (2012) Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J Agric Food Chem 60(36): 91809187.
- Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, et al. (2015) Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-kappaB both in vitro and in vivo. J Mol Cell Cardiol 79: 1-12.
- Maithili Karpaga SN, Sridhar MG, Swaminathan RP, Sripradha R (2014) Curcumin Attenuates Oxidative Stress and Activation of Redox- Sensitive Kinases in High Fructose-and High-Fat-Fed Male Wistar Rats. Sci Pharm 83(1): 159-175.
- Panchal SK, Poudyal H, Brown L (2012) Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J Nutr 142(6): 1026-1032.
- Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Kucuk O, et al. (2010) Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci 87(7-8): 240-245.
- Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, et al. (2009) Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin- induced nephrotoxicity in Wistar rats. Eur J Pharmacol 606(1-3): 162171.
- Ramprasath T, Senthamizharasi M, Vasudevan V, Sasikumar S, Yuvaraj S, et al. (2014) Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J Physiol Biochem 70(2): 407-415.
- Esmaeili MA, Alilou M (2014) Naringenin attenuates CCl4-induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin Exp Pharmacol Physiol 41(6): 416-422.
- Bhakkiyalakshmi E, Shalini D, Sekar TV, Rajaguru P, Paulmurugan R, et al. (2014) Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br J Pharmacol 171(7): 1747- 1757.
- Hassan NA, El-Bassossy HM, Mahmoud MF, Fahmy A (2014) Caffeic acid phenethyl ester, a 5-lipoxygenase enzyme inhibitor, alleviates diabetic atherosclerotic manifestations: effect on vascular reactivity and stiffness. Chem Biol Interact 213: 28-36.
- Sandireddy R, Yerra VG, Komirishetti P, Areti A, Kumar A (2016) Fisetin Imparts Neuroprotection in Experimental Diabetic Neuropathy by Modulating Nrf2 and NF-kappaB Pathways. Cell Mol Neurobiol 36(6): 883-892.
- Hur KY, Kim SH, Choi MA, Williams DR, Lee YH, et al. (2010) Protective effects of magnesium lithospermate B against diabetic atherosclerosis via Nrf2-ARE-NQO1 transcriptional pathway. Atherosclerosis 211(1): 69-76.
- Song Y, Wen L, Sun J, Bai W, Jiao R, et al. (2016) Cytoprotective mechanism of ferulic acid against high glucose-induced oxidative stress in cardiomyocytes and hepatocytes. Food Nutr Res 60(10): 30323.
- Tzeng TF, Liou SS, Chang CJ, Liu IM (2013) Zerumbone, a tropical ginger sesquiterpene, ameliorates streptozotocin-induced diabetic nephropathy in rats by reducing the hyperglycemia-induced inflammatory response. Nutr Metab (Lond) 10(1): 64.
- Imran K, Waqar A, Nasiara K, Manzoor A, Munsaib K, et al. (2017) Antidiabetic activity and histopathological analysis of carnosol isolated from Artemisia indica linn in streptozotocin-induced diabetic rats. Med Chem Res 26(2): 335-343.
- Mellbye FB, Jeppesen PB, Hermansen K, Gregersen S (2015) Cafestol, a Bioactive Substance in Coffee, Stimulates Insulin Secretion and Increases Glucose Uptake in Muscle Cells: Studies in Vitro. J Nat Prod 78(10): 2447-2451.
- Arafat SY , Nayeem M, Jahan S, Karim Z, Reza HM, et al. (2016) Ellagic acid rich Momordica charantia fruit pulp supplementation prevented oxidative stress, fibrosis and inflammation in liver of alloxan induced diabetic rats. Orient Pharm Exp Med 16(4): 267-278.
- Garud MS, Kulkarni YA (2017) Eugenol ameliorates renal damage in streptozotocin-induced diabetic rats. Flavour Fragr J 32: 54-62.
- Al-Numair KS, Chandramohan G, Veeramani C, Alsaif MA (2015) Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Report 20(5): 198-209.






























