Onset of Early Menarche as A Predictor of The Metabolic Syndrome Formation Among Women of Reproductive Age
Suplotova Ludmila1*, Smetanina Svetlana2 and Khramova Yelena3
1 Head of endocrinology course of a Therapy department, Tyumen State Medical University, Russia
2Department in pediatrics faculty, Tyumen State Medical University, Russia
3Department in pediatrics faculty, Tyumen State Medical University, Russia
Submission: January 16, 2018; Published: September 25, 2018
*Corresponding author: Mohammad Suplotova Ludmila, Professor, Head of endocrinology course of a Therapy department, Tyumen State Medical University, Ministry of Healthcare of the Russian Federation, Tyumen, Russia.
How to cite this article: Suplotova L, Smetanina S, Khramova Y. Onset of Early Menarche as A Predictor of The Metabolic Syndrome Formation Among Women of Reproductive Age. Curre Res Diabetes & Obes J. 2018; 8(5): 555750. DOI: 10.19080/CRDOJ.2018.08.555750.
Abstract
Purpose: To provide an analysis of clinical, hormonal-metabolic and molecular-genetic associations among Russian women of reproductive age with early menarche, obesity and metabolic syndrome.
Materials and methods:Non-serial prospective study of 537 women of the Russian population of reproductive age from 18 to 45 years was conducted. The group with obesity included 177 people with a BMI ≥ 30 kg / m2 and 232 people were included into the group with a metabolic syndrome. The control group consisted of 128 healthy women without overweight and dysmetabolism. Biochemical and hormonal parameters of blood plasma were determined. Genotyping of polymorphic markers of insulin resistance candidate genes and insulin deficiency was carried out based on the laboratory of molecular diagnostics and genomic dactyloscopy “GosNII Genetika”, Moscow (chief – Dr. habil. (in Biology), professor Nosikov V.).
Results:Early menarche relates to the development of obesity and metabolic syndrome among women of reproductive age and is associated with polymorphic markers of candidate genes of insulin resistance and insulin deficiency TCF7L2 (rs7903146), ADIPOQ (rs1501299), ADIPOR2 (rs16928751).
Conclusion:Hormonal-metabolic and molecular-genetic associations of early menarche, obesity and metabolic syndrome among reproductive age women of the Russian population were identified.
Keywords:Obesity; Metabolic syndrome; Menarche; Molecular-genetic markers
Introduction
In recent decades in many countries around the world there has been a progressive increase of patients with overweight and obesity reaching the parameters of endemia. According to the World Health Organization (WHO), obesity is one of the top ten risk factors for immature deaths from cardiovascular disease [1]. The formation of obesity in childhood and adolescence has an adverse effect on physical and psychosocial health, both in the short and long term, determining the high-level risk of metabolic disorders both with patient and his/her offspring.
The pubertal period is controlled by a complex of neuroendocrinal factors leading to physical, mental and reproductive maturity of the organism. In the period of puberty, there are evident changes in the composition of the body caused by the acceleration of skeleton growth, an increase in muscle and adipose tissue [2,3]. Menarche or the first menstrual bleeding in women is the result of ovarian response to multiple hormone-metabolic changes occurring in the body during the pubertal period, which is influenced by endogenous and exogenous factors [4,5]. In the XIX-XX centuries in European countries and in Russia the age of menarche as an optimal discrete feature, that allows to analyze historical trends in the development of children, subsided on average by 2-3 months every decade and decreased from 17 to 12 years [6].
At present there is some evidence that with an excess of nutrients because of the interaction of leptin and insulin-like growth factor-1, the compositional structure of the body changes, the growth rate increases, the bone matures early, and the early onset of menarche [1,4]. The scientific literature actively discusses the interaction between early menarche and the risk of cardiovascular disease in women in old age [2-4].Nevertheless, the pathogenetic mechanisms of early menarche with excessive body weight and obesity in women, genotypic and phenotypic associations, as well as long-term health effects remain unknown to this day.
Purpose of research: to analyze the association of early menarche with molecular-genetic markers of insulin resistance and insulin deficiency in women of reproductive age with obesity and metabolic syndrome.
Tasks of research: to determine associations between the early age of menarche, hormonal-metabolic parameters and molecular-genetic markers of candidate genes of insulin resistance and insulin deficiency in women of reproductive age with obesity and metabolic syndrome.
Methods and Materials
The study was conducted based on the University multidisciplinary clinic of Higher Education State Institution “Tyumen State Medical University” of the Healthcare Ministry of Russia (Chief doctor – Bagirov R.). The study protocol was approved by the Ethics Committee of Higher Education State Institution “Tyumen State Medical University” of the Healthcare Ministry of Russia. During the period from 2010 up to 2015 a one-stage prospective study of 537 women of the Russian population from 18 to 45 years was conducted, the criteria for a woman to be included in the study was the availability of voluntary informed consent. The control group included women with a BMI of 18.5-24.9kg/m2 without metabolic abnormalities (n = 128), 177 women with a BMI≥30kg/m2 without metabolic abnormalities were included in the second study group, and 232 patients within the third group had metabolic syndrome. IDF criteria were used for MS diagnosis [7]. The mean age of those surveyed was 33.0 (28.0; 37.0) years and did not differ between groups (р = 0,32). Exclusion criteria were decompensated somatic diseases, malignant neoplasm, acute or exacerbation of chronic infectious disease, use of glucocorticosteroids, pregnancy and lactation, the presence of a verified diagnosis of T1D, the presence of secondary diabetes due to pancreatitis, hemochromatosis and other diseases.
The following survey methods are used in the study: collection and interpretation of complaints and anamnesis, physical examination, measurement of blood pressure, height, body weight, waist circumference, body mass index (BMI). The diagnosis of obesity in women was carried out considering the BMI (WHO, 1997). Laboratory diagnostics was carried out on the automatic analyzer “Chem Well +” manufactured by Awareness Technology (USA). Blood sampling was carried out from the medial cubital vein after 10-12 hours of fasting with the determination of biochemical and hormonal parameters of blood plasma, including cholesterol, low- and high-density lipoproteins, triglycerides, as well as leptin and adiponectin.
Evaluation of carbohydrate metabolism is based on the results of an oral glucose-tolerant test with a load of 75g. glucose according to WHO criteria (1999). Genotyping of polymorphic markers of several candidate genes of insulin resistance and insulin deficiency of biological samples of frozen venous blood was carried out on the basis of the Laboratory of Molecular Diagnostics and Genomic Dactyloscopy of the State Scientific Center of the Russian Federation “GosNII Genetika”, Moscow (Head – Dr. Nosikov V.). Polymorphic markers of candidate genes for insulin resistance and insulin deficiency were studied, which in various populations demonstrated association with MC components: TCF7L2 (rs7903146), ADIPOQ (rs1501299), ADIPOR (rs1692875). Identification of alleles of polymorphic markers of genes was carried out using polymerase chain reaction [8,9].
Statistical analysis of the obtained data was carried out using STATISTICA software package (StatSoft Inc. version 10.0, USA), Microsoft Excel, version 7.0. Quantitative indicators are presented as the mean and standard deviation (M±s), median and interquartile range (Me (25%,75%)). When comparing two independent samples, the “Student” test and the Mann- Whitney U-test were used. Calculation of odds ratio (OR) and 95% confidence interval (95%CI) was used to estimate the probability of development of the studied event in the research groups. To determine the relationship between qualitative and/ or quantitative indicators, the Spearman correlation coefficient is used. The analysis of qualitative indicators is carried out by the method of calculating absolute and relative frequencies with the construction of contingency tables. A comparison of the corresponding frequencies in the study groups was carried out using the exact Fisher test and the χ2-test. Statistically significant differences were considered when p <0.05.
Results and discussion
It was found that for women with MS the earlier age of menarche (12.8±1.30 years) is a definitive characteristic in comparison with the control group (13.3±1.29 years) and with obesity (13.0±1.38 years) p1-3 <0.001. In obese women and MS, the incidence of early menarche was 13.3% and 15.5% properly and was higher than in the control group (5.4%) - p1-2 = 0.04; p1-3 = 0.01 (Figure 1). It was found that early menarche increases the risk of obesity (OR = 2.69, CI 1.12-6.49) and MS (OR = 3.23, 95% CI 1.34-7.81) in women of reproductive age. In the presented study we did not find any associations of late-age menarche with the development of obesity and MS.
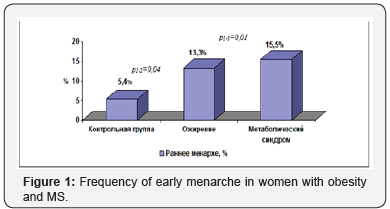
Control group
I. obesity
II. Metabolic syndrome
III. Early menarche %
It was determined that women with menarche at the age of 10.7±0.53 years had a higher BMI at 18 years and maintained it during the reproductive age (25.1±5.4kg/m2 and 35.6±7.5kg/ m2) in comparison with the group in which the mean age of the menarche was 13.3±1.1 years (22.8±6.4kg/m2 and 30.9±7.9 kg/ m2) - p = 0, 02; p <0.001.
The study showed that early menarche is most typical for carriers of risk genotypes of polymorphic markers of candidate genes of insulin resistance and insulin deficiency. Thus, the polymorphic marker rs7903146 of gene TCF7L2 of the transcription factor 7 plays an important role in the embryonic development of various organs and tissues, affects all key stages in insulin synthesis and adipogenesis [3,7]. In persons with the T/T genotype, the age of menarche was 12.5±1.17 years, compared with the age of menarche in carriers of the C/T genotype of 13.2±1.6 years (p = 0.03). The differences were probably associated with a leptin level, which was 44.2 (24.95; 64,50) ng/ml in persons with the T/T genotype as compared to carriers of the C/T genotype (24.3 (10.30, 43.90) ng/ml) - p = 0.008.
The ADIPOQ gene product – adiponectin – is the most studied protein among those synthesized by adipocytes. The action of adiponectin on cells is mediated by receptors that are found in many tissues, including the central nervous system, ovaries, fallopian tubes, and endometrium. With obesity, the level of ADIPOQ gene expression in adipocytes is reduced, and low levels of adiponectin receptors can significantly reduce the biological effect of its action and, thus, further exacerbate the negative metabolic effect [9].
The earlier age of menarche (12.6±1.3 years) was determined in women with the G/G genotype of the polymorphic marker rs1501299 of the ADIPOQ gene compared to the carriers of the G/T and T/T genotypes (13.1±1.7 and 14, 3±1.1 years relatively) – p1-3=0,0001. The association of the age of menarche with the risk genotype G/A of the polymorphic marker rs16928751 of the receptor gene for adiponectin type 2 (ADIPOR2) was proved. The heterozygous carriers of the polymorphic marker rs16928751 of the adiponectin type 2 receptor gene (ADIPOR2) determined an earlier age of menarche (12.2±1.2 years) than in homozygous carriers (13.0±1.4 and 14.5±2, 1 years) – p = 0.02. Probably the differences are associated with hypoadiponectinemia, proven in individuals with the G/A genotype (p = 0.02).
Thus, it is determined that the early age of menarche raises the risk of obesity and metabolic syndrome in women of reproductive age and is associated with polymorphic markers genes candidate of insulin resistance and insulin deficiency TCF7L2 (rs7903146), ADIPOQ (rs1501299), ADIPOR2 (rs16928751). The relationship between leptin and adiponectin with the activation of a reproductive system and an age of menarche requires further study
References
- Noncommunicable Diseases Country Profiles (2014) World Health Organization, Geneva, Switzerland.
- Canoy D (2015) Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation 131(3): 237-244.
- Charalampopoulos D, McLoughlin A, Elks CE, Ong KK (2014) Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol 180(1): 29-40.
- Lakshman R (2009) Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 94: 4953–4960.
- Lee СY, Lee CH, Tsai S, Huang CT, Wu MT, et al. (2009) Association between serum leptin and adiponectin levels with risk of insulin resistence and impaired glucose tolerance in non-diabetic women. J Med Sci 25(3): 116-125
- Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, et al. (2012) Recent Secular Trends in Pubertal Timing: Implications for Evaluation and Diagnosis of Precocious Puberty. Horm Res Paediatr 77: 137–145.
- The IDF consensus worldwide definition of the metabolic syndrome (2006) International Diabetes Federation.
- Grant SF (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38(3): 320-323.
- Potapov VA, Chistiakov DA, Dubinina A, Shamkhalova MS (2008) Adiponectin and adiponectin receptor gene variants in relation to type 2 diabetes and insulin resistance-related phenotypes. Rev Diabet Stud 5(1): 28-37.






























