“Cardiovascular Outcomes Trials in Diabetes: A Necessity or Wastage of Time and Money?”
Samit Ghosal11* and Binayak Sinha2
1 Department of Endocrinology, Nightingale Hospital, India
2 Department of Endocrinology, AMRI Hospitals, India
Submission: July 24, 2018; Published: August 22, 2018
*Corresponding author: Samit Ghosal, Nightingale Hospital, 11 Shakespeare Sarani, Kolkata, India.
How to cite this article: Samit Ghosal, Binayak Sinha. “Cardiovascular Outcomes Trials in Diabetes: A Necessity or Wastage of Time and Money?”. Curre Res Diabetes & Obes J. 2018; 8(3): 555738. DOI: 10.19080/CRDOJ.2018.08.555738.
Abstract
The last decade can be branded as the decade of cardiovascular outcomes trials (CVOTs) as far as diabetes-related research is concerned. With the publication of several CVOTs we have seen changes creeping into guidelines as well as package inserts of diabetes-related medications. This has become a bit challenging for the physicians as it has now become a norm to have a good grasp over CVOTs to choose an anti-hyperglycemic agent. However, CVOTs have also been exposed to a variety of criticisms. For example, they do not help answer the mechanistic basis for understanding of diabetes or its related complications. Moreover, can we justify such a large expenditure of funds on CVOTs? This mini review investigates certain core issues related to CVOTs and addresses these concerns accordingly
Keywords: Cardiovascular outcomes trials; Type 2 diabetes; Safety; Regulatory bodies; FDA
Abbrevations: CVOTs: Cardiovascular Outcomes Trials, FDA: Food and Drug Administration, GLP1-RA: Glucagon Like Peptide 1-Receptor Analogs, SGLT-2i: Sodium-Glucose Linked Transporter -2 Inhibitor, HDL-C: High Density Lipoprotein–Cholesterol, hHF: Hospitalization Due to Heart Failure, MACE: Major Adverse Cardiovascular Events, EASD: European Association for the Study of Diabetes
Introduction
In the year 2008 the publication of a meta-analysis looking into the outcomes associated with rosiglitazone alarmed the regulatory bodies regarding safety signals of drugs [1]. Subsequently we saw the several regulatory bodies (FDA, EAMA, etc.) widen their focus from the narrow efficacy window to include safety issues as a prime outcomes measure. The financial fallout was obvious, since it became mandatory for the pharmaceutical industry to follow the directives (safety) to get marketing rights. The CVOTs trials done with DPP-4 inhibitors were all neutral from the primary outcomes point of view raising questions whether it’s worth spending such huge amounts on CVOTs. This argument was buttressed by the fact that none of the RCTs documented a cardiovascular benefit with intensive glucose lowering. However, the publication of EMPA REG outcomes trial changed the picture completely [2]. Now we can expect a molecule related impact on cardiovascular outcomes (irrespective of intensive glucose control). Moreover, all these outcomes trials made us aware of several unknown adverse effects associated with anti-hyperglycemic agents. With this background this mini-review investigates the rationale of conducting CVOTs.
The quantum of financial investment in CVOT
A very common criticism about CVOTs is that a huge amount of fund is diverted away from other important research areas. So, what should be our response? Should the funds have allocated for CVOTs reduced?
Let us take an in-depth look at this issue.
Is it justified investing big in CVOTs?
Recent trends suggest a reversal of trend favoring non-communicable disease over communicable disease as far as global burden of disease is concerned [3]. By the year 2030 CVD would feature as the major non-communicable disease of interest. It is predicted that by this timeframe we are looking at approximately 24 million deaths annually from heart attack and stroke [3]. The health care cost is going to skyrocket if we consider the morbidities associated with the above-mentioned conditions in the survivors. The Emerging Risk Factors Collaboration study documented loss of 6 years of life in type 2 diabetic patients above 50 years of age [4]. With recent data with SGLT-2i and GLP1-RA documenting decreased CV & all-cause mortality, we can give back some of those lost years. Hence is it not justified to invest heavily in CVOTs?
Investment in CVOTs versus other research areas
Contrary to conventional belief, the largest number of new molecule applications including fastest FDA approvals is happening with oncology drugs [3]. If we look at the innovative compounds, drugs targeting the cardiovascular system come a distant fifth (behind oncology, CNS, anti-infectives including vaccines, and genito-urinary drugs) [3]. Taking the epidemiological projections and the actual spending on preventing cardiovascular deaths it seems justified to conduct more CVOTs rather than cutting down on the budget.
Primum non noce: CVOT in diabetes - Only CV centric?
A major misconception as far as judging CVOTs are concerned is looking at them from a myopic cardiovascular perspective [5]. These are multiple end studies, which assesses several diabetes-related outcomes. One of the major areas of focus is safety of the therapeutic agent in question. The advantage of such an assessment lies in the fact that these studies are done in a randomized, controlled, blinded fashion minimizing biases as much as possible [6]. Although the duration of follow up is small, it does help in generating a hypothesis from the secondary end points which can subsequently be tested in a large observational real-life study and post-marketing surveillance study. There have been past instances where drugs got approval after conducting efficacy trials only to be withdrawn later due to life threatening adverse events, the classical examples being terfenadine & HDL-C raising agents (Table 1).
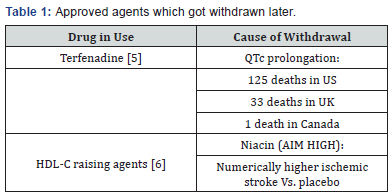
As a matter of fact, post AIM HIGH, other studies assessing the impact of raising HDL-C on CVD also hit similar roadblocks. Prominent among them is the ILLUMINATE trial [7]. This led to a reassessment of the CV risk status conferred on low HDH-C. In the year 2016 reassessment of the Framingham Offspring study failed to demonstrate isolated low HDL-C as a CV risk factor [8]. And what about the scary terfenatide data? Do we need to wait for such disastrous consequences before initiating safety measures/trials? The above-mentioned examples illustrate the utility of CVOTs in not only assessing the safety/superiority of a agent from a cardiovascular perspective but also in raising numerous important hypothesis needing further research and clarifications [9-10].
CVOTs: Outcomes beyond CVD
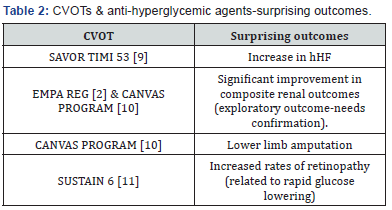
Cardiovascular outcomes trials with gliptins, GLP1-RA and SGLT-2i sprang a few surprises both related and unrelated to the cardiovascular system (Table 2). In view of the possibility of renal benefits associated with SGLT-2i dedicated trials were initiated [11]. The topline results of CREDENCE assessing renal outcomes with canagliflozin are already in public domain [12]. The results are encouraging. So, what did CVOTs have to offer apart from pure CV outcomes? A lot [13-15].
CVOT: The way to progress
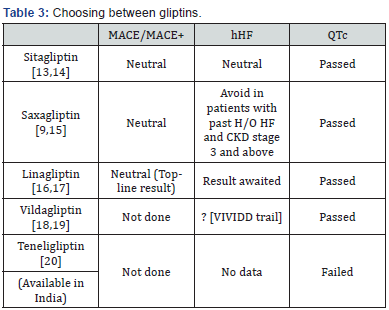
The regulatory authorities have responded in a justifiable manner making it mandatory for new drug applicants to follow a stringent recommendation focused on safety concerns [16,17]. Every single molecule will now have to prove their safety from a CV and QTc prolongation point of view. As physicians we need to be vigilant whether such data are available with new molecules prior to prescribing them [18,19]. Let us take the example of DPP-4 inhibitors [20]. How do we choose a safe agent in a type 2 diabetes patients with established cardiovascular disease? (Table 3)
Conclusion
The issue is no longer whether CVOTs are justified or not. But how to modify them. The EASD study group has been organizing CVOT summit on an annual basis [21]. The main objective of this meet is to assess and modify the way the scope of CVOTs can be expanded. They recommend bringing all medications under the scrutiny of CVOTs. Even the older agents like metformin and sulfonylureas should not be exempted from this scrutiny. Not only the field of diabetes, but medicines in other disciplines of medicine likes nephrology, cardiology, neurology etc. should be brought under the purview of CVOT.
References
- Nissen SE, Wolsky K (2007) Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N Engl J Med 356(24): 2457-2471.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373(22): 2117-2128.
- Fordyce CB, Roe MT, Ahmad T, Libby P, Borer JS, et al. (2015) Cardiovascular Drug Development. Is it Dead or Just Hibernating? J Am Coll Cardiol 65(15): 1567–1582.
- Seshasai SRK, Kaptoge S, Thompson A, Angelantonio ED, Gao P, et al. (2011) Diabetes Mellitus, Fasting Glucose, and Risk of Cause-Specific Death the Emerging Risk Factors Collaboration. N Engl J Med 364(9): 829-841.
- Rangno R (1997) Terfenadine therapy: Can we justify the risks? Can Med Assoc J 157: 37-38.
- Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. (2011) Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy The AIM-HIGH Investigators. N Engl J Med 365: 2255-2267.
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, et al. (2007) Effects of Torcetrapib in Patients at High Risk for Coronary Events. N Engl J Med 357(21): 2109-2122.
- Bartlett J, Predazzi IM, Williams SM, Bush WS, Kim Y, et al. (2016) Is Isolated Low High-Density Lipoprotein Cholesterol a Cardiovascular Disease Risk Factor? New Insights from the Framingham Offspring Study. Circ Cardiovasc Qual Outcomes 9: 206-212.
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, et al. (2011) The design and rationale of the Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus–Thrombolysis in Myocardial Infarction (SAVOR-TIMI) 53 Study. Am Heart J 162(5): 818-825
- Neal B, Perkovic V, Mahaffey KW, Zeeuw D, Fulcher G, et al. (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377(7): 644-657.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jo ÅLdar E, et al. (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375(19): 1834–1844
- Phase 3 CREDENCE Renal Outcomes Trial of INVOKANA® (canagliflozin) is Being Stopped Early for Positive Efficacy Findings.
- Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, et al. (2015) “Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes,” N Engl J Med 373(3): 232–242.
- Bloomfield DM, Krishna R, Hreniuk D, Hickey L, Ghosh K, et al. (2009) A Thorough QTc Study to Assess the Effect of Sitagliptin, a DPP4 Inhibitor, on Ventricular Repolarization in Healthy Subjects. J Clin Pharmacol 49(8): 937-946.
- Pollack PS, Chadwick KD, Smith DM, Billger M, Hirshberg B, et al. (2017) Nonclinical and clinical pharmacology evidence for cardiovascular safety of saxagliptin. Cardiovasc Diabetol 16: 113
- Boehringer Ingelheim and Lilly announce Tradjenta’s CARMELINA cardiovascular outcome trial meets primary endpoint
- Ring A, Port A, EGraefe-Mody EU, Revollo I, Iovino M, et al. (2011) The DPP-4 inhibitor linagliptin does not prolong the QT interval at therapeutic and supratherapeutic doses. Br J Clin Pharmacol 72(1): 39-50.
- McMurray JJV, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, et al. (2018) Effects of Vildagliptin on Ventricular Function in Patients with Type 2 Diabetes Mellitus and Heart Failure A Randomized Placebo- Controlled Trial. J Am Coll Cardiol HF 6: 8-17
- He YL, Zhang Y, Serra D, Wang Y, Ligueros-Saylan M, et al. (2011) Thorough QT study of the effects of vildagliptin, a dipeptidyl peptidase IV inhibitor, on cardiac repolarization and conduction in healthy volunteers. Curr Med Res Opin 27(7): 1453-1463.
- Singh AK (2017) Efficacy and safety of teneligliptin. Indian J Endocr Metab 21: 11-17.
- Schnell O, Standl E, Catrinoiu D, Genovese S, Lalic N, et al. (2016) Report from the 1st Cardiovascularv Outcome Trial (CVOT) Summit of the Diabetes & Cardiovascular Disease (D&CVD) EASD Study Group. Cardiovasc Diabetol 15: 33.






























