Abstract
Agroecological transition is a major societal challenge and it is urgent to support technical solutions to answer farmers demand towards the necessary changes. The project RESTORE - biopReparados Em SisTemas prOdução agRoEcológicos aimed to study and validate the production, conservation, and use of seven biopreparations by characterizing and testing them in lettuce nurseries. The biopreparations used include prickly pear vinegar, purslane vinegar, fermented orange juice, garlic extract, nettle infusion, horsetail decoction and a biopreparation of garlic and chilli. The in vitro microbiological characterization and chemical analysis of the biopreparations, which included phenolic composition and antioxidant capacity, allowed us to understand their properties and to propose the most appropriate conservation strategy. The tests were conducted in four different moments: during the biopreparations’ manufacturing, after six months of frozen storage, and after three and six months of fresh storage.
One-Way ANOVA in SPSS 29.0 and PCA comparison tests were used to analyze the results. Fermented orange juice and vinegars were the biopreparations that showed the greatest outcomes in terms of antioxidant capacity and phenolic composition. Purslane vinegar had the highest average inhibition against germs.
The concentration of phenolic compounds, the antioxidant capacity, and the antibacterial activity of the biopreparations varied with time and type of conservation. It is crucial to conduct more trials, using different species and recipes, to support farmers’ decision making and adoption of agroecological solutions.
Keywords: Agroecology; Antioxidants; Microbiology; Phenolics; Horsetail; Chilli; Nettle; Prickly pear; Purslane; Garlic
Abbreviations: BAP - Biopreparation of garlic and chilli; DC - Horsetail Decoction; EA - Garlic Extract; IU - Nettle Infusion; SFL - Fermented Orange juice; VB - Purslane vinegar; VF - Pricky Pear Vinegar
Introduction
Due to the use of plant protection products and the metabolites produced by all agricultural activities, agriculture is one of the economic sectors that most contributes to environmental pollution and climate change [1-2]. Using biopreparations-mixtures of made from natural resources found on the farm-as alternatives to synthetic pesticides and fertilizers, could be a game changer in agriculture and its environmental impact, especially if the farmers could produce and storage their homemade products with plants which they have in their farming [3]. Plants are the living beings with the highest production of chemical substances known, which is why it is important to study them, as well as their compounds, to understand their functionalities and their uses, particularly in agriculture [4]. The effect of different biopreparations is related to its phenolic content and antioxidant capacity. Phenolic compounds frequently have fungicidal and bactericidal properties, directly affecting microorganism resistance [5].
In this context, biopreparations emerge as solutions to reduce agricultural impacts on water, soil, and food, while maintaining productivity. Depending on the biomaterial used (plant species, organic residues), recipe, storage, and lifetime, biopreparations effects can go from biostimullation to crop protection [6-7]. Biopreparations can be an alternative to the use of synthetic pesticides or fertilizers, but they require studies to prove their effectiveness and a lower social, economic and environmental impact, especially on the health of the farmer and the environment [8-9].
Plants can have different compositions according to the soil and climate conditions in which they are raised and collected, as well as different compositions according to the plant organ in use and/or being analysed [10-11]. On the other hand, the compounds that are extracted from different plants depend on the extraction method and the solvent [10-11]. Also, after some time of fermentation, and depending on the method of fermentation, the content of phenolic compounds and antioxidant capacity grows when comparing with extracts without fermentation [12]. The content of the extracts can change during time in different phases of fermentation [13]. On other hand, the type of conservation is important for the results obtained of phenolic content and antioxidant activity [14]. Some studies show that nettle extracts could be an answer to control Escherichia coli [15-17], but not for Penicillium sp. [16]. In terms of inhibiting microbial activity, ethanol and methanol extracts perform poorly, while water extracts (nettle infusion) achieve more promising results in terms of inhibiting E. coli [18]. For horsetail extracts, some studies demonstrate that could be a possibility for controlling E. coli [19, 20], but some others do not [21]. About orange extracts, there are studies that indicate that this biopreparation is a support for the growth of acid lactic bacteria [22]. The same work demonstrated that orange fermentation with acid lactic bacteria has phenolic compounds and antioxidant capacity [22]. About antimicrobial activity by fermented juice orange, we did not find studies like ours.
Purslane has some compounds which have antioxidant and antimicrobial activities, namely against E. coli [23-27]. Studies show that this plant is very versatile, and it has important capacity to inhibit bacteria [27]. Prickly pear vinegar has been studied because of its importance on food and human health and there are studies with some information about antioxidant and antimicrobial activities [13,28,29]. Depending on the method of extraction and the cultivar, antioxidant capacity could be more expressive, but some works indicate that it is important to smash garlic because the inner substances transform themselves in others with antioxidant potential [30,31].
The current study is part of the RESTORE - biopReparados Em SisTemas prOdução agRoEcológicos project, which aimed to identify the main biopreparations used by Portuguese farmers; detail the preparation methods; chemical characterization and microbiological activity of biopreparations; evaluate of its effects in vivo; and provide farmers with technical information on how to prepare and use biopreparations. Based on the most used by Portuguese farmers, seven biopreparations were selected, namely purslane vinegar (PV), prickly pear vinegar (PPV), fermented orange fruit juice (FJO), garlic extract (GE), nettle infusion (NI), decoction of horsetail (HD), and garlic and chilli biopreparation (GCP).
Methods
The biopreparations under study were produced according to the farmers recipes, collected in 2023 in six farms (Table 2). All were preserved fresh and by freezing.
The tests were conducted in four different moments: during the biopreparations’ manufacturing, after six months of frozen storage, and after three and six months of fresh storage.
For the chemical study of biopreparations, the analysis of total phenolic compounds was carried out, using the Folin-Ciocalteu method and the analysis of antioxidant activity, using the ABTS•+ (2,2′-Azino-bis(3) -Ethylbenzothiazoline-6-Sulfonic Acid)) and DPPH (2,2-Diphenyl-1-picrylhydrazyl). For each method, it was necessary to proceed with the calibration line, so gallic acid was used for the calibration line for the Folin-Ciocalteau method and trolox for the ABTS•+ and DPPH methods as a standard antioxidant. The results of total phenolic compounds are presented in relation to the corresponding gallic acid concentration. ABTS•+ and DPPH results were presented in correspondence with the percentage of inhibition.
In terms of microbial activity, the impregnated disc technique was performed. It used sterilized paper discs with a diameter of five milimeters, and it had used three repetitions for which biopreparation and per microorganism. Gram negative bacteria (Escherichia coli), Gram positive bacteria (lactic acid bacteria), fungi and yeasts (Penicillium spp.), and red-pigmented yeasts (Rhodotorula spp.) were selected for the in vitro tests.
Data was analyzed using the Statistical Package for the Social Science (SPSS) 29.0. Normality tests were carried out using the Kolmogorov-Smirnova Test, the homogeneity test was carried out using One-Way ANOVA and mean comparison tests were carried out to understand possible significant differences between the means, using the LSD tests for tests with homogeneous variances and Tamhane for inhomogeneous variances. For results that do not respond to normality, the Kruskal-Wallis non-parametric test was performed. Regarding microbiology evaluation and time and treatment, data were analyzed using principal components analysis (PCA) for understanding the correlation between time and treatment.
Results
Total phenolic compounds and antioxidant capacity
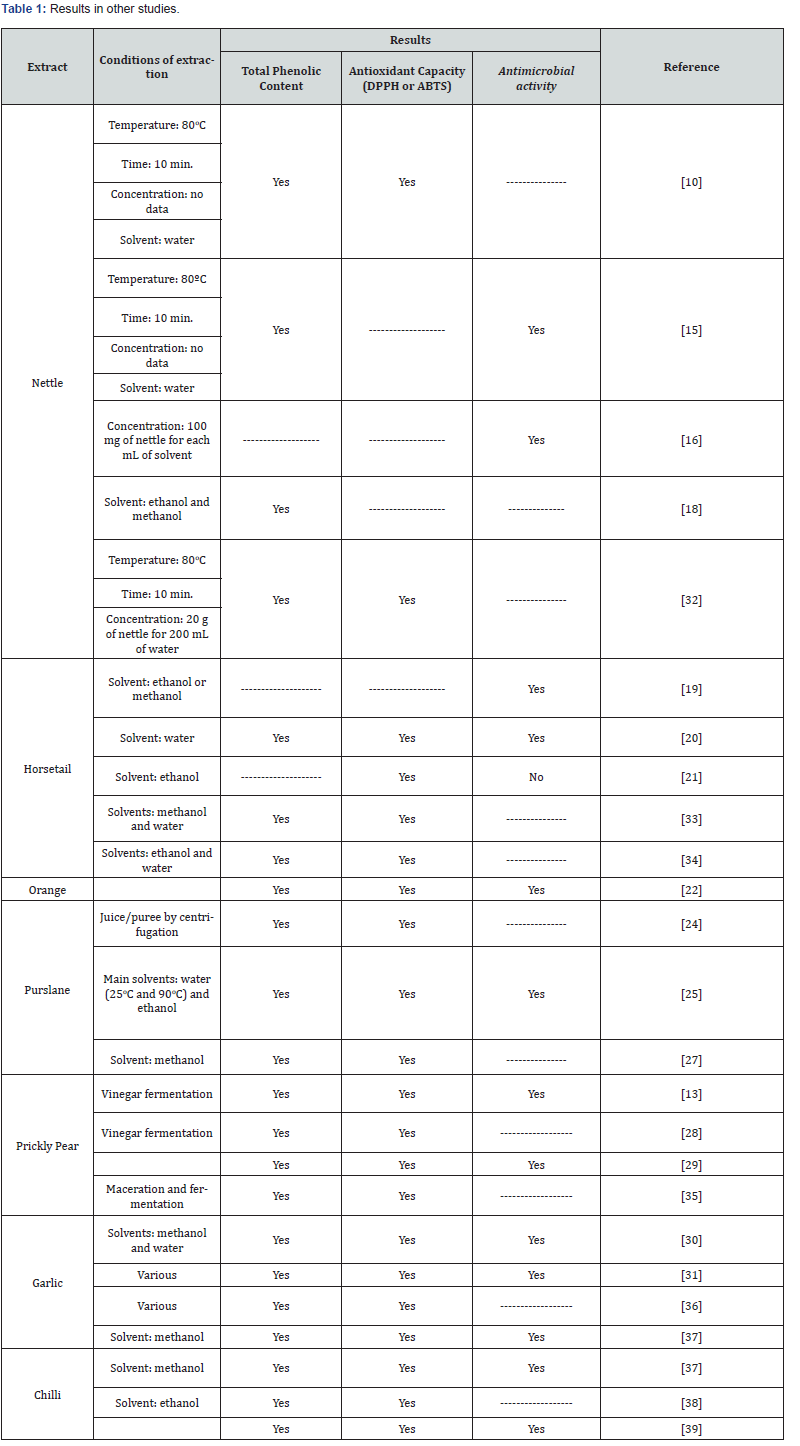
In average terms of total phenolic compounds, PPV has the highest phenolic content, followed by PV and FJO. All others show residual results. The biopreparation with the lowest average content of phenolic compounds was HD (Figure 1).
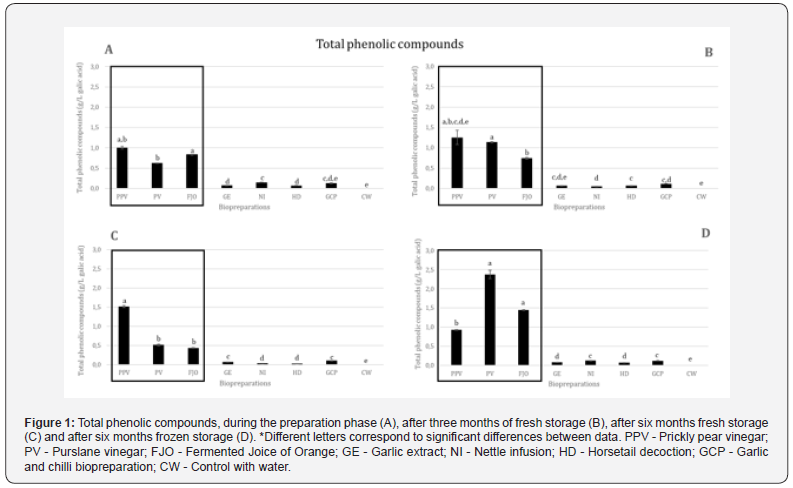
In Figure 1, we can see that the biopreparations with the best results over time and in all analyzes were PPV, PV and FJO; only after six months of conservation (Figure 1C), PPV did not obtain the highest phenolic content, demonstrating a small decrease in the phenolic content. PV has the third highest phenolic composition, at the time of preparation (Figure 1A), behind FJO, but after three months of fresh storage, like PPV, it presents an increase in phenolic compounds, which results in a higher content than FJO. The PV loses around 2/3 after six months of fresh storage, 1/6 of the content through freezing after six months (Figure 1D). All the others show, in general, a decrease in their phenolic compounds over time, except FJO, which shows an increase in phenolic content after six months of freezing. GCB appears very stable over time. HD and GE presented the lowest phenolic contents, except for three months of fresh storage, when NI presented the lowest phenolic content.
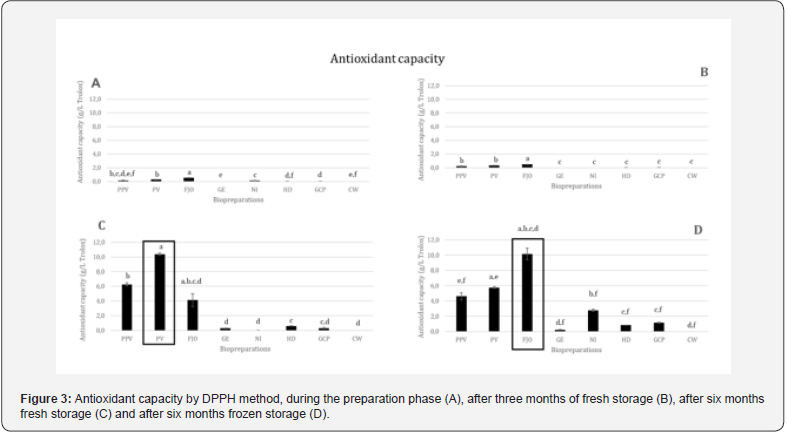
Regarding the antioxidant capacity by the ABTS•+ method and by the DPPH method, on average, the biopreparations with the highest capacity, in descending order, are FJO, PV and PPV. All others have residual antioxidant capacity (Figures 2 & 3).
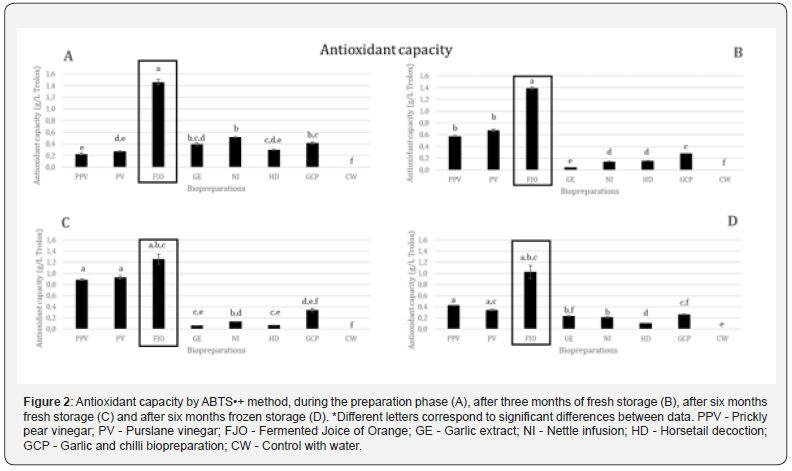
The specific results obtained by ABTS•+ method are in the Figure 2. FJO is the biopreparation that presents a greater antioxidant capacity in all ABTS•+ analyses, despite its decrease over time. At the time of finalizing the preparation (Figure 2A), NI has the highest antioxidant capacity after FJO with a large significant difference, followed by GCP. Throughout the process, its antioxidant capacity decreases. BAP decreases after three months of storage (Figure 2B), but its antioxidant capacity increases again when fresh, improving better than when frozen. PPV and PV biopreparations have the next highest antioxidant capacity, around 1/7 of the FJO, but, at the time of biopreparation completion, they are the ones that demonstrate the lowest antioxidant capacity. All biopreparations, with the exception of these, show that after three months of fresh storage they lose their antioxidant capacity; PPV and PV increase your antioxidant capacity to practically triple. At six months (Figure 2C), and compared to the initial moment, both increase their antioxidant capacity, but present greater antioxidant capacity, double and around triple when fresh than when frozen (Figure 2D), respectively for PPV and PV.
The specific results obtained by DPPH method (Figure 3) show that FJO is the biopreparation with the highest antioxidant capacity in all analyzes carried out, except in the analysis of fresh conservation six months after preparation (Figure 3C), in which PV has the highest antioxidant capacity. FJO decreases antioxidant capacity from the time of preparation (Figure 3A) to the three months of fresh storage (Figure 3B). However, after six months, both in fresh (Figure 3C) and frozen (Figure 3D) storage, the antioxidant capacity increases to values higher than at the time of preparation. Although both are beneficial, freezing storage, in terms of antioxidant capacity, is twice as good as keeping it fresh for six months. PPV and PV biopreparations, over time and regardless of the type of conservation, increase their antioxidant capacity. The best results are obtained after six months when kept cool. For the remainder, the antioxidant capacity is significantly lower in any of the analyses.
Microbiological activity inhibition
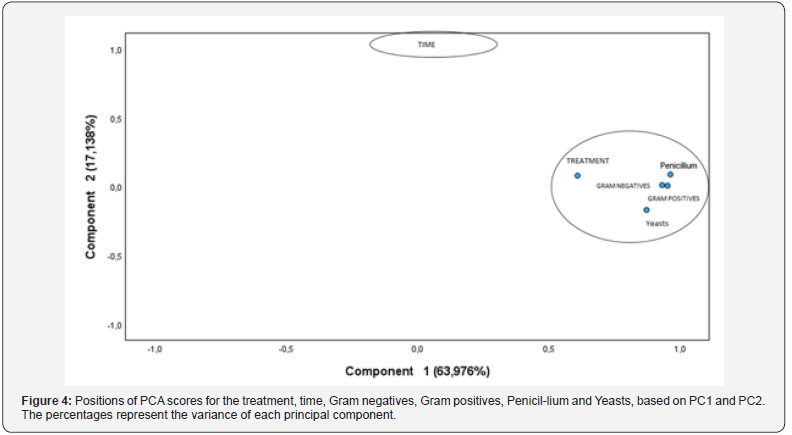
Regarding the correlation between data obtained using principal components analysis (PCA), between the microbiology evaluation and time and treatment, it is indicated that the greatest relationship established is precisely with the treatment and that time will have little or no effect on the results, which also justifies the results presented above. The PCA produced two interpretable components that together explain 81.115% of the total variance of the variables (Figure 4). The parameters were associated in two groups (KMO = 0.762). The most important component (PC1) explained 63.976% of the total variance. PC1 and is related to the antimicrobial effect on red pigmented yeasts, penicillium treatment, Gram negative and Gram-positive bacteria. PC2 explained 17.138 of the total variance and correlates over time.
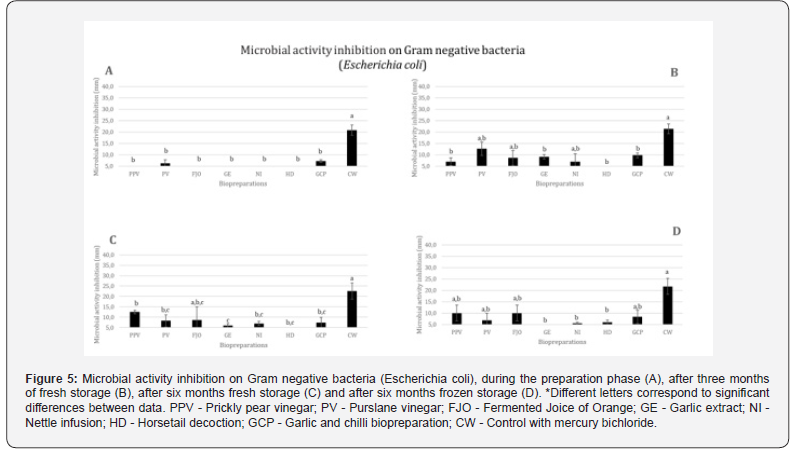
Generally speaking, PPV and PV have the greatest average values, while FJO and GCP show little variation. There are variations between these and GE, NI, and HD, but nothing noteworthy is shown. Practically minimal inhibitory activity is shown by HD.
At the time of preparation (Figure 5A), only PV and GCP show microbial inhibition. After three months of fresh storage (Figure 5B), only HD shows no inhibition. All the rest show inhibition, with PV and GCP standing out as those with the greatest inhibition. PV increases inhibition in the first three months of storage but decreases after six months of storage (Figure 5C). FJO shows inhibition after 3 months of fresh storage and decreases in the following months; Frozen (Figure 5D) is its biggest inhibition. VVP shows the best results after six months of fresh storage and the same inhibition as FJO when frozen. GE presents its best inhibitory result after three months of fresh storage, which decreases when fresh and does not show any inhibition when frozen. HD shows only residual inhibition after six months of freezing.
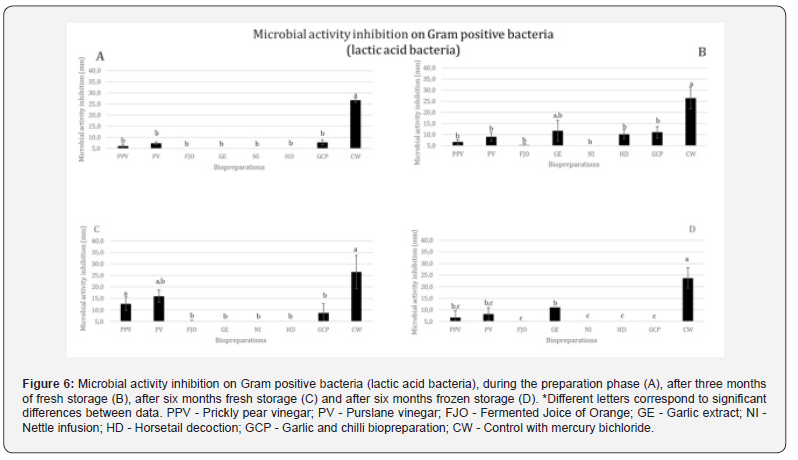
Regarding the general average results, for Gram positive bacteria and lactic acid bacteria, PV presents the highest average inhibition, seconded by PPV, GE and GCP. DC shows a residual medium inhibition and FJO and NI show no inhibition (Figure 6).
Only PPV, PV, and GCP exhibit inhibition of Gram-positive activity at the time of biopreparations finalization (Figure 6A), with PPV exhibiting persistent inhibition. The highest levels of inhibition are seen by GPC, HD, and GE after three months of fresh storage (Figure 6B); it should be emphasized that HD only exhibits inhibition during this period for Gram positives. FJO now exhibits highly residual inhibition, which is maintained under the same circumstances for up to six months (Figure 6C). NI never shows any inhibition.

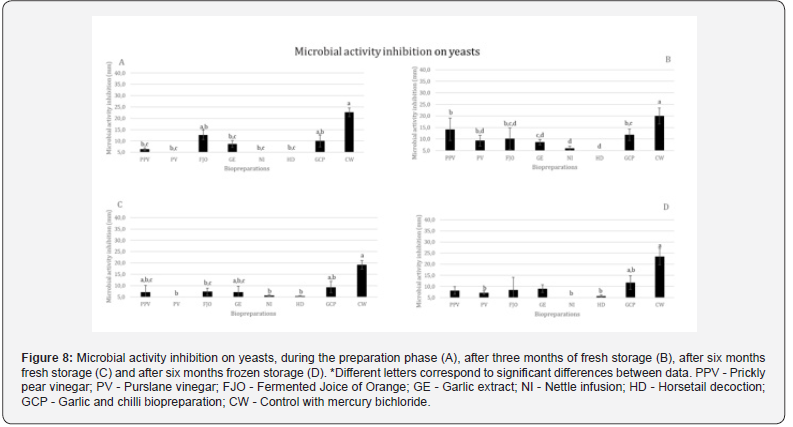
When it comes to the findings on fungi, assessed as the average inhibition of Penicillium spp., GCP exhibits the best results; in contrast, NI and HD show either no inhibition at all or very little inhibition. Results from PPV, PV, FJO, and GE are similar (Figure 7).
As shown in Figure 7A, only FJO, GE, and GCP exhibit inhibition to Penicillium at the time of production. Following three months of conservation, FJO completely lost its ability to inhibit, while NI and HD continue to display no inhibition, and GE exhibits a modest reduction in its inhibitory activity (Figure 7B). After six months in fresh storage, only PPV, PV, and GCP exhibit Penicillium inhibition (Figure 7C). Only NI exhibits no inhibition at all after six months of freezing (Figure 7D); DC exhibits very residual inhibition; GE exhibits the same inhibition as it did during preparation; GCP exhibits its largest inhibitory halo; and FJO exhibits less inhibition than it did at first. Results from PPV are superior to those from three months of fresh storage, however.
Overall, they all exhibit yeast inhibition, which is very residual in the case of HD. GCP is the biopreparation that exhibits the highest level of inhibition, followed by FJO and PPV in that order (Figure 8).
At the time of preparation (Figure 8A), PV, NI and HD do not show any inhibition to yeast. FJO presents the greatest inhibition of yeast at the time of preparation and loses this capacity over time, including through freezing. HD only shows an inhibition, albeit residual, after six months, both frozen and fresh. PV shows inhibition frozen for six months and fresh after three months of storage (the latter with the greatest inhibition). When stored fresh for three months (Figure 8B), PPV (greatest inhibition) and GCP (second greatest inhibition) increase their inhibitory capacity, compared to the initial one. However, three months later (Figure 8C), they show slightly lower results than those obtained at the time of preparation. In terms of freezing conservation (Figure 8D), PPV and GE do not show any inhibition.
Discussion
Vinegars contain many phenolic and antioxidant compounds, some of which have properties that inhibit microbial development [13,40], such as acetic acid. Purslane can be used to treat bacterial infections [27,41] and has a high content of phenolic compounds and substances with antioxidant capacity [26,27,41]. The same is indicated in the literature about oranges [5]. During the different phases of the process of fermentation of the prickly pear vinegar, the content of total phenolics and antioxidant capacity changes [13], which this study can show. Thus, this could explain the results and the different effects in microorganisms. PPV, PV and FJO are the biopreparations with the best results in terms of total phenolic composition and antioxidant capacity, which were those that included sugar in their composition and went through a fermentation process, lasting at least one week to ninety days. The results obtained on FJO, PPV and PV find similarities with other studies [22,26-28].
Regarding conservation, PPV can be stored for at least six months in a dark, cool environment maintaining its phenolic content; PV and FJO exhibit superior performance following a sixmonth freeze, so it might make sense to store them in this manner as well. Although garlic and chilli are described in the literature as a product with components capable of inhibiting microbial development and as a bactericide and fungicide [5,31,37], some indicate that garlic extract with water or methanol or ethanol as solvents do not have any antimicrobial effects on E. coli [31]. The results obtained were less expressive than expected, but they are consistent at the preparation moment and after freezing conservation on E. coli with GE, but GCP do not demonstrate a big difference for this biopreparation. However, GE and GPC demonstrate more antimicrobial effects against fungi. About the total phenolic content and antioxidant capacity, although studies show that garlic and chilli extracts have some [5,30,31,37], our results show low content and antioxidant activity for GE and GCP and the results has decreased after fresh and frozen conservation, which is according to other studies [14,36].
Compared to other studies, the results in terms of phenolic compound content and antioxidant capacity of the nettle infusion were at least ten times higher, as can be seen when comparing the results of this study with Table 1. According to the results obtained in studies on the extraction of compounds from extracts with ethanol or other solvents than water, it may make sense to use extracts based on ethanol and/or methanol in studies to further verify the potential effects, as it shows better results in terms of antioxidant capacity [18].
Studies indicate that horsetail extracts with water or ethanol as solvents have antioxidant capacity and phenolic compounds [19,20,33,34,42]. However, the horsetail decoction, in oxidising terms, fell short of our expectations. Regarding horsetail decoction, studies indicate that horsetail extract can have antimicrobial effect [19,20,42], but there are some that demonstrate that horsetail extract does not have antimicrobial activity for E. coli [19]. In our case, horsetail decoction does not have a significant antimicrobial effect. Most of the studies indicate that horsetail should be dried and/or sterilized [19-21,33,42], which it could be an important factor for a major extraction of its compounds and antimicrobial effects. In our studies, the horsetail was not dried before the procedure to the extraction with decoction.
Conclusion
The concentration of phenolic compounds, the antioxidant capacity, and the antibacterial activity of the seven studied biopreparations vary with time and depending on the type of conservation. Given the rise in phenolic compounds in PPV cases, fresh conservation is preferred; nonetheless, the use of the biopreparation is always more intense after some time of its elaboration.
About antioxidant capacity, only PPV and PV are worthy of keeping fresh over time. All the rest, even though they seek to preserve their antioxidant capacity over time, are not worth using over time due to their antioxidant capacity, as they are being lost.
One of the best biopreparations in terms of antibacterial activity is PPV. Conversely, HD and NI are among the biopreparations that exhibit the poorest outcomes across nearly all evaluations.
Only these two biopreparations are worth keeping fresh over time. All the rest, even though they seek to preserve their antioxidant capacity over time, are not worth using over time due to their antioxidant capacity, as they are being lost. It is important to continue the study of these and other biopreparations, focused on seed germination and plant growth, use of other plant species and natural substances, test other recipes, effects on different ecosystem components, such as soil, water and biodiversity.
Nevertheless, this work was an important step towards in the search for proven information to be systematized and compiled for better understanding and use in agriculture.
Acknowledgement
The authors would like to acknowledge the financial support from national funds to the Centre for Natural Resources, Environment and Society Studies (CERNAS) [project UIDB/00681/2020] funded by the Foundation for Science and Technology (FCT), PT and to project HARVEST – Valorizar a Horta fAmiliar de forma a educaR para uma dieta mediterrânica, saudáVel E SustenTável [PRR-C05-i03-I-0001].
References
- Castellanos L, Baldovino A, Céspedes N, Rivera X (2020) Biopreparations for the control of foliar diseases of strawberry, Pamplona, Columbia, even a partial solution. JONNPR 5(9): 933-951.
- Gonçalves MS (2005) Organic Waste Management (1st) Sociedade Portuguesa de Inovação (SPI), Portugal.
- Toundou O, Palanga KK, Simalou O, Abalo M, Woglo I, et al. (2020) Biopesticide Plants species of the mining area of Tokpli (South-Togo) effects on Okra (Abelmoschus esculentus) protection against Aphtona spp. Int J Biol Chem Sci 14(1): 225-238.
- Anarat CG, Sattely ES (2014) The Chemical Logic of Plant Natural Product Biosynthesis. Curr Opin Plant Biol 19: 51-58.
- Regnault Roger C, Philogène BJR, Vincent C (2010) Biopesticides of plant origin (en-edition). Ediciones Mundi-Prensa.
- Centro de Educación y Tecnologia (CET) INIA (2017) Biopreparations for the ecological management of pests and diseases. Chile.
- Lira AI (2011) Manual of Biopreparations for Organic Agriculture. Center for Education and Technology (CET), Santiago, Chile.
- Filho JGS, Almeida AS, Pinto-Zevallos D, Barreto IC, Cavalcanti SCH, et al. (2023) From plant scent defense to biopesticide discovery: Evaluation of toxicity and acetylcholinesterase docking properties for Lamiaciae monoterpenes. Crop Protection 164(106126): 1-12.
- Pino-Otín MR, Ballestero D, Navarro E, González-Coloma A, Val J (2019) Ecotoxicity of a novel biopesticide from Artemisia absinthium on non-target aquatic organisms. Chemosphere 216: 131-146.
- Jeszka SM, Zgola-Grzeskowiak A, Frankowski R, Grzeskowiak T, Jeszka AM (2022) Variation in the Content of Bioactive Compounds in Infusions Prepared from Different Parts of Wild Polish Stinging Nettle (Urtica dioica). Molecules 27(4242): 1-12.
- Repajic M, Cegledi E, Zoric Z, Pedisic S, Garofulic IE (2021) Bioactive compounds in Wild Nettle (Urtica dioica) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habit variations. Foods 10(190): 1-18.
- Saritas S, Portocarrero ACM, López JMM, Lombardo M, Koch W, et al. (2024) The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules 29(3941): 1-29.
- Hassen W, Alibi S, Hassen A, Mansour HB (2022) Assessment of physicochemical, microbiological and sensory quality of new product of cactus fruit (Opuntia ficus-indica) vinegar flavored with ginger and cinnamon. Journal of new sciences, Agriculture and Biotechnology 89(5): 5040-5051.
- Al-Dabbas M, Joudeh K, Abughoush M, Al-Dalali S (2023) Effect of processing and preservation methods on total phenolic contents and antioxidant activities of garlic (Allium sativum). J Chil Chem Soc 68(2): 5822-5826.
- Elez Garofulic I, Malin V, Repajic M, Zoric Z, Pedisic S (2021) Phenolic Profile, Antioxidant Capacity and Antimicrobial Activity of Nettle Leaves Extracts Obtained by Advanced Extraction Techniques. Molecules 26(6153): 1-16.
- Modarresi Chahardehi A, Ibrahim D, Fariza-Sulaiman S, Mousavi L (2012) Screening antimicrobial activity of various extracts of Urtica dioica. Rev Biol Trop 60(4): 1567-1576.
- Gülçin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME (2003) Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica). J Ethnopharmacol 90(2004): 205-215.
- Kukric ZZ, Topalic-Trivunovic LN, Kukavica BM, Matos SB, Pavicic SS (2012) Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica). APTEFF 43: 257-272.
- Krystsova M, Koscová J, Kohuch T, Savenko M, Spivak N (2021) Antimicrobial, Antibiofilm-forming Properties of Equisetum arvense L. Shoot Extracts. Curr Pers MAPs 4(1): 50-57.
- Mir MA, Ahraf MW, Mir BA (2021) Antimicrobial and Antifungal and Phytochemical Analysis of Various Extracts of Equisetum Diffusum. Trends Biomater Artif Organs 35(2): 186-189.
- Pallag A, Filip GA, Olteanu D, Clichici S, Baldea I, et al. (2018) Equisetum arvense L. Extract Induces Antibacterial Activity and Modulates Oxidative Stress, Inflammation, and Apoptosis in Endothelial Vascular Cells Exposed to Hyperosmotic Stress. Oxid Med Cel Long 2018: 1-14.
- Razola-Díaz MC, Montijo-Prieto S, Guerra-Hernandéz EJ, Jiménez-Valera M, Ruiz-Bravo A, et al. (2024) Fermentation of Orange Peels by Lactic Acid Bacteria: Impacto n Phenolic Composition and Antioxidant Capacity. Foods 13 (1212): 1-15.
- Xu L, Gao G, Zhou Z, Wei Z, Sun W, et al. (2024) Fermented Purslane (Portulaca oleracea) Supplementation Enhances Growth and Immune Function Parallel to the Regulation of Gut Microbial Butryate Production in Weaned Piglets. Microorganisms 12 (1403): 1-15.
- Cagno RD, Filannino P, Vincentini O, Cantatore V, Cavoski I, et al. (2019) Fermented Portulaca oleracea Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury. Nutrients 11(248): 1-18.
- El-Sayed MI, Ibrahim AA, Awad S (2019) Impact of Purslane (Portulaca oleracea) Extract as Antioxidant and Antimicrobial Agent on Overall Quality and Shelf Life of Greek-Style Yoghurt. Egypt J Food Sci 47(1): 51-64.
- Souza PG, Rosenthal A, Ayres EMM, Teodoro AJ (2022) Potential Functional Food Products and Molecular Mechanisms of Portulaca oleracea on Anticancer activity: A Review. Oxidative Medicine and Cellular Longevity 2022: 1-9.
- Fernández-Poyatos MP, Llorent-Martínez EJ, Ruiz-Medina A (2021) Phytochemical Composition and Antioxidant Activity of Portulaca oleracea: Influence of the Steaming Cooking Process. Foods 10(94): 1-14.
- Wit M, Toit A, Osthoff G, Hugo A (2020) Antioxidant Content, Capacity and Retention in Fresh and Processed Cactus Pear (Opuntia ficus-indica and robusta) Fruit Peels from Different Fruit-Colored Cultivars. Front Sustain Food Syst 4(133): 1-14.
- Giraldo-Silva L, Ferreira B, Rosa E, Dias ACP (2023) Opuntia ficus-indica Fruit: A Systematic Review of its Phytochemicals and Pharmacological Activities. Plants 12(543): 1-31.
- Petropoulos S, Fernandes A, Barros L, Ciric A, Sokovic M (2017) Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chemistry 245(2018): 7-12.
- Kallel F, Driss D, Chaari F, Belghith L, Bouaziz F (2014) Garlic (Allium sativum) husk waste as a potential source of phenolic compounds: Influence of extracting solvents on its antimicrobial and antioxidant properties. Industrial Crops and Products 62(2014): 34-41.
- Karakaya S, El SN (2006) Total Phenols and Antioxidant Activities of Some Herbal Teas and In Vitro Bioavailability of Black Tea Polyphenols. GOÜ Ziraat Fakültesi Dergisi 23(1): 1-8.
- Mimica-Dukic N, Simin N, Cvejic J, Jovin E, Orcic D, et al. (2008) Phenolic Compounds in Field Horsetail (Equisetum arvense) as Natural antioxidants. Molecules 13: 1455-1464.
- Ataseven S, Misirli D, Uzar F, Türkan NN, Elmastas M (2021) Determination of Phenolic compound composition of Water and Ethanol Extracts of Horsetail (Equisetum arvense). Journal of Integrative and Anatolian Medicine 2(2): 3-9.
- Çaglar A, Akarca G, Tomar O, Ekmekçi E (2020) Some Physicochemical and Sensory Properties of Cactus Fruit (Opuntia ficus-indica) Vinegar produced by Traditional Method. European Journal of Science and Technology 18: 952-957.
- Bozin, B, Mimica-Dukic N, Samojlik Goran A, Igic R (2008) Phenolics as antioxidant in garlic (Allium sativum, Alliaceae). Food Chemistry 111(2008): 925-929.
- Nagy M, Socaci SA, Pop C, Muresan C, Pop A (2015) Determination of Total Phenolics, Antioxidant Capacity and Antimicrobial Activity of Selected Aromatic Spices. Food Science and Technology 72(1): 82-85.
- Wangcharoen W, Morasuk W (2007) Antioxidant Capacity and Phenolic Content of Chillies. Kasetsart J (Nat Sci) 41: 561-569.
- Singh N, Yadav SS (2022) A review on health benefits of phenolics derived from dietary spices. Current Research in Food Science 5: 1508-1523.
- Soares VCC (2022) Antimicrobial and antioxidant properties of fruit vinegars. Master Thesis, University of Algarve, Portugal.
- Plants for a Future (2023) Portulaca Oleraceae technical sheet.
- Boeing T, Moreno KGT, Junior AG, Silva LM, Souza P (2021) Phytochemistry and Pharmacology of the Genus Equisetum (Equisetaceae): A Narrative Review of the Species with Therapeutic Potential for Kidney Diseases. Hindawi Evidence-Based Complementary and Alternative Medicine p: 1-17.






























