Larvicidal and Pupicidal Activity of Indigenous Entomopathogenic Nematodes Against Soil-dwelling Stages of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Under Laboratory and Greenhouse Conditions in Saudi Arabia
Mohamed Baha Saeed1,2,4,5,6*, Yousef A Algarzaee1, Abdulaziz M Alnasser1, Mohamed A Aloqayli1, Khalid A Alhudaib3 and Mark D Laing6
1Natural Organic Agriculture Center, Ministry of Environment Water and Agriculture, Saudi Arabia
2Saudi Organic Farming Association, Ministry of Environment Water and Agriculture, Saudi Arabia
3Pests and Plant Diseases Unit, College of Agriculture and Food Sciences, King Faisal University, Saudi Arabia
4Department of Crop Protection, Faculty of Agriculture, University of Khartoum, Sudan
5Center of Excellence for Pesticides and Plant Health, Faculty of Agriculture, University of Khartoum, Sudan
6Discipline of Plant Pathology, School of Agricultural, Earth and Environmental Science, University of KwaZulu-Natal, South Africa
Submission: August 07, 2023; Published: August 16, 2023
*Corresponding author: Mohamed Baha Saeed, Natural Organic Agriculture Center, Saudi Organic Farming Association, Ministry of Environment Water and Agriculture, Saudi Arabia, Department of Crop Protection, Faculty of Agriculture, Center of Excellence for Pesticides and Plant Health, Faculty of Agriculture, University of Khartoum, Sudan, Discipline of Plant Pathology, School of Agricultural, Earth and Environmental Science, University of KwaZulu-Natal, South Africa
How to cite this article: Mohamed Baha S, Yousef A A, Abdulaziz M A, Mohamed A A, Khalid A A, et al. Larvicidal and Pupicidal Activity of Indigenous Entomopathogenic Nematodes Against Soil-dwelling Stages of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Under Laboratory and Greenhouse Conditions in Saudi Arabia. Agri Res & Tech: Open Access J. 2023; 27 (4): 556386. DOI: 10.19080/ARTOAJ.2023.27.556386
Abstract
The tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is a devastating pest worldwide affecting tomato production. The efficacy of soil treatments of the entomopathogenic nematode (EPN) Heterorhabditis indica strains NOAC.N1 and NOAC.N2 were determined against the fourth instar larvae (L4 larvae) and pupae at one, three, and five days old of T. absoluta. In the laboratory, each EPN strain was applied at a dose of 0.00, 50,100, and 150 infective juveniles (IJs) cm2. Both strains reduced the adult emergence to <50%, even at the lowest doses of 50 IJs cm2 when applied against L4 larvae. No adults of T. absoluta emerged from their pupae at one day old after treatment with both strains of EPNs at doses of 50, 100, and 150 IJs cm2, whereas more than adults emerged from 93% of the pupae of the control treatment (0.00 IJs cm2). Strain NOAC.N2 performed better than Strain NOAC.N1 and reduced adult emergence to <50% at a dose of 150 IJs cm2 against pupae at three and five days old. In the greenhouse, both strains were applied at a dose of 150 IJs cm2 against L4 larvae and pupae at different ages. Strain NOAC.N2 consistently provided a greater reduction in adult emergence than Strain NOAC.N1 and the control treatment. Therefore, the indigenous EPN strain NOAC.N2 could be used as a biological agent to control the soil-dwelling stages of T. absoluta in greenhouse conditions if it can be registered and commercialized for use by farmers.
Keywords: Biocontrol; Tuta absoluta; Heterorhabditis indica; EPN; adult emergence; pupae; L4 Larvae; Tomato
Introduction
Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) has invaded more than 90 countries outside of South America (EPPO 2021), thus becoming a serious threat to tomato (Solanum lycopersicum L.) production worldwide and, to a lesser extent, a pest of other economically important solanaceous crops, including potato (Solanum tuberosum L.), eggplant (Solanum melongena L.), pepper (Capsicum annum L.), and tobacco (Nicotiana tabacum L.) [1-4]. Tomato crops grown in greenhouses in the Middle East are under constant threat due to widespread infestations of T. absoluta [5].
The life cycle of this insect comprises four development stages: egg, larva, pupa, and adult. Females usually lay eggs on the underside of leaves or stems. Neonate larvae penetrate leaves, stems, or fruits, on which they feed and develop. Last instar larvae usually drop to the ground on a silk thread and pupate in the soil. Pupae complete their development in the soil and after a few days, adults emerge from the soil [6]. The impact of T. absoluta on tomato plants is as a result of direct feeding of larvae on photosynthetic areas of leaves and damage to fruit. In addition, feeding on the growing tips of plants shoots results in reducing and stopping plant development, with yield losses reaching 100% on tomatoes [3,4,7].
The economic importance of T. absoluta has led to management strategies being focused on pesticides [8]. However, some integrated pest management programs in tomato crops against T. absoluta are also based on biological control. Macrolophus pygmaeus (Rambur) (Hemiptera: Miridae) and Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae) have already been tested for their suitability as predators of T. absoluta [9-11]. These studies showed that such predators might be able to regulate T. absoluta populations under field conditions when a certain number is present in the crop.
Many entomopathogens such as Bacillus thuringiensis (Berliner) Sandeep Kumar et al. [12], Beauveria bassiana (Vuill.) and Metarhizium robertsii (Sorokin) Abdel-Baky et al. [13] have been tested against T. absoluta in laboratory, greenhouse and open staked-tomato field experiments. However, these biocontrol agents only target the stages of T. absoluta on the aerial parts of the plant (leaves, stems or fruits). Thus far, few biocontrol strategies have been developed to control the soilborne stages of T. absoluta, including the last instar larvae, pupae, and emerged adults.
Entomopathogenic nematodes (EPNs) are important biological control agents for a variety of economically important pests [14,15]. These nematodes, belonging to the families Steinernematidae and Heterorhabditidae, are obligate parasites that kill insects with the help of mutualistic bacteria that inhabit the intestine of the infective juveniles (IJs) [16]. They have been used with variable success against insects occupying different habitats. Most success has been achieved against soil dwelling pests or pests in cryptic habitats such as inside galleries in plants where IJs find protection from hostile environmental factors [17,18]. Although EPNs have been used against a number of soil-inhabiting coleopterous insects, less information is available about their use against lepidopterous insects in soil. This is the first study we are aware of to evaluate the activity of indigenous strains of entomopathogenic nematodes of Heterorhabditis. indica to control the soil-dwelling stages of T. absoluta in laboratory and greenhouse trials under Saudi Arabia conditions.
Material and methods
Isolation and maintenance of nematodes
The nematodes strains NOAC.N1 and NOAC.N2 used in this study were isolated from soil samples from the National Organic Agriculture Center (NOAC) and Algosamy organic farm, Algassium region, Saudi Arabia, using Galleria mellonella (L.) (Lepidoptera: Pyralidae) as a live insect bait [19]. The nematodes were identified using PCR by Macrogen Inc., Korea (https://www.macrogen.com/en/main, accessed on 27 January 2023). IT5 primer (5'- GGAAGTAAAAGTCGTAACAAGG-3') and the reverse ITS4 primer (5'-TCCTCCGCTTATTGATATGC-3') were used to amplify the entire ITS region [20]. The accession number with Macrogen Inc., are (OQ427656) and (OQ427655) for NOAC.N1 and NOAC.N2, respectively. Both H. indica strains NOAC.N1 and NOAC.N2 were cultured in G. mellonella, which were reared on artificial media according to [21]. The IJs were recovered in tap water according to Kaya & Stock [22], upon emergence, stored at 12-14 oC, and used within two weeks of harvest.
Laboratory experiments
Effects of entomopathogenic nematode against absoluta L4 larvae
indica strains NOAC.N1 and NOAC.N2 were tested for their virulence against T. absoluta L4 larvae. For each strain, a dose of 0.00, 50,100, 150 IJs cm2 were used. 1 kg of sun sterilized soil with an initial soil moisture content of 0.97%, was added in plastic boxes (15x 10 x 5 cm3), EPNs were pipetted onto the soil surface in each plastic box in a 50 ml of sterile tap water. The final moisture level was at field capacity (23.2% v/w). Twenty-four hours later, fifteen T. absoluta L4 larvae, collected from a naturally infested tomato plantation (cultivar Newton, cultivated at the NOAC under greenhouse conditions) were released on the soil surface of each box. All boxes were incubated at 25 ± oC. Three replicates were used for each dose. The Control (0.00 IJs) was treated with sterile tap water only. To evaluate the larval mortality, nine days post-inoculation, the number of emerging adults was calculated every day for four days. Treatments were arranged in a 2 x 4 factorial experiment, arranged in a randomized complete block design (RCBD).
Effect of entomopathogenic nematode against different pupae ages of absoluta
Both H. indica strains were tested against pupae at one, three, and five days old (time after the larvae were placed on the soil). To ensure the exact pupae age, fifteen T. absoluta L4 larvae were released on plastic boxes (15x 10 x 5 cm3) containing 1 kg of soil, and then each group of pupae were challenged at one, three or five days later. For each pupal age, a separate experiment was conducted using both strains at a dose of 0.00, 50,100, 150 IJs cm2. The EPNs were pipetted onto the soil surface in each plastic box in 50 ml of sterile tap water. Each dose was replicated three times. The Control was with water only. The experiments were a 2x4 factorial experiment arranged in a randomized complete block design (RCBD). To evaluate pupae mortality, nine, seven- and five-days post-inoculations, the number of emerged adults was counted every day for four days from pupae at one, three and five days old, respectively.
Greenhouse experiments
Effects of entomopathogenic nematode against absoluta L4 larvae and different pupae ages
For both strains, the best dose of 150 IJs cm2 from the previous experiments was tested against T. absoluta L4 larvae in greenhouse conditions. Plastic boxes of (20x 15 x 10 cm3) were used in this experiment. Each box was opened from the lower side using a sharp knife. The upper side of the boxes was covered with insect net material to facilitate air circulation. The boxes were then dug into the soil surface under growing tomato plants. EPNs were added on the soil surface in each plastic box in a 100 ml of tap water, using 10 ml medical syringe. Twenty-four hours later, twenty T. absoluta L4 larvae were released in each plastic box. Treatments were replicated three times. The control treatment received tap water only. For pupae experiment, the same two strains of EPNs were tested at doses of 0.00 and 150 IJs cm2. Different pupae ages were prepared as mentioned previously, with using twenty L4 larvae. Nematode application was as discussed previously, and pupae mortality data was collected as mentioned previously. The experiment was a 2x2 factorial experiment arranged in a randomized complete block design (RCBD). Treatments were replicated three times. The control treatment received tap water only.
Statistical analysis
Mortalities data of T. absoluta L4 larvae and pupae at different ages were subjected to analysis of variance (ANOVA). Means were compared using Duncan multiple range test at a 5.0% level of significance. The analyses were conducted using GenStat for Windows, 21th edition.
Results
Laboratory trial
Effect of EPNs against absoluta L4 larvae
Highly significant differences were observed between the tested doses (F= 286.39; P<0.001; df = 3). However, there were no significant differences between the two EPN strains (F= 2.57; P= 0.131; df = 1), and there was no interaction effect between doses and strains (F= 2.10; P= 0.147; df = 3) for their efficacy against T. absoluta L4 larvae.
A polynomial response to dose was observed with R2 > 0.9. Adult emergence reduced as a function of dose increase (Figure 1). H. indica strains NOAC.N1 and NOAC.N2 were pathogenic against T. absoluta L4 larvae and reduced adult emergence at all doses compared to the control treatment. Strain NOAC.N2 at the highest dose of 150 IJs cm2 reduced the adult emergence to 22.6% compared to 25% by Strain NOAC.N1. Both strains caused reductions in adult emergence <50% even at the lowest doses of 50 IJs cm2 (Figure 2). Observation by stereo microscope (Euromex, Netherland) showed the emergence of IJs from the dead cadaver (Figure 3).
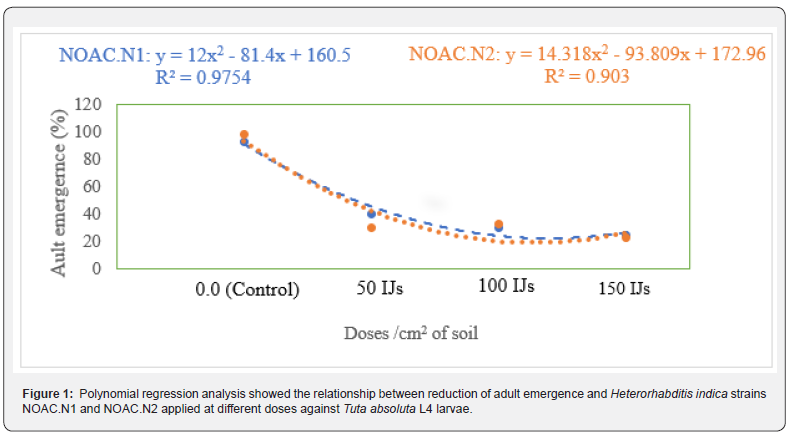
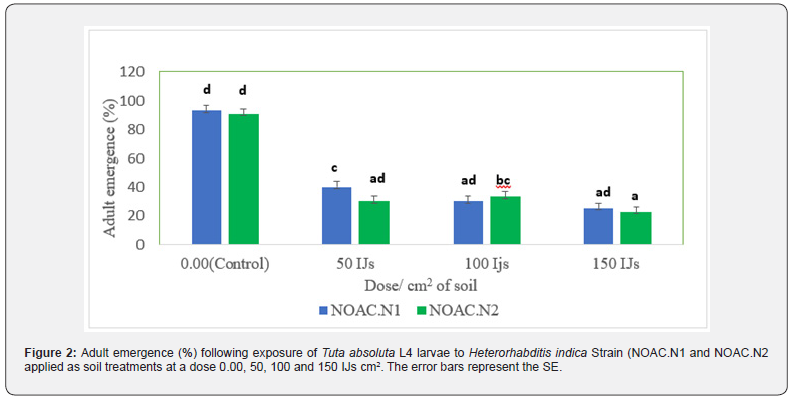

Effects of entomopathogenic nematode against absoluta L4 larvae and different pupae ages
For all tested pupal ages, no significant differences were observed between the EPN strains. However, highly significant differences were observed between doses. No significant interactions effects were found between strains and doses (Table 1). Generally, pupae at one day old were more susceptible to the nematode infection, followed by pupae at three days old and five days old. No adult emergence was observed from pupae at one day old after treatment with H. indica strains NOAC.N1 and NOAC.N2 at a dose of 50, 100 and 150 IJs cm2. In contrast, >93% of adults emerged in the control treatment (0.00 IJs) (Table 1) Increased levels of adult emergence were observed on pupae at three and five days old following exposure to the same strains at the same doses. H. indica Strain NOAC.N2 at a high dose of 150 IJs cm2 reduced the adult emergence to <50% against pupae at three and five day old (Table 1). A linear response to dose was observed with all tested pupal ages and strains with R2 > 0.6. Adult emergence reduced as the result of EPN dose increases (Figure 4).
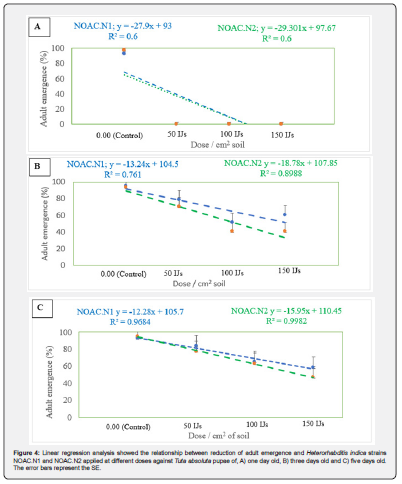
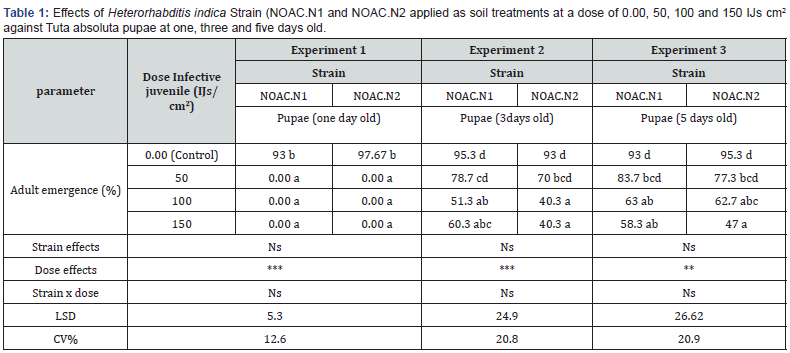
Means followed by the same letter do not differ significantly at P ˂0.05 according Duncan multiple range test. asterisks indicate significant differences at *** P<0.001 **P<0.01
Greenhouse experiment
Effect of entomopathogenic nematode against absoluta L4 larvae and different pupae ages
There were significant differences between the two tested Strains (F= 12.48; P<0.012; df = 1). Doses, (F= 97.13; P<0.001; df = 1). and the interaction between Strain and Dose effects were also significant (F= 12.48; P<0.012; df = 1). Application of H. indica strain NOAC.N2 caused a reduction in adult emergence to 35.3% compared to 67% by Strain NOAC.N1 when applied at a dose of 150 IJs cm2 against L4 larvae in greenhouse conditions. With the Control treatment, 95.3% of adults emerged (Figure 5). H. indica Strain NOAC.N2 performed better than Strain NOAC.N1 in reducing the adult emergence following application pupae at three and five days old However, both strains reduced adult emergence to <30% when applied against pupae at one day old (Table 2). Strain NOA.N2 at a dose of 150 IJs cm2 reduced the adult emergence to the level of 51.67% even against pupae at five days old, compared to a higher level of emergence of 85% after treatment with Strain NOAC.N1. Again, >93% of adults emerged after the control treatment (Table 2).
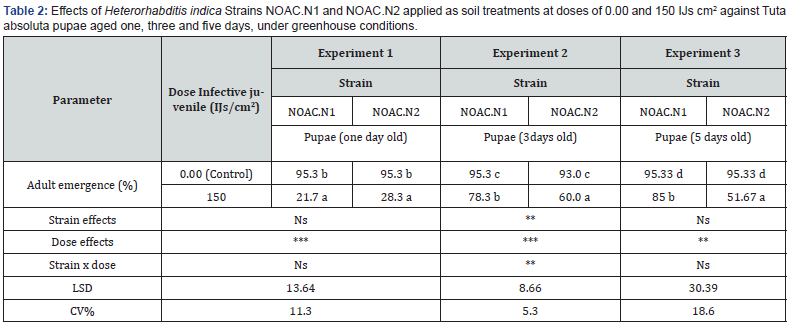
Means followed by the same letter do not differ significantly at P ˂0.05 according Duncan multiple range test. asterisks indicate significant differences at ***P<0.001 **P<0.01*P<0.05
Discussion
Our study demonstrated the potential of two indigenous EPNs strains when applied as IJs as a soil treatment to control L4 larvae and pupae of T. absoluta in both laboratory and greenhouse experiments. Low levels of adult emergence of 22.6% and 25% were observed following soil treatments at a dose of 150 IJs cm2 by H. indica strains NOAC.N1 and NOAC.N2, respectively. Both strains caused a reduction in adult emergence of less than 50%, even at the lowest dose of 50 IJ cm2. Our results agree with those of Garcia-del-Pino, Alabern, & Morton [23], who studied the ability of EPNs to find and infect L4 larvae of T. absoluta in soil. We also observed the emergence of fresh IJs from dead cadavers, which would have the potential to extend the epizootic to other larvae and pupae, reflecting the ability of EPNs as biocontrol agents to multiply in their target hosts and extend the scale and efficacy of control as long as there are target insects in the soil. Although the pupal stage has been considered to be less susceptible to EPNs than other life stages Malan, Knoetze, & Moore [24], Steyn, Malan, & Addison [25], Vicente-Díez et al [26], in our trial, no adults emerged from pupae at one day old after treatment with H. indica strains NOAC.N1 and NOAC.N2 at a dose of 50, 100, and 150 IJs cm2, whereas adults emerged from more than 93% of the pupae of the control treatment. We postulate that the efficacy of the EPNs against pupae was because the pupae are sessile, and at only one day into the pupation process, the silk covering of the pupae would have been incomplete. Combined, these two factors would have made it relatively easy for IJs to find, penetrate and kill the pupae.
Interestingly, both EPNs strains were able to infect and kill three and five day old pupae, with Strain NOAC.N2 killing >50% of the pupae. In doing so, the EPN’s demonstrated an ability penetrate the completed silk cocoons of mature pupae, and to infect the pupae. This result contradicts the finding of Garcia-del-Pino et al. [23], who observed no mortality of in the T. absoluta pupal stage following exposure to three EPNs (Steinernema carpocapsae, S. feltiae, and Heterorhabditis bacteriophora). Batalla-Carrera, Morton, & García-del-Pino [27] also observed that pupae were relatively resistant to the IJs of EPNs in laboratory trials conducted in Petri dishes on filter paper. Another observation in this study was that some adults were infected and killed by IJs after they emerged from the soil, which enhanced the overall efficacy of the two strains of EPNS as biocontrol agents.
Overall, the environmental conditions greenhouse tomato production appears to be compatible with the environmental requirements for these EPNs to achieve high levels of infection of the soilborne stages of T. absoluta. H. indica Strain NOAC.N2 performed better than NOAC.N1 against L4 larvae and three pupal ages of T. absoluta, and reduced the adult emergence to 35.3% when applied against L4 larvae. Interestingly, reduced the adult emergence to the level of 51% was also recorded with H. indica Strain NOAC.N2 at a dose of 150 IJs cm2 when applied against pupae at five days old, compare to an 85% adult emergence after treatment with Strain NOAC.N1, and more than 95% adult emergence in the control treatment. This result confirmed that the pupal stage is less susceptible to EPNs than the L4 larvae, and required higher doses of IJs. In similar research Andaló et al. [28] documented a mortality level of 80% against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) pupa after they were treated with IJs of Heterorhabditis amazonensis (Andalo et al.) at doses of 600 and 800 IJs pupa-1. Vicente-Díez et al. [26] also found that killing pupae required more IJs than were needed to control larvae of the grapevine moth, Lobesia botrana (Denis & Schiffermuller) (Lepidoptera: Tortricidae).
In the present study, H. indica strain NOAC.N2 demonstrated its potential to control the soilborne stages of T. absoluta. Therefore, it could be incorporated into management programs for greenhouse-grown tomatoes as soil applications, if the EPN is successfully registered and manufactured for farmers’ use. Some additional greenhouse studies will be necessary to optimize the efficacy of EPNs for T. absoluta with regards to application volumes, use of adjuvants, timing of applications regarding the presence of different pest stages, and importance of foliar versus soil applications, and a cost-benefit analysis of using both foliar and soil applications.
References
- Aatif HM, Afzal A, Idrees A, Mansha MZ, Hanif CMS, et al. (2023) Entomopathogenic nematodes for the control of oriental fruit fly Bacterocera dorsalis (Diptera: Tephritidae). Journal of King Saud University-Science 35(1): 102428.
- Abdel-Baky NF, Alhewairini SS, Al-Azzazy MM, Qureshi MZ, Al-Deghairi MA, et al. (2021) Efficacy of Metarhizium anisopliae AND Beauveria bassiana against Tuta absoluta (Lepidoptera: Gelechiidae) Eggs Under Laboratory Conditions. Pakistan Journal of Agricultural Sciences 58(2).
- Andaló V, Faria LS, Carvalho FJ, Assis GA, Santos V, et al. (2021) Entomopathogenic nematodes for the control of Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) pupae. Arquivos do Instituto Biológico 88(8): e00742019.
- Arno J, Sorribas R, Prat M, Matas M, Pozo C, et al. (2009) Tuta absoluta, a new pest in IPM tomatoes in the northeast of Spain. IOBC/ WPRS Bull 49: 203-208.
- Bajracharya ASR, Bhat B (2018) Life cycle of South American tomato leaf miner, Tuta absoluta (Meyrick, 1917) in Nepal. Journal of Entomology and Zoology Studies 6(1): 287-290.
- Batalla CL, Morton A, García-del-Pino F (2010) Efficacy of entomopathogenic nematodes against the tomato leafminer Tuta absoluta in laboratory and greenhouse conditions. BioControl 55(4): 523-530.
- Bedding R, Akhurst R (1975) A simple technique for the detection of insect paristic rhabditid nematodes in soil. Nematologica 21(1): 109-110.
- Begley J (1990) Efficacy against insects in habitats other than soil. Entomopathogenic Nematodes in Biological Control (GAUGLER, R. & KAYA, HK, Eds) CRC Press, Boca Raton, FL, pp. 215-231.
- Biondi A, Guedes RNC, Wan FH, Desneux N (2018) Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: past, present, and future. Annual review of entomology 63: 239-258.
- Campos MR, Biondi A, Adiga A, Guedes RN, Desneux, N (2017) From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. Journal of Pest Science 90(3): 787-796.
- De Backer L, Megido RC, Haubruge E, Verheggen FJ (2014) Macrolophus pygmaeus (Rambur) as an efficient predator of the tomato leafminer Tuta absoluta (Meyrick) in Europe. A review. BASE.
- Desneux N, Luna MG, Guillemaud T, Urbaneja A (2011) The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. Journal of Pest Science 84(4): 403-408.
- Desneux N, Wajnberg E, Wyckhuys KA, Burgio G, Arpaia S, et al. (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. Journal of Pest Science 83(3): 197-215.
- Garcia-del-Pino F, Alabern X, Morton A (2013) Efficacy of soil treatments of entomopathogenic nematodes against the larvae, pupae and adults of Tuta absoluta and their interaction with the insecticides used against this insect. BioControl 58(6): 723-731.
- Illakwahhi DT, Srivastava BBL (2017) Control and management of tomato leafminer-Tuta absoluta (Meyrick)(Lepidotera, Gelechiidae). A review IOSR J Appl Chem 10: 14-22.
- Kaya HK, Stock SP (1997) Techniques in insect nematology Manual of techniques in insect pathology. Elsevier, pp. 281-324.
- Klein M (1990) Efficacy against soil-inhabiting ınsect pests: Entomopathogenic Nematodes in Biological.
- Malan AP, Knoetze R, Moore SD (2011) Isolation and identification of entomopathogenic nematodes from citrus orchards in South Africa and their biocontrol potential against false codling moth. Journal of invertebrate pathology 108(2): 115-125.
- Mama SS, Akutse KS, du Plessis H, Aigbedion PO, Mohamed SA, et al. (2022) Interactions between the Entomopathogenic Fungus Metarhizium anisopliae ICIPE 20 and the Endoparasitoid Dolichogenidea gelechiidivoris, and Implications for Combined Biocontrol of Tuta absoluta. Biology 11(9): 1323.
- Mansour R, Cherif A, Attia BS, Zappalà L, Grissa LK (2019) Tuta absoluta in Tunisia: ten years of invasion and pest management. Phytoparasitica 47(4): 461-474.
- Metwally HM, Hafez GA, Hussein MA, Hussein M, Salem H, et al. (2012) Low cost artificial diet for rearing the greater wax moth, Galleria mellonella L.(Lepidoptera: Pyralidae) as a host for entomopathogenic nematodes. Egyptian Journal of Biological Pest Control 22(1): 15.
- Mokrini F, Laasli SE, Benseddik Y, Joutei AB, Blenzar A, et al. (2020) Potential of Moroccan entomopathogenic nematodes for the control of the Mediterranean fruit fly Ceratitis capitata Wiedemann (Diptera: Tephritidae). Scientific reports 10(1): 1-11.
- Payne RW, Murray DA, Harding SA, Baird DB, (2019) GenStat for Windows, 21th VSN International, Hemel Hempstead, UK.
- Poinar GO (1990) Taxonomy and Biology of Steinernematidae and Heterorhabditidae. Entomopathog. Nematodes Biol Contro 54(2): 1-42.
- Sandeep KJ, Jayaraj J, Shanthi M, Theradimani M, Venkatasamy B, et al. (2020) Potential of standard strains of Bacillus thuringiensis against the tomato pinworm, Tuta absoluta (Meyrick)(Lepidoptera: Gelechiidae). Egyptian Journal of Biological Pest Control 30(1): 1-7.
- Steyn VM, Malan AP, Addison P2021) Efficacy of entomopathogens against Thaumatotibia leucotreta under laboratory conditions. Entomologia Experimentalis et Applicata 169(5): 449-461.
- Vicente DI, Blanco PR, Chelkha M, Puelles M, Pou A, et al. (2021) Exploring the use of entomopathogenic nematodes and the natural products derived from their symbiotic bacteria to control the grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). Insects 12(11): 1033.
- White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18(1): 315-322.






























