Morphological and TRAP Marker Data Integration for Early Stage Assessment of Salinity Tolerance in Some Sugarcane (Saccharum spp.) Varieties
Nour MMI1*, Moustafa M Eldakak2, Allabbody AHS1 and Mohamed M Yacout2
1Sugar Crops Institute, Agricultural Research Center, Giza, Egypt
2Department of Genetics, Faculty of Agriculture (El Shatby) Alexandria University, Alexandria, Egypt
Submission: July 26, 2023; Published: August 09, 2023
*Corresponding author: Nour MMI, Sugar Crops Institute, Agricultural Research Center, Giza, Egypt
How to cite this article: Nour M, Moustafa M E, Allabbody A, Mohamed M Y. Morphological and TRAP Marker Data Integration for Early Stage Assessment of Salinity Tolerance in Some Sugarcane (Saccharum spp.) Varieties. Agri Res & Tech: Open Access J. 2023; 27 (4): 556385. DOI: 10.19080/ARTOAJ.2023.27.556385
Abstract
Salinity stress poses a significant challenge to sprouting and formative growth stages of sugarcane, impacting both crop establishment and subsequent productivity. In order to enhance and develop more salt tolerance sugarcane verities, it is crucial to understand the genetic and morphological factors associated with salinity tolerance during the early stages. This study aims to explore the integration of morphological traits data and 14 combination of four TRAP markers related salinity tolerance ESTs and three arbitrary primers. Six varieties were examined, i.e. three commercial varieties (GT.54-9, G.3, and G.4), one salinity-tolerant (Bo34), one drought-tolerant (Co 419), and one salinity-sensitive variety F161. All varieties were evaluated under five salinity levels (0, 25, 50, 75, and 100mM NaCl) during 90 DAP. Statistical analysis confirmed the significant effects of both variety and treatments on most of the measured traits. Bo34 and Co419 displayed better salinity tolerance compared to other varieties, while F161 exhibited high susceptibility to salinity stress. Phenotypic similarities and dissimilarities among the sugarcane varieties were determined through Euclidean distance analysis. Co419 and G.3 showed the least dissimilarity, whereas Bo34 and F161 exhibited the greatest dissimilarity. In the TRAP marker analysis, out of 90 alleles, 88 were found polymorphic (97.78%). In conclusion, using phenotypic and genetic similarity derived from both morphological and TRAP marker data could be more useful tools for identifying individuals with more salinity-tolerance traits and for selecting parents in breeding programs.
Keywords: Sugarcane; Salinity Tolerance; TRAP Marker; Phenotypic Similarities; Genetic Similarity
Abbreviations: TRAP: Target Region Amplification Polymorphism; PCR:polymerase chain reaction; SCRI: Sugar Crops Research Institute; ARC: Agricultural Research Center; CRD: completely randomized design; ANOVA: UPGMA: analysis of variance; un-weighted pair group method with arithmetic average; GS: genetic similarity; PIC: Polymorphism Information Content; Rp: resolving power; HAT: Histone Acetyltransferase; GCN5: General Control Nonderepressible 5; CTL1: Chitinase-Like 1
Introduction
Sugarcane (Saccharum spp.) belongs to the Poaceae family and is one of the major sugar crops in the world. Sugarcane genotypes (Saccharum spp. hybrids) are complex polyploid plants 2n=100-130 [1]; These current cultivars are the result of interspecific crosses between S. spontaneum, which shows higher plant vigor and resistance to several pests and abiotic stresses, and S. officinarum, a species with high sugar content [2,3]. According to [4]) chromosome in situ hybridization revealed that S. officinarum has a basic chromosome number of x=10 and S. spontaneum has a basic chromosome number of x=8, indicating that two distinct chromosome organizations coexist in contemporary cultivars.
Abiotic stresses have a huge negative effect on plant development and production worldwide. These abiotic stresses include many types such as drought, salinity, chilling, and high temperature. In spite of these various types of abiotic stresses, salinity comes on the top regards how much effect and damage it can cause to various crops [5]. It has been estimated that almost 20 % of cultivable lands (including 6% of irrigated lands) in the world are affected by salinity (expected to reach 50% of lands by 2050) [6]. In Egypt, salinity affects around 0.9 million hectares (33% of irrigated lands) [7]. In Egypt, sugarcane is a major crop occupying approximately 136 thousand hectares of cultivated land, contributing to about 34% of the country’s total sugar production in the 2021-2022 (USDA, 2022). Sugarcane is considered a moderately salt-sensitive species that can endure electrical conductivity ECe levels up to 1.7 dS m-1. However, increasing salinity beyond this level causes a significant decrease in plant growth and development at all stages and probably cane yield at the end [8,9]. At the early growth stage (Formative Growth) Vasantha et al. [9] found that salinity reduces leaf area index (LAI) by 36%. Moreover, they found a decline in biomass accumulation by 44% during the same stage. It has been found that the highest reduction in shoot and root biomass accumulation in sugarcane sprouts occurs when the level of salinity increases from normal to 120mM NaCl [10]. Similarly, the increase in NaCl levels led to a reduction in shoot, root length, root volume, and leaf area of sugarcane seedlings by 36-41%, 29-42%, and 52-66%, respectively. Additionally, chlorophyll contents also decreased by approximately 20.0-45.0% [11] In breeding programs, the efficient detection of genetic variability and selection of desirable traits are crucial [12]. Traditional methods that integrate agronomic and morphological characteristics have allowed for the identification and description of differences among members of the Saccharum complex [13], However, members of the Saccharum complex are primarily outcrossed and propagated vegetatively, resulting in highly plastic and heterozygous phenotypic expressions of traits. Although morphological characteristics can be used to identify and classify clones, the majority of these characteristics are influenced by the growing or selection environment.
Molecular markers have proven to be highly valuable in crop breeding programs for the effective assessment of genetic diversity at the DNA level. Specifically, salinity-related markers have played a crucial role in assisting breeders in selecting salt-tolerant plants. [14]. In terms of defining genetic diversity, gene-targeted molecular markers may be more promising and meaningful than random DNA markers. Gene-targeted markers are derived from polymorphisms within genes and thus reflect functional polymorphism, whereas random DNA markers are derived from polymorphic sites throughout the genome [15].
The Target Region Amplification Polymorphism (TRAP) developed by [16]) takes advantage of the expanding availability of EST sequences from various cDNA libraries. TRAP is a straightforward marker system based on polymerase chain reaction (PCR) that makes use of the sequence information in the EST database to generate polymorphic markers that target candidate genes. It basically combines an arbitrary primer that targets the intron or exon region (AT- or GC- rich core) with an 18-mer primer derived from the EST sequence [17]. The polymorphism that is produced as a result of TRAP’s use of anchored and unanchored primers to amplify the genome’s coding regions ought to reflect the diversity of functional genes.
TRAP markers have been successfully employed to assess genetic variation in sugarcane genotypes related to agronomic and stress-tolerant genes. For instance, [18] used RAP markers with CDPK and Aqua candidate genes associated with stress tolerance to evaluate genetic variation among 10 sugarcane genotypes. Similarly, Farsangi et al. [19] conducted a study using 18 salinitytolerant and susceptible genotypes of sugarcane, applying 30 combinations of 5 TRAP markers targeting salinity-tolerant ESTs and three arbitrary primer combinations for molecular characterization. This research aims to investigate the integration of morphological traits Performance data and TRAP genetic marker for sprouting and formative growth stages assessment (90 DAP) of salinity tolerance in sugarcane in order to screen different morphological responses and genetic variability between selected varieties.
Materials and Methods
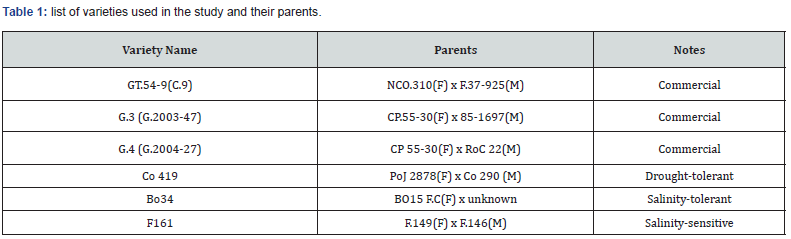
The study was conducted in 2021 at the greenhouse of El- Sabahia Research Station in Alexandria governorate, Egypt. The coordinates of the research station are latitude 31° 12’ N and longitude 29.57° E. The sugarcane varieties used in this study were obtained from The Sugar Crops Research Institute (SCRI), Agricultural Research Center (ARC) in Giza, Egypt (Table 1).
Experimental Design
The experimental design was a completely randomized design (CRD) with a 6 x 5 factorial design (6 varieties and five treatments: 0, 25, 50, 75, and 100mM NaCl). Each treatment had 6 replicates. The experiment was conducted in March 2021 in the greenhouse using clay pots filled with pre-washed sand. The dimensions of the pots were 21cm in height and 25cm in diameter. Healthy singlebud cuttings were horizontally planted with two buds per pot and irrigated with tap water every three days for 90 days. After 15 days, the emerging seedlings were fertilized with NPK (2.5ml/L) once a week. The average high temperatures during the three-month experimental period (March, April, and May 2021) were 33.4 °C, 39.1 °C, and 41.8 °C, respectively, while the low temperatures were 13.6 °C, 16.7 °C, and 19.2 °C, respectively, under greenhouse conditions.
Morphological and Physiological Data
At age 90 days after planting (except for sprouting at 45 days after planting), six samples were taken from each treatment, and data were recorded for the following traits: sett sprouting percentage, seedling shoot and root length, number of leaves, leaf area, shoot and root dry weight, chlorophyll content using SPAD index, and mortality percentage.
DNA Extraction
For DNA extraction, fresh young leaves were collected from six sugarcane varieties at the age of 90 DAP. Tissue samples were homogenized in liquid nitrogen, and genomic DNA was isolated using the DNeasy™ Plant Mini Kit from QIAGEN™ according to the manufacturer’s protocol. The DNA was quantified using spectrophotometer, and its quality was assessed using 1% agarose gel electrophoresis.
TRAP Markers PCR and Electrophoresis of PCR Product
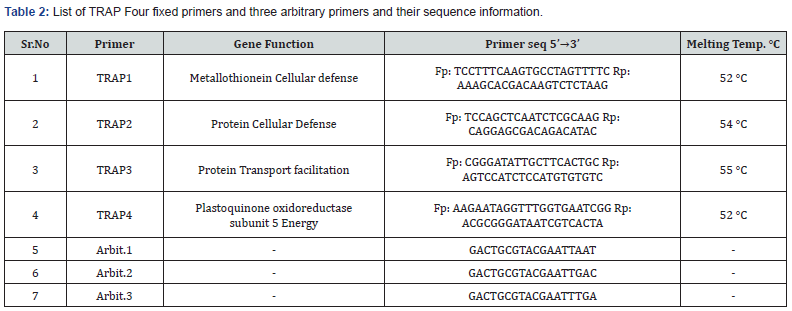
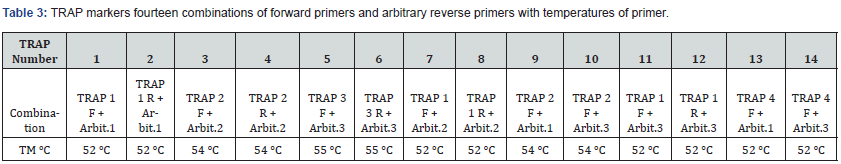
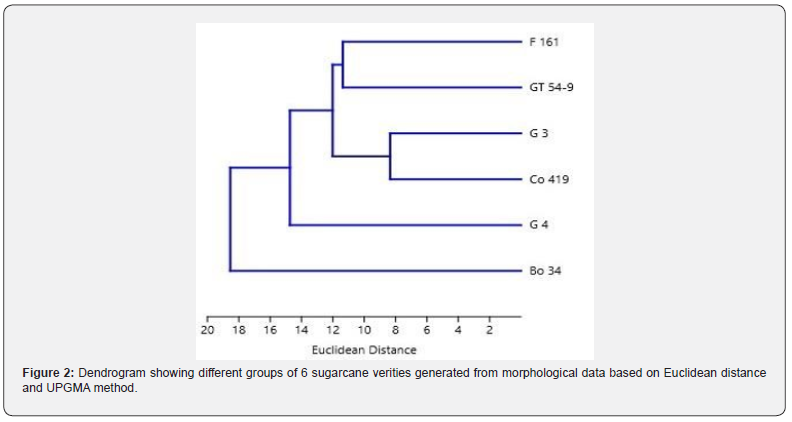
A total of fourteen combinations of forward primers and arbitrary reverse primers of TRAP were selected for amplification (Table 2 & 3). The primer lengths ranged from 18 to 22 nucleotides and were designed based on previous studies [20,19,16,21]. PCR amplification was performed using a reaction mixture consisting of PCR master mix TOPsimple™ PCR DyeMIX-nTaq from Ezynomics® (12.55 μM), 1.5 μM of a fixed forward primer and an arbitrary reverse primer, and 50 ng of template DNA. The PCR process was carried out in a Peqlab Hain Primus 25® Thermal cycler with the following cycling conditions: an initial temperature of 94°C for 4.30 minutes (1 cycle), followed by 35 cycles of 1 minute at 94°C, 1 minute at 52-55°C, and 2 minutes at 72°C. The final extension cycle lasted for 7 minutes at 72°C. The PCR products of TRAP were separated on a 1.5% (w/v) agarose gel using 1× TBE buffer. The gel was stained with Ethidium Bromide (EtBr) and visualized under a UV transilluminator. To determine the size of the amplified DNA fragments, a 3000 bp DNA molecular marker was employed.
Data analysis
The morphological data of the different varieties were analyzed using a two-way analysis of variance (ANOVA). Subsequently, phenotypic similarities and dissimilarities among the sugarcane varieties were determined through Euclidean distance analysis [22]. A dendrogram was generated using Euclidean distance and the un-weighted pair group method with arithmetic average (UPGMA) algorithm implemented in Past 4.11 software. For the TRAP marker data, the amplified products that displayed consistent and distinguishable bands were considered as identical bands. The DNA bands obtained from each combination of TRAP markers were converted into binary numbers, where 1 indicated the presence of DNA bands and 0 indicated their absence. Only well-defined bands were considered for further analysis. The genetic similarity (GS) between the genotypes was determined using Jaccard’s similarity coefficient. Based on this coefficient, a dendrogram was constructed using the UPGMA algorithm in Past 4.11 software.
The allelic diversity at specific loci was assessed using the Polymorphism Information Content (PIC). Additionally, the capability of the primers to differentiate between genotypes was evaluated by calculating their resolving power (Rp) using popgene-1.32 software. Statistical analysis for the study was performed using IBM SPSS Statistics 26, incorporating appropriate tests and methods as required.
Results and Discussion
Effect of Salinity on Morphological and Physiological Traits
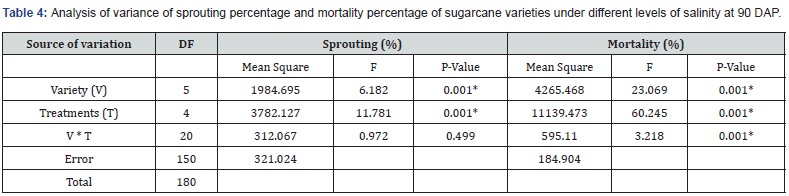
The results of the analysis of variance (ANOVA) for the sprouting percentage and mortality rate are presented in Table 4. The analysis evaluated the sources of variation, including the Variety (V), Treatments (T), and their interaction (V * T). Additionally, the mean squares, F-values, and corresponding p-values are provided to assess the significance of each factor
*sinificant at P < 0.05
The sprouting percentage was significantly influenced by the variety of plants (F (5,150) = 6.182, p < 0.001*), suggesting that different varieties had distinct sprouting capabilities. Similarly, the mortality rate was significantly affected by the variety factor (F (5,150) = 23.069, p < 0.001*), indicating that different varieties exhibited varying levels of mortality. In addition to the variety factor, the Treatments (T) factor also showed significant effects on the sprouting percentage and the mortality rate. The various treatments significantly impacted the sprouting percentage (F (4,150) = 11.781, p < 0.001*) and the mortality rate (F (4,150) = 60.245, p < 0.001*), suggesting that specific treatments had a notable influence on both parameters.
Regarding the interaction between Variety and Treatments (V * T), it was found to be statistically non-significant for the sprouting percentage (F (20,150) = 0.972, p = 0.499). This indicates that the combined influence of variety and treatment on sprouting was not statistically significant. However, for the mortality rate, the interaction between Variety and Treatments was found to be statistically significant (F (20,150) = 3.218, p < 0.001*), suggesting that the combined effect of variety and treatment had an impact on the mortality rate (Table 5).
Moving on to the analysis of other plant growth parameters, As shown in (Tables 6 & 7), the Variety (V) factor exhibited significant effects on shoot length (F (5,140) = 3.665, p = 0.004*), root length (F (5,140) = 37.569, p < 0.001*), number of leaves (F (5,140) = 2.873, p = 0.017*), leaf area (F (5,140) = 14.585, p < 0.001*), shoot dry weight (F (5,140) = 49.120, p < 0.001*), root dry weight (F (5,140) = 26.662, p < 0.001*), and chlorophyll levels (F (5,140) = 5.121, p = 0.001*). This suggests that different varieties significantly influenced these plant growth parameters. Similarly, the Treatments (T) factor demonstrated significant effects on shoot length (F (5,140) = 45.467, p < 0.001*), root length (F (4,140) = 24.361, p < 0.001*), number of leaves (F (4,140) = 10.112, p < 0.001*), leaf area (F (4,140) = 84.379, p < 0.001*), shoot dry weight (F (4,140) = 107.910, p < 0.001*), root dry weight (F (4,140) = 76.541, p < 0.001*), and chlorophyll levels (F (4,140) = 61.186, p < 0.001*). These findings indicate that the specific treatments significantly influenced the measured plant growth parameters. The interaction between Variety and Treatments (V * T) had a significant effect on root length (F (18,140) = 1.886, p = 0.022*), shoot dry weight (F (18,140) = 3.982, p < 0.001*), and chlorophyll levels (F (18,140) = 1.837, p = 0.026*). This suggests that the combined effect of variety and treatment played a role in shaping these aspects of plant growth. However, the interaction did not have a significant effect on shoot length, number of leaves, leaf area, and root dry weight.
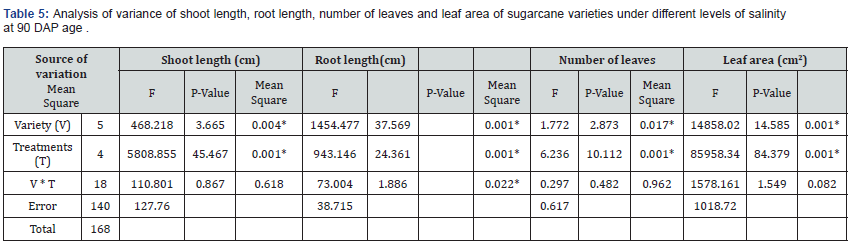
*sinificant at P < 0.05
Comparison between Sugarcane Varieties Morphological and Physiological Traits Performance under salinity stress
Effect of salinity on sprouting percentage, shoot and root length
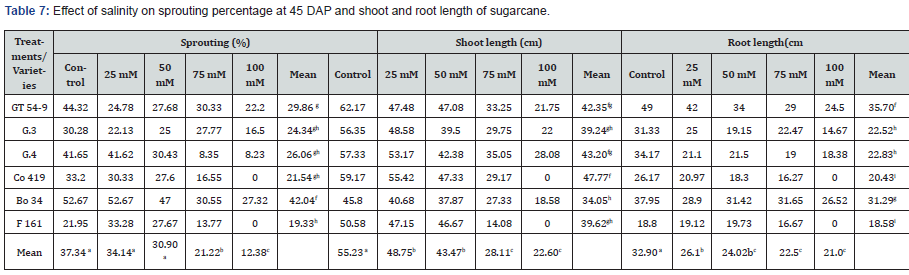
The results obtained from the experiment are presented in Table 8, which shows the sprouting percentage, shoot length, and root length for different treatments and varieties. The treatments included various concentrations of NaCl (control, 25mM, 50mM, 75mM, and 100mM) while the varieties examined were GT 54- 9, G.3, G.4, Co 419, Bo 34, and F 161. Regarding the sprouting percentage, the control group exhibited an average of 37.34%. Bo 34 had the highest sprouting percentage (52.67%), while F 161 had the lowest (21.95%). Among the treatments, the 25mM concentration showed the highest sprouting percentage and 100mM were the lowest. Significant differences were observed among the varieties, with Bo 34 displaying the highest sprouting percentages across all treatments. In contrast, F 161 exhibited the lowest sprouting percentage at the 100mM concentration (0%), with a mean of 19.33% across all treatments.
In terms of shoot length, GT 54-9 had the longest average shoot length in the control group (62.17cm), while Bo 34 had the shortest (45.80cm). The different NaCl concentrations also had a significant impact on shoot length, with the 25mM concentration resulting in a mean shoot length of 48.75cm, and 100mM (22.60cm). Varietal differences were observed as well, with Co 419 exhibited the highest mean shoot length (59.17cm), while Bo 34 having the lowest (45.80cm). Root length analysis revealed that GT 54-9 had the highest average value (49cm), while F 161 had the lowest (18.58 cm). The different NaCl concentrations also influenced root length, with lower concentrations leading to longer roots. Across all treatments, GT 54-9 had the longest mean root length (35.70cm), whereas F 161 had the shortest (18.58cm) (Figure 1).
Effect of salinity on number of leaves, leaf area and shoot dry weight
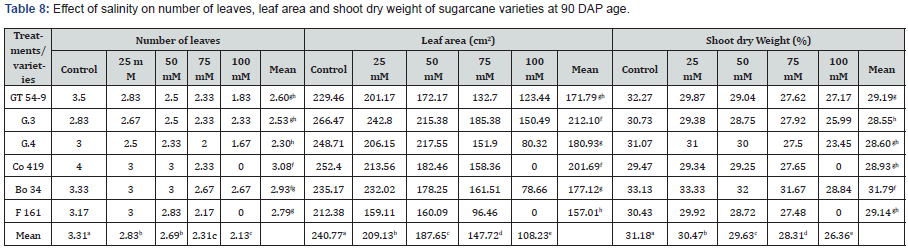
The number of leaves varied among the treatments and plant varieties. As shown in Table 9, the control group had an average of 3.31 leaves per plant. Co 419 exhibited the highest number of leaves (4), while G.3 had the lowest (2.83). As the NaCl concentration increased, the mean number of leaves decreased. Co 419 had the highest mean number of leaves (3.08), while G.4 had the lowest (2.30). Statistically significant differences were observed among the treatments and varieties at (p < 0.05). Leaf area demonstrated significant variations among the plant varieties and treatments. In the control group, G.3 displayed the highest leaf area (266.47cm²), while F 161 had the lowest (183.73cm²). The control group also had the highest overall leaf area (240.77cm²), while the 100mM concentration had the lowest (108.23cm²). Among the varieties, G.3 exhibited the highest average leaf area (212.10cm²), whereas F 161 had the lowest (157.0cm²). The results of shoot dry weight showed significant differences among the plant varieties and treatments. The control group exhibited the highest shoot dry weight, with an average of 31.18%, while the 100mM group had the lowest, measuring 26.36%. Bo 34 had the highest shoot dry weight (33.13%), whereas G.3 had the lowest (28.55%).
Effect of salinity on root dry weight, chlorophyll SPAD index and mortality percentage
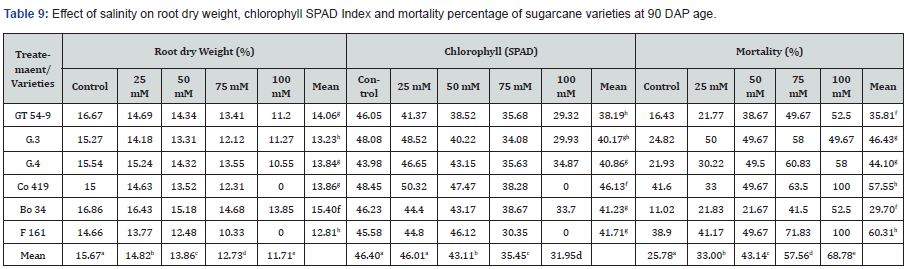
As exhibited in Table 10, Root dry weight exhibited variations across the different varieties and treatments. On average, the control group had a root dry weight of 15.67%, whereas F 161 displayed the lowest value (14.66%). Bo 34 exhibited the highest root dry weight (16.86%). The control group resulted in the highest root dry weights among the treatments, while the 100 mM concentration had the lowest (11.71%). Likewise, Bo 34 had the highest root dry weight (15.40%), whereas F 161 had the lowest (12.81%) among the varieties across all concentrations.
*sinificant at P < 0.05

Chlorophyll content, measured using the SPAD index, also showed variations among the varieties and treatments. On average, the highest chlorophyll content was observed in Co 419 (48.45 SPAD units), while the lowest was found in G.4 (43.98 SPAD units). Among the treatments, the Control group and the 25mM concentration resulted in the highest chlorophyll content, while the 100mM concentration had the lowest. Variation among varieties found to be significant as well with Co 419 (46.13 SPAD units) as the highest value and GT 54-9 displaying the lowest (38.19 SPAD units). Mortality rate, indicating the percentage of plants that did not survive, also varied among the varieties and treatments. On average, the control group had the lowest mortality rate of 25.78 %, while Bo 34 had the lowest (11.02%). F 161 exhibited the highest mortality rate (38.90%). Increasing NaCl concentrations led to higher mortality rates, with the 100mM concentration resulting in the highest. There were significant differences among the varieties as well, with the lowest average rate recorded in Bo 34 (29.70%) and the highest observed in F 161 (60.31%). Notably, the NaCl concentration of 100 mM was found to be completely lethal for two varieties (Co 419 and F 161).
In general, significant variations were observed among the treatments and varieties for all parameters measured. The results indicate that salinity stress negatively impacted plant growth and development, with higher NaCl concentrations leading to reduced sprouting percentage, shoot length, root length, number of leaves, leaf area, shoot dry weight, root dry weight, and chlorophyll content. The control group consistently exhibited the highest values for most parameters, while Bo 34 and Co 419 displayed better tolerance to salinity stress compared to other varieties. F 161 was found to be highly susceptible to salinity, as evidenced by its low values across multiple parametersure 1. Roots growth of six sugarcane verities at age 30 DAP under different NaCl treatments.
Phenotypic Similarity and Dissimilarities between Varieties
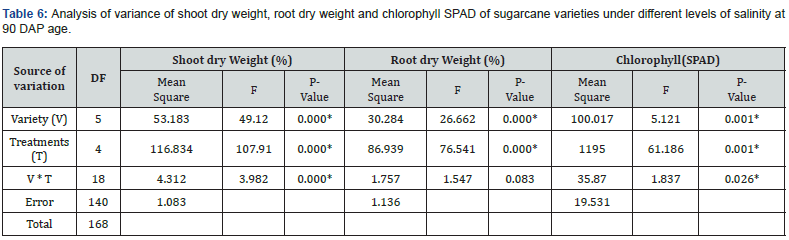
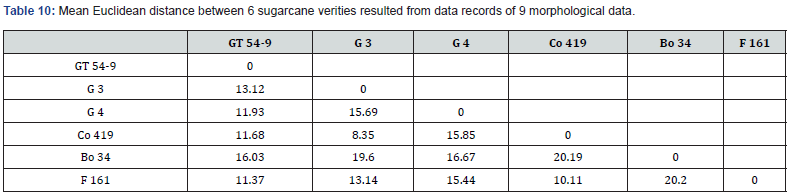
Phenotypic similarities and dissimilarities between six sugarcane varieties (GT.54-9, G.3, G.4, Co 419, Bo 34, and F 161) were analyzed based on morphological data. The similarities were quantified using the Euclidean distance, and the results are presented in Table 10. The Euclidean distance is a measure of dissimilarity that quantifies the difference between two varieties based on their standardized means of morphological traits. A smaller Euclidean distance indicates a higher similarity between the varieties, while a larger distance indicates greater dissimilarity.
Upon analyzing the results, the distances between varieties ranged from 8.35 to 20.20, reflecting the variability in their morphological traits. Among the studied varieties, Co 419 and G.3 exhibited least dissimilarity, as indicated by a Euclidean distance of 8.35. In contrast, Bo 34 (salinity-tolerant) and F 161 (susceptible) displayed the largest dissimilarity, with a distance of 20.20. The dendrogram in Figure 2 provides a visual representation of the phenotypic relationships among the sugarcane varieties. By using the Euclidean distance matrix, we gained insights into the similarities and dissimilarities between the varieties. From the dendrogram, we can observe distinct clusters and branch lengths that reflect the similarities and dissimilarities between the varieties. Bo 34 stands out as the most dissimilar variety, as it has the longest branch length foliowed by G.4. On the other hand, the varieties F 161, GT.54-9 and Co 419, G.3 respectively form a relatively closer twso clusters in the dendrogram suggesting a higher similarity between them in terms of the analyzed morphological traits.
TRAP Markers PCR and Cluster Analysis
The results of the molecular characterization of six sugarcane varieties using fourteen combinations of forward primers and arbitrary reverse primers of TRAP are presented in Figure 3 and Table 11. The table provides information on TRAP combination number , primer combinations, primer temperatures (TM), total alleles (TA), polymorphic alleles (PA), monomorphic alleles (MA), percentage polymorphism (P%), polymorphic information content (PIC), resolving power (RP), and discriminating power (D) for each primer combination. As shown in Table 11 a total of 14 TRAP combinations were evaluated, with varying primer combinations and temperatures resulted in total number of alleles (TA) of 90, out of which 88 alleles were polymorphic (PA), while 2 alleles were monomorphic (MA). The percentage of polymorphism (P %) was calculated to be 97.78%.The total number of alleles ranged from 2 to 10 in TRAP 3R+Arbit.3 and TRAP 2F + Arbit.3 respectively, with the mean of 6.43 alleles per primer. The mean of polymorphic information content (PIC) was determined to be 0.36, while the resolving power (RP) values were ranged from 1.67 to 7.33 in TRAP 3R+Arbit.3 and TRAP 2F + Arbit.3 respectively with an average of 3.97. Discriminating power (D) ranged from 0.56 to 0.93 in TRAP 2R+Arbit.2 and TRAP1F+ Arbit.2 respectively with an average of 0.8.
Genetic Similarity between Varieties
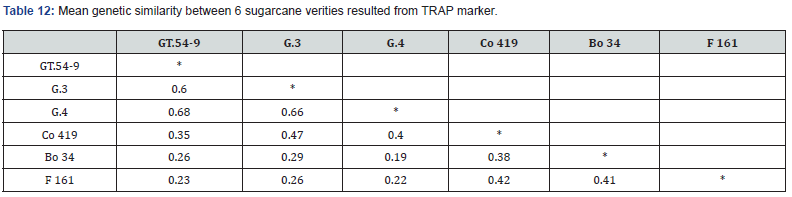
The genetic similarity analysis using the TRAP marker provided insights into the relationships between the six sugarcane varieties examined in this study. The mean genetic similarity values presented in Table 12 reveal the degree of genetic resemblance between each pair of varieties. Among the varieties, GT.549 and G.4 demonstrated the highest mean genetic similarity coefficient of 0.68 while lowest similarity coefficient was observed 0.19 between Bo 34 and G.4 with an average of 0.39. The dendrogram divided the six sugarcane varieties into two clusters (Figure 4). The first cluster consisted of GT.54-9, G.3 and G.4 exhibiting a genetic similarity of 0.61. Cluster two comprised other three varieties Co 419, F161 and Bo 34 with 0.041 similarities. Furthermore, each cluster branched into two sub-clusters, GT.54-9 and G.4 (0.68 similarity) and G.3 in first cluster. Second cluster divided into sub-cluster contains Co 419 and F 161 with similarity 0.42 among them, and the other sub-cluster with variety Bo 34 Table12. Mean genetic similarity between 6 sugarcane verities resulted from TRAP marker Figure 4. Dendrogram showing different distinct groups of 6 sugarcane verities resulted from TRAP marker based on Jaccard’s similarity coefficients and UPGMA method.
Discussion
The present study aimed to investigate the early stage salinity tolerance of six sugarcane varieties through the integration of morphological and TRAP genetic marker data. The findings provide valuable insights into the impact of salinity stress on the growth and development of selected sugarcane varieties, as well as the genetic relationships among the tested varieties. The results of our study are consistent with previous research on the negative effects of salinity stress on sugarcane growth and development. Our findings align with the study conducted [8-10], which reported reduced shoot length, root length, leaf area and shoot and root biomass under salinity stress in sugarcane varieties. Similarly, Anitha et al. [11] found that salinity stress negatively affected the chlorophyll content .The observed variations in the measured parameters can be attributed to both variety and treatment factors. This is consistent with the findings of previous studies reported significant interaction effects between sugarcane varieties and salinity treatments on various growth and physiological traits.
Phenotypic similarity successfully discriminates between the salinity-tolerant variety (Bo34) and the salinity-sensitive variety (F 161) with largest Euclidean distance of 20.20. The Euclidean distance is a measure of dissimilarity that quantifies the difference between two varieties based on their standardized means of morphological traits. A smaller Euclidean distance indicates a higher similarity between the varieties, while a larger distance indicates greater dissimilarity. In this study, the molecular characterization of six sugarcane varieties using TRAP markers and cluster analysis was conducted. The results presented in this study provide valuable insights into the genetic characteristics and relationships among the examined sugarcane genotypes. Our study utilized fourteen combinations of forward and arbitrary reverse primers in PCR amplification, resulting in a total of 90 alleles, out of which 88 alleles were polymorphic and 2 alleles were monomorphic. The high percentage of polymorphism (97.78%) indicates a significant level of genetic diversity among the analyzed varieties, this percentage is much higher than the polymorphism percentage of 65 % that reported by Farsangi et al. [19] and 73% by Devarumath et al. [20]. The difference in polymorphism percentage can be attributed to the use of 14 of TRAP combinations with the highest reported polymorphism (100%) from a previous study of Farsangi et al. [19], moreover , we examined 6 different sugarcane varieties against 18 in the same mentioned study. The average polymorphic information content (PIC) value in our study was 0.36, which is near to the PIC value of 0.30 that obtained by Farsangi et al. [19] and 0.28 [23] among 18 and 64 sugarcane genotypes respectively.
Genetic similarity among the tested varieties in our study ranged from 0.19 to 0.68, with an average of 0.39. In comparison, Farsangi et al. [19] reported a range of 0.53 to 0.91, with an average of 0.72. These results indicate higher genetic variation among the six sugarcane varieties in our study compared to the previous study. In contrast to phenotypic distance, the genetic similarity analysis did not show a clear distinction among varieties in terms of their response to salinity stress. Interestingly, the salinitysensitive variety (F161), salinity-tolerant variety (Bo 34) and the drought-tolerant variety (Co 419) all were grouped together in the same cluster, indicating a higher level of genetic similarity between these varieties compared to the other three commercial varieties. This finding highlights that these salinity and drought tolerant varieties share more genetic similarities despite their different stress tolerance characteristics performance.
The difference between phenotypic and genetic similarity arises from the fact that phenotypic traits can be influenced by environmental factors, which may lead to variations among individuals even if they share similar genetic backgrounds. In contrast, genetic similarity focuses solely on the genetic information and is less influenced by environmental conditions [24]. Molecular markers, including TRAP, RAPD, and SSR markers, play a crucial role in providing insights into the genetic diversity and relatedness among sugarcane varieties, particularly in terms of salinity tolerance. These markers enable the detection of genetic variations associated with specific stress-responsive genes or pathways, even when there are no significant morphological differences.
Means that share at least one common letter are considered statistically non-significant (p < 0.05) based on the Duncan Test.
Means that share at least one common letter are considered statistically non-significant (p < 0.05) based on the Duncan Test.
Means that share at least one common letter are considered statistically non-significant (p < 0.05) based on the Duncan Test.
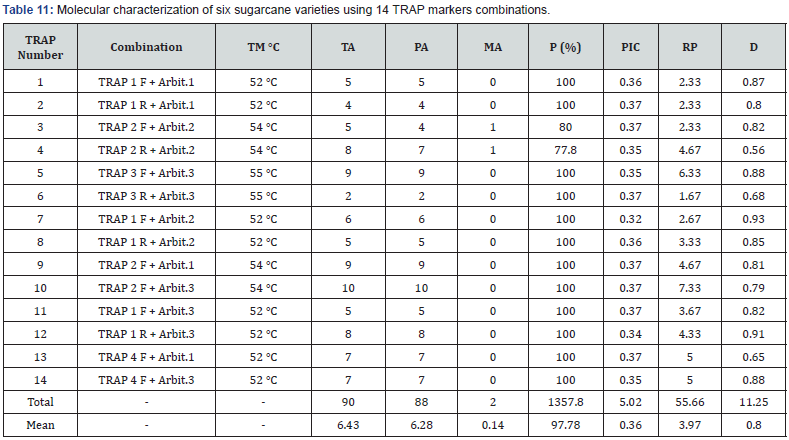
Note: TM: Temperatures of primers. TA: Total Alleles. PA: Polymorphic Alleles. MA: Monomorphic Alleles. P: Percentage Polymorphism. PIC: Polymorphic Information Content. RP: Resolving Power. D: Discriminating power
Interestingly, our study revealed significant morphological trait variation among the sugarcane varieties under salinity stress. However, the genetic similarities based on the TRAP markers analysis for these varieties differed from the phenotypic similarity and dissimilarity analysis. One plausible explanation for this discrepancy is that morphological traits, which typically rely on observable characteristics, may not accurately reflect the underlying genetic similarities or differences at the molecular level. This could be attributed to the occurrence of epigenetic changes without significant alterations in morphology. While considerable research has been conducted on the mechanisms underlying abiotic stresses, our understanding of the role of epigenetic regulation in these processes remains relatively limited [25]. Epigenetic modifications, such as DNA methylation and histone modifications can affect gene expression and regulate stress-responsive pathways. These changes can be heritable and play a role in the adaptation of plants to different environmental conditions [26-28]. For example, salt stress in plants leads to increased accumulation of salt contents, primarily sodium ions (Na+), which can cause ionic toxicity. This stress negatively affects plant growth and development and can result in secondary oxidative stress [29,30]. The role of Histone acetyltransferase (HAT) in regulating salt tolerance has been studied in A. thaliana, where it was found that the mutant strain lacking the GENERAL CONTROL NONDEREPRESSIBLE 5 (GCN5) gene exhibited higher uptake and accumulation of Na+ compared to wild-type plants. This increased Na+ content impaired the growth of the mutant under salt stress conditions. Furthermore, GCN5 was shown to interact with genes involved in cell wall synthesis, such as CHITINASE-LIKE 1 (CTL1) and MYB54. The reduced concentrations of H3K9ac and H3K14ac, which are associated with GCN5, in the mutant under salt stress indicated that GCN5 acts as a conserved epigenetic regulator [31- 33]. Therefore, it is possible that sugarcane varieties with similar morphological traits performance in terms of salinity tolerance may exhibit different genetic similarities at the molecular level due to variations in their epigenetic profiles.
Conclusion
This study provides valuable information on the early stage salinity tolerance of six sugarcane varieties, which aligns with previous findings. Integrating morphological and TRAP marker data offers a comprehensive understanding of the mechanisms underlying salinity tolerance in sugarcane varieties. The use of phenotypic and genetic distances derived from both morphological and molecular data can be more useful for identifying individuals with more salinity-tolerant traits and for selecting parents in breeding programs.
References
- Piperidis N, D’Hont A (2020) Sugarcane genome architecture decrypted with chromosome‐specific oligo probes. The Plant Journal 103(6): 2039–2051.
- Grivet L, D’Hont A, Roques D, Feldmann P, Lanaud C, et al. (1996) RFLP mapping in cultivated sugarcane (Saccharum spp.): Genome organization in a highly polyploid and aneuploid interspecific hybrid. Genetics 142(3): 987-1000.
- Grivet L, Arruda P (2002) Sugarcane genomics: Depicting the complex genome of an important tropical crop. Current Opinion in Plant Biology 5(2): 122-127.
- D’Hont A, Ison D, Alix K, Roux C, Glaszmann JC, et al. (1998) Determination of basic chromosome numbers in the genus Saccharum by physical mapping of ribosomal RNA genes. Genome 41(2): 221-225.
- Hussain S, Shaukat M, Ashra, M Zhu, C, Jin Q, et al. (2019) Salinity Stress in Arid and Semi-Arid Climates: Effects and Management in Field Crops. In S. Hussain (Ed.), Climate Change and Agriculture. IntechOpen.
- FAO (2009) High level expert forum-How to feed the world in 2050, economic and social development. Food and Agricultural Organization of the United Nations Rome.
- Ghassemi F, Jakeman AJ, Nix HA (1995) Salinisation of land and water resources: Human causes, extent, management and case studies. CAB international, pp. 526.
- de Santana MJ, Carvalho J de A, de Souza KJ (2007) Efeitos da Salinidade da Água de Irrigação na Brotação e desenvolvimento Inicial da Cana-de-açúcar (Saccharum Spp) e em solos com diferentes níveis texturais. Ciênc agrotec 31(5): 7.
- Vasantha S, Gomathi R, Brindha C (2017) Growth and Nutrient Composition of Sugarcane Genotypes Subjected to Salinity and Drought Stresses. Communications in Soil Science and Plant Analysis 48(9): 989-998.
- Akhtar S, Wahid A, Rasul E (2003) Emergence, growth and nutrient composition of sugarcane sprouts under NaCl salinity. Biologia Plantarum 46(1): 113-116.
- Anitha R, Mary PCN, Savery M, Sritharan N, Purushothaman R S, et al. (2015) Differential responses of sugarcane (Saccharum officinarum L.) varieties exposed to salinity under a hydroponic system. Plant Archives 15(2): 1055-1060.
- SINGH A, Bhatnagar PK, Khan AQ, Shrotria PK (2002) Variability and heritability for cane yield, its components and quality characters in sugar cane (Saccharum spp complex). Indian Sugar 51(10): 717-719.
- Skinner JC, Hogarth DM, Wu KK (1987) Selection methods, criteria, and indices. In Developments in crop science 11: 409-453. Elsevier.
- Saxena P, Srivastava RP, Sharma ML (2010) Studies on salinity stress tolerance in sugarcane varieties. Sugar Tech 12(1): 59-63.
- Andersen JR, Lübberstedt T (2003) Functional markers in plants. Trends in Plant Science 8(11): 554-560.
- Jinguo Hu, Brady A Vick (2003) Target region amplification polymorphism: A novel marker technique for plant genotyping. Plant Molecular Biology Reporter 21(3): 289-294.
- Li G, Quiros CF (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theoretical and Applied Genetics 103: 455-461.
- Amein K A, El-Aref HM (2015) GENETIC VARIABILITY AND RELATIONSHIPS AMONG SUGARCANE VARIETIES BASED ON TRAP MARKERS OF STRESS TOLERANCE CANDIDATE GENES AND SOME AGRONOMIC TRAITS. Journal of Agricultural Chemistry and Biotechnology 6(11): 545-557.
- Farsangi FJ, Thorat AS, Devarumath RM (2018) Molecular characterization of sugarcane genotypes for their salinity and susceptibility using trap markers.
- Devarumath RM, Kalwade SB, Bundock P, Eliott FG, Henry R, et al. (2013) Independent target region amplification polymorphism and single‐nucleotide polymorphism marker utility in genetic evaluation of sugarcane genotypes. Plant Breeding 132(6): 736-747.
- Khan IA, Bibi S, Yasmeen S, Seema N, Khatri A, et al. (2011) Identification of elite sugarcane clones through trap. Pakistan Journal of Botany 43(1): 261-269.
- Pocovi MI, Collavino NG, Gutiérrez Á, Taboada G, Castillo V, et al. (2020) Molecular versus morphological markers to describe variability in sugar cane (Saccharum officinarum) for germplasm management and conservation. Revista de La Facultad de Ciencias Agrarias 52(1): 40-60.
- Suman A, Ali K, Arro J, Parco AS, Kimbeng CA, et al. (2012) Molecular Diversity Among Members of the Saccharum Complex Assessed Using TRAP Markers Based on Lignin-Related Genes 197-205
- Hasan N, Choudhary S, Naaz N, Sharma N, Laskar RA, et al. (2021) Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J Genet Eng Biotechnol 19(1): 128.
- Raza A (2021) Eco-physiological and biochemical responses of rapeseed (Brassica napus L.) to abiotic stresses: Consequences and mitigation strategies. Journal of Plant Growth Regulation 40(4): 1368-1388.
- Karan R, DeLeon T, Biradar H, Subudhi PK (2012) Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PloS One 7(6): 40203.
- Pandey G, Yadav CB, Sahu PP, Muthamilarasan M, Prasad M, et al. (2017) Salinity induced differential methylation patterns in contrasting cultivars of foxtail millet (Setaria italica L.). Plant Cell Reports 36(5): 759-772.
- Saeed F, Chaudhry UK, Bakhsh A, Raza A, Saeed Y, Bohra, et al. (2022) Moving Beyond DNA Sequence to Improve Plant Stress Responses. Frontiers in Genetics 13: 874648.
- Chaudhry UK, Junaid MD, Gökçe AF (2021) Influence of environmental adversities on physiological changes in plants. 1st edition, Developing climate-resilient crops, CRC Press, pp. 85-110.
- Hafeez MB, Raza A, Zahra N, Shaukat K, Akram MZ, et al. (2021) Gene regulation in halophytes in conferring salt tolerance. In Handbook of bioremediation 341-370.
- Zheng M, Liu X, Lin J, Liu X, Wang Z, et al. (2019) Histone acetyltransferase GCN 5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. The Plant Journal 97(3): 587-602.
- Nazarul H, Sana C, Neha N, Nidhi S, Rafiul AL (2021) Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. Engineering and Biotechnology 19(1): 128.
- Andru S, Kazim A, Jie A, Arnold SP, Collins AK (2011) Molecular Diversity Among Members of the Saccharum Complex Assessed Using TRAP Markers Based on Lignin-Related Genes. BioEnergy Research 5(1): 197-205.






























