Effect of Bacillus insecticidal proteins on the Japanese beetle, Popillia japonica (Scarabaeidae)
Fionna Knecht1 , Yolanda Bel2 , Dafne Toledo2 , Giselher Grabenweger1 and Baltasar Escriche2 *
1Agroscope, Research Group Extension Arable Crops, Department of Plants and Plant Products, Zurich, Switzerland
2Laboratory of Biotechnological Control of Pests, Instituto BioTecMed, Departamento de Genética, Universitat de València, Dr. Moliner 50, 46100-Burjassot, València, Spain
Submission: February 03, 2023; Published: March 13, 2023
*Corresponding author: Baltasar Escriche, Laboratory of Biotechnological Control of Pests, Instituto BioTecMed, Departamento de Genética, Universitat de València, Dr. Moliner 50, 46100-Burjassot, València, Spain
How to cite this article: Fionna Knecht, Yolanda Bel, Dafne Toledo, Giselher Grabenweger and Baltasar Escriche. Effect of Bacillus insecticidal proteins on the Japanese beetle, Popillia japónica (Scarabaeidae). Agri Res & Tech: Open Access J. 2023; 27 (3): 556375. DOI: 10.19080/ARTOAJ.2023.27.556375
Abstract
The Japanese beetle, Popillia japonica, is a minor pest in Japan but has become a serious invasive species in several parts of the world, causing damage in a variety of crops and high costs for its control. Many different methods of control have already been applied, including chemical and cultural measures, as well as mass-trapping. The efficacy of biological control attempts, using parasitoids, nematodes, fungi, or bacteria has also been discussed especially with regard to added value of lower ecological impact. The effect of Bacillus thuringiensis (Bt) treatments is probably attributable to the proteins accumulated in the parasporal body of the bacteria. In this context, a study was conducted to assess the insecticidal activity against P. japonica of the Bt 3 domain non-parasporal body accumulated Cry1Ia protein, and the structurally distant Bt proteins Mpp23Aa and Xpp37Aa. Their activity was tested against P. japonica larvae using a modified leaf-dip protocol, and only the Cry1Ia showed some activity. The results suggest that additional studies on Bacillus proteins are needed for developing new biological control strategies against P. japonica.
Keywords: Japanese Beetle; Cry1I; Cry23; Cry37; Bioassay; Insecticidal Activity; Larval Stage; Bacillus thuringiensis; Coleoptera
Introduction
The Japanese beetle, Popillia japonica, Newman, 1841, (Coleoptera: Scarabaeidae) is an invasive pest to North America and Europe. Their larvae are typical “grubs”, with a creamy white and c-shaped body and a yellowish brown head, with strong, dark-colored mandibles. They look similar to native grubs in the invaded areas and are only identifiable to species level with the help of a stereomicroscope. The adult beetle has bright coppery-brown elytra and a shiny metallic pronotum and head. A row of white tufts on each side of the abdomen is characteristic for this species, and is easily visible with the naked eye (Figure 1).

Third instar larvae overwinter in the soil and re-start feeding for four to six weeks before they start pupating in late April. Adults emerge from mid-May onwards, and the peak swarming period is usually reached in the first half of July. A female lays about 40 to 60 eggs in its average lifespan (30-45 days), and first instar larvae appear from August onwards. The life cycle is usually completed within a year [1]. The larvae feed on the roots of grasses and herbs and can be found in any kind of grassland [2]. They are, however, most abundant in regularly irrigated lawns and golf courses. Adults are very polyphagous and have been described as feeding on the leaves, flowers, buds and fruits of at least 300 species of plants from 79 plant families [3].
P. japonica originates from Japan, as its name suggests, and is a minor pest in its native range because natural enemies suppress populations and the terrain is generally unsuitable for larval development [4]. Unintentional, human-assisted transport likely played a role for its invasion of North America, where it established successfully about hundred years ago. High population densities of P. japonica in suitable habitats caused severe damage and enormous costs for control that are estimated to exceed $460 million/year. Consequently, P. japonica was declared a quarantine pest by USDA/APHIS [5]. Large parts of Eastern Asia, Europe, South America, sub-Saharan Africa, New Zealand, and Australia are considered as suitable for the Japanese beetle and the area at risk may even be enlarged in the course of the global climate change [6]. In fact, P. japonica is a pest of quarantine concern worldwide.
The Japanese beetle was already found on European territory decades ago, in the Azores islands in the 1970s, but has only recently reached continental Europe in 2014 [7]. It is an A2 quarantine organism in the EPPO region [8], which means that the pest is present but not widely distributed. The damage potential and economic importance of the Japanese beetle have triggered intensive studies on various means of control. Treating susceptible plants with carbamates, organophosphates, or more recently, pyrethroid insecticides [9] has been effective. More recently, the ban of persistent insecticides pushed research about other methods such as mass trapping [4,9,10]. In addition, the application of biological control has been investigated in detail, including the use of parasitoids (Tiphia spp.) [11], entomopathogenic nematodes (genera Steinernema and Heterorhabditis) [12], entomopathogenic fungi (Metarhizium spp.), [13], and bacteria (Bacillus spp.) [14]. Spores of Paenibacillus (=Bacillus) popilliae were found to be the primary causal agent of milky disease in P. japonica, and several proteins accumulated in the parasporal body of Bacillus isolates (called Cry proteins) have proven toxicity [15]. Extensive screening of different strains led to the discovery of new Cry proteins with toxicity to Coleopteran pests, such as proteins from the family Cry1I and the ones codifying in a single operon Mpp23 and Xpp37 (called Cry23 and Cry37 in the old nomenclature).
Test insects for this study were collected in an irrigated maize field in the north Italian region of Piedmont, close to the village of Mottalciatta (45°31'24.7"N 8°12'30.7"E) in September 2022. Third instar larvae were removed from soil between roots of maize stalks, isolated in six-well plates and provided with peat as substrate and a carrot slice as food. They were stored in a climatized quarantine chamber at 23°C, 60% humidity and a day-night cycle of 16:8 h until use in experiments.
Protoxins from a Cry1Ia were prepared as described by Khorramnejad et al. [16], using the same transformed Escherichia coli strain containing cry1Ia38 allele. This 3-domain Cry protein is excreted into the medium instead of being accumulated in the parasporal body, and shows dual toxic activity against some species of Lepidoptera and Coleoptera. The other proteins (Mpp23 and Xpp37) can be found in the Bt parasporal body but show secondary structures different to other 3 domain Cry proteins. Both proteins were considered a binary toxin but their effect in a study with Cylas puncticollis was additive [17]. The Mpp23 and Xpp37 proteins were prepared from the EG10327 strain, which expresses Mpp23Aa1 and Xpp37Aa1, following Rodríguez-González et al. [18]. The solubilized Bt proteins were analyzed by SDS-PAGE and quantified by the Bradford method [19]. The preparations were frozen at -20 oC for transport and defrosted for the bioassays.
A modification of the leaf-dip protocol described by Ruiz de Escudero et al. [20] was used in the experiment, since the study was conducted with the soil-dwelling larvae of P. japonica. Carrots were cut into 1 cm slices (2,5 cm2 surface) and 100 µl of toxin solution (1 mg/ml) or buffer was applied topically to the slices and then left to dry for 1 h in a sterile bench. Afterwards carrot slices were turned around and the procedure repeated (application of 200 µl/slice or 40 µg/cm2). Inoculated carrot slices were put singly into small cups (Tube PS 90 ml, Semademi Plastics Group), filled with moist peat and containing single third instar larvae of P. japonica. After a week of incubation in the quarantine chamber (see above), the inoculated carrot slices were removed, and exchanged with untreated slices if the larvae were still alive. Mortality of larvae was checked weekly during a period of eight weeks and old carrots were exchanged weekly for fresh ones in cups with living larvae. Larval mortality for each observation date was corrected using Abbott's formula [21] and the mean value and the standard error of the mean were obtained. 15 cups/ larvae were used per treatment, and the whole experiment was performed twice.
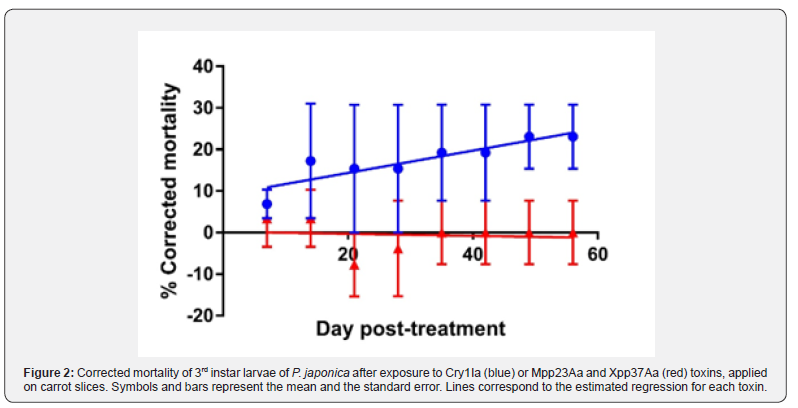
The mean mortality in controls (carrots treated with buffer) was lower than 20% during the observation period. Mortality in insects treated with toxin-inoculated carrot slices showed different responses related to the toxin applied (Figure 2).
Treatments with Mpp23Aa and Xpp37Aa toxins exhibited no difference to the control, but Cry1Ia treatments show an increase in mortality up to a value of 23%. Statistical regression analysis confirmed no significant differences in mortality of the control and the Mpp23Aa and Xpp37Aa treatment (r2=0.03, slope not significantly different from 0, F= 0.08, df= 1,6, P=0.78). The Cry 1Ia treatment did not show a significant difference in mortality to the control at the end of the treatment (Fisher's exact test, P=0.15), probably due to the variability between the replicates, but the regression analysis was significant (r2=0.77, slope significantly different from 0, F= 22, df= 1,6, P=0.04) with slope values of 0.27±0.06 %mortality/day post-treatment. This indicates a toxicity of the Cry1Ia to P. japonica larvae, even though it is low when considering the high application dose.
Lepidoptera have been used more extensively than Coleoptera for tests with Bacillus insecticidal proteins, probably due to the different biology of the insects which facilitates the toxicological study in the former compared to the latter. Nevertheless, several proteins from the 3-domain types (i.e. Cry1, Cry3, or Cry7) and other types (i.e. App6, Mpp34, Xpp35) have shown high efficacy to control certain species of Coleoptera. Beetles of the Scarabaeidae family have shown high susceptibility to several types of 3-domain Cry8 protein, including P. japonica [15]. The same is true for other types of Bacillus proteins with different molecular structures, including a mixture of Mpp23Aa and Xpp37Aa [15]. The results of the present work did not confirm the previously reported toxicity of these proteins to P. japonica. This may have methodological reasons, e.g., the modified bioassay protocol, or biological ones, e.g., different susceptibility towards the toxic proteins depending on the pest’s developmental stages. Interestingly, the Cry1I protein, which has never been tested before against P. japonica, exhibited some toxicity against Japanese beetle larvae. Testing different variants of this protein may open up an interesting resource for future biocontrol applications against P. japonica.
Conclusion
The Japanese beetle, P. japonica, is a globally important invasive pest with a broad host range and a high capability to damage many different crops. Since synthetic pesticides are mostly unavailable, there is a dire need for environmentally friendly methods to control this pest and prevent or at least slow down its spread in continental Europe. Bacillus strains expressing insecticidal proteins are an interesting option for biological control of P. japonica. The uncommon proteins Cry1Ia, Mpp23Aa, and Xpp37Aa tested in the present work did not enhance Japanese beetle larval mortality significantly, and additional screening efforts are needed to obtain more effective toxins.
Acknowledgement
We thank Rosa Maria Gonzalez-Martinez for her laboratory assistance in protein preparations. This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 861852. D.T. was supported by a grant from Spanish MCIN PRE2019-089628. This research was funded by grants from Generalitat Valenciana (grant number PROMETEO/2020/010); the grant PID2021-122914OB-100 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”, by the European Union.
References
- Shanovich HN, Dean AN, Koch RL, Hodgson EW (2019) Biology and management of Japanese beetle (Coleoptera: Scarabaeidae) in corn and soybean. J Integr Pest Manag 10(1): 9.
- Redmond CT, Potter DA (2010) Incidence of turf-damaging white grubs (Coleoptera: Scarabaeidae) and associated pathogens and parasitoids on Kentucky golf courses. Environ. Entomol. 39(6): 1838-1847.
- Fleming WE (1972) Biology of the Japanese beetle. USDA Technical Bulletin, USDA, Washington DC, USA, (1449).
- Potter DA, Held DW (2002) Biology and management of the Japanese beetle. Ann Rev Entomol 47: 175-205.
- USDA/APHIS (2016) Japanese beetle program manual. Washington DC, USA: United States Department of Agriculture - Animal and Plant Health Inspection Service.
- Kistner TEJ (2019) The potential global distribution and voltinism of the Japanese beetle (Coleoptera: Scarabaeidae) under current and future climates. J Insect Sci 19(2): 16.
- EPPO (2017) First report of Popillia japonica in Switzerland. In: EPPO Reporting Service, No. 09.
- EPPO (2019). EPPO standards: EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests. EPPO, PM 1/2(28) Paris, France.
- Cramer ME, Demchak K, Marini R, Leskey T (2019) UV-blocking high-tunnel plastics reduce Japanese beetle (Popillia japonica) in red raspberry. HortScience 54(5): 903-909.
- Switzer PV, Cumming RM (2014) Effectiveness of hand removal for small-scale management of Japanese beetles (Coleoptera: Scarabaeidae). J Econ Entomol 107: 293-298.
- Gagnon M, Giroux M (2019) Records of the Japanese beetle and its parasitoid Istocheta aldrichi (Mesnil) (Diptera: Tachinidae) in Quebec, Canada. The Tachinid Times 32: 53-55.
- Marianelli L, Paoli F, Torrini G, Mazza G, Benvenuti C, et al. (2017) Entomopathogenic nematodes as potential biological control agents of Popilia japonica (Coleoptera, Scarabaeidae) in Piedmont Region (Italy). J Appl Entomol 142(3): 311-318.
- Vega FE, Dowd PF, Lacey LA, Pell JK, Jackson DM, et al. (2007) Dissemination of beneficial microbial agents by insects. In: Lacey LA, Kaya HK (Eds.), Field manual of techniques in invertebrate pathology, Springer, Dordrecht, Netherlands, pp. 127-148.
- Jackson TA, Klein MG (2006) Scarabs as pests: a continuing problem. Coleopterists Society Monograph, pppp. 5102-5119.
- Domínguez AM, Villanueva M, Escriche B, Ancín AC, Caballero P (2020) Insecticidal activity of Bacillus thuringiensis proteins against Coleopteran pests. Toxins 12(7): 1-29.
- Khorramnejad A, Domínguez AM, Caballero P, Escriche B, Bel Y (2020) Study of the Bacillus thuringiensis Cry1Ia protein oligomerization promoted by midgut brush border membrane vesicles of lepidopteran and coleopteran insects, or cultured insect cells. Toxins 12: 133.
- Hernández MP, Khorramnejad A, Prentice K, Andrés G A, Vera-Velasco, NM, et al. (2020) The independent biological activity of Bacillus thuringiensis Cry23Aa protein against Cylas puncticollis. Front Microbiol 11: 1-9.
- Rodríguez GÁ, Porteous-Álvarez AJ, Del Val M, Casquero PA, Escriche B (2020) Toxicity of five Cry proteins against the insect pest Acanthoscelides obtectus (Coleoptera: Chrysomelidae: Bruchinae). J Invertebr Pathol 169: 107295.
- Bradford MM (1976) A Rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Ruiz de Escudero I, Estela A, Porcar M, Martínez C, Oguiza JA, et al. (2006) Molecular and insecticidal characterization of a Cry1I protein toxic to insects of the families Noctuidae, Tortricidae, Plutellidae and Chrysomelidae. Appl Environ Microbiol 72(7): 4796-4804.
- Abbott WS (1925) A method of computing the effectiveness of on insecticide. J Econ Entomol 18(2): 265-267.






























