Copper-Chitosan Nanoparticles Boost Plant Growth Stimulation and Induced Resistance against Fusarium Wilt of Banana (Musa sp.)
Vasantha Kumar N1, Vinay B Raghavendra2, Anusha Narayana Murthy3 and Lokesh S1*
1Department of Studies in Biotechnology, University of Mysore, Mysuru, India
2P G Department of Biotechnology, Teresian college, Mysuru, India
3Vijnana Bhavan, University of Mysore, Mysuru, India
Submission: January 31, 2023; Published: February 15, 2023
*Corresponding author: Lokesh S, Department of Studies in Biotechnology, University of Mysore, Mysuru, India
How to cite this article: Vasantha Kumar N, Vinay B Raghavendra, Anusha Narayana Murthy and Lokesh S. Copper-Chitosan Nanoparticles Boost Plant Growth Stimulation and Induced Resistance against Fusarium Wilt of Banana (Musa sp.). Agri Res & Tech: Open Access J. 2023; 27 (4): 556373. DOI: 10.19080/ARTOAJ.2023.27.556373
Abstract
In banana cultivation, the search for biopolymer-derived compounds is seeking huge demand to replace chemicals harmful to the environment. In the present study, synthesized Cu-Ch Nps showed potential activity from 30 to 240 days after treatment in in vivo studies against Fusarium wilt of banana caused by Fusarium oxysporum f. sp. cubense (Foc). Cu-Ch Nps treated banana plants exhibited a growth promotor effect through enhanced chlorophyll stability index (CSI), chlorophyll (a and b) content, leaf succulence (LS), and also the vitamin C contents of banana fruits in vivo conditions. In the field experiments, plant height, the number of leaves, total hands/bunch of whole fingers, and bunch weight were found to enhance through Cu-Ch NPs (0.20%). Control of disease and plant growth enhancement was favored when Cu directly released from Cu-Ch NPs. Furthermore, Cu-Ch NPs treated plants showed significant defense responses in chitinase, peroxidase, phenylalanine ammonia lyase, and polyphenol oxidase enzymes. This gives better natural elicitation towards the plant defense and disease protection and hence enhances the sustainable growth of the banana crop.
Keywords: Cu-ChNPs; Chlorophyll; Chlorophyll Stability Index (CSI); leaf succulence (LS); vitamin C; Defense Enzymes
Introduction
Banana (Musa sp,) is one of the most popular fruits worldwide and stands second most important fruit crop in India. Banana contains important nutrients which are essential to human health. At present, India is the largest producer of bananas. According to FAO data, India produces 29 million tonnes of bananas annually, followed by China (11 million tonnes), the Philippines (7.5 million tonnes), and Brazil on average [1]. A substantial source of carbohydrates and vitamins, particularly B and C, are found in bananas. It is a significant fruit with high calcium, magnesium, potassium, and phosphorus [2]. The nutrients in bananas support several benefits, including stress relief and a decrease in cancer and cardiovascular disorders. Along with treating ulcers, it has also been said to prevent kidney stones [3]. Additionally, banana is known for antibacterial, anticancer, antifungal, antidiabetic, anti-inflammatory and antioxidant effects [4]. Among the fungal diseases of bananas, Fusarium wilt, also known as Panama wilt, caused by Fusarium oxysporum f. sp. cubense (Foc), is one of the most severe diseases, highly epidemic among banana plants if they are not protected properly [5]. It reduces the crop’s physiological maturity, biomass and yield. The pathogen damages the banana crop at every stage of development, from seedling through fruiting.
With the use of nanomaterial, modern agriculture is evolving into precision agriculture, which is helping to maximize the output from these technologies. There are various ways to increase crop yield and crop nutraceutical quality with the technology that is now available. The use of nanotechnology to increase productivity is one of them [6,7]. Due to nanotechnology, disease management, genetic improvement, nutrient management, and plant growth promotion have been improved through nanomaterials such as nanoparticles, nano fertilizers, and nano pesticides [6]. Materials with at least one dimension smaller than 100nm are referred to as nanoparticles (NPs) and nanomaterials (NMs). This small size results in different properties from those displayed by most of the same composition material. In fact, cellular responses of different metallic elements such as Fe, Ag, Cu, Ce, and Ti when induced by ionic forms as opposed to nanometric forms [8]. The number of studies on nanoparticles (NPs) in crop plants has expanded along with the development of nanotechnology [9]. Fertilizers are required to increase crop yield, but numerous studies have shown that they can reduce soil fertility by upsetting the mineral balance in the soil [10]. Pesticides, fertilizers and medicines are frequently sprayed and can flow off effectively. These insecticides and fertilizers are also produced at a very high cost, which needs no alteration. The use of nanoparticles in agriculture seeks to minimize production costs to optimize output, decrease product amounts for plant protection and reduce nutrient losses to boost yields [11]. More emphasis is being placed on chitosan-based nanotechnology in agrochemical delivery systems and research into utilizing chitosan as a carrier of active chemicals is ongoing. Molybdenum (Mo), Copper (Cu), Iron (Fe), Nickel (Ni), Manganese (Mn), and Zinc (Zn) are some of the micronutrients needed by plants, and they are required considerably in lesser quantities than macronutrients for the proper growth of crops [12].
In contrast, copper is a crucial metal for plants because it plays various roles in respiration and photosynthesis, including the movement of electrons [13,14]. On the other hand, copper has lower plant toxicity and is frequently employed as a fungicide against several infections. In addition, copper packed in NPs improves pathogen inhibition, the efficiency of fertilizer usage, and nutrient bioavailability when compared to other conventional ions and salts, leading to less environmental degradation [15]. Considering the beneficial effects reported, this study’s has been emphasized to ascertain how the foliar application of copper nanoparticles (cu NPs) on the accumulation of bioactive compounds in banana fruits affected the positive effects on the increase of bioactive compounds as observed in other crops.
Materials and Methods
Crop Development
“Nanjangudrasabale” (NRB) banana cultivar was used for this experiment. After the transplant of NRB plants, flowering took place after 9 months after the crop was established under field conditions. The crop was maintained with a single stem till the end of the experiments from the transplant date to obtain fruits for the evaluations. A substrate composed of a mixture of perlite–peat moss (1-1) and N:P:K (20:20:20) was used accordingly [16].
Application of Treatments
The treatments consisted of foliar applications of five different Cu-ChNPs concentrations, as mentioned below. For positive control, SAAF fungicide was used, whereas distilled water was applied as a control. Cu-ChNPs applications were carried out during the development of the crop. The first application was made 30 days after the transplant, the second one at 60 DAT third treatment at 120 DAT, and the final treatment at 240 days. In total, 30 mL of solution was applied per plant, corresponding to different concentrations of Cu-ChNPs per plant in each treatment. The copper nanoparticles were synthesized in the Institute of Excellence (IOE), University of Mysore, Mysuru, Karnataka, India, following the methodology described by Vasanth. [17].
a. Control (dw)
b. Bulk Chitosan (BCH) (0.2%).
c. Copper sulfate(CuSO4)(0.2%).
d. Fungicide (SAAF) (0.2%).
e. Cu-ChNPs (0.01, 0.05, 0.1, 0.15 and 0.20mg/mL).
The plants were maintained as per standard agronomic practices. Observation days start from 30 days till the fruiting ends are considered. Meanwhile, disease symptoms, leaf senescence, number of leaves/plant, plant height, fruits/bunch, and weight/plant were preferred to record at maturity (365 days) time as suggested by Campbell and Madden, [18]. Field condition ware maintained to accord percent wilt index: external wilt symptoms were taken from 1 to 5 scales as per International Musa Program Testing (IMPT) rating.
Effect of Cu-ChNPs on fusarium wilt disease in field condition
After transferring treated plants (Nanjanagudurasabale, a susceptible banana cultivar) from pot to the field condition [17]. Plants were treated with fungal spores (5×103 conidia mL-1)inoculated on banana plants as per the proposed procedure [18]. After the 30th day of inoculation, symptoms were recorded for up to 240 days, and the treatment was continued every 30 days at intervals up to 180 days.Fusarium wilt, the disease was evaluated based on the yellowing, and wilted leaves were considered to calculate the disease severity according to the standard rating 0-5 grades [18]. Further, the Percentage Efficacy of Disease Control (PEDC) was calculated using the formula [19].

Plant physiological parameters
Cu-Ch Nps treated banana plants were evaluated for chlorophyll stability index (CSI), chlorophyll content (CC), leaf K (LK), and leaf succulence (LS) [20].
Chlorophyll content measurement
The chlorophyll content of the leaves of the banana plant was measured from the 30th, 60th, 90th, 120th,and 240th DAT. The banana leaves were extracted with the solution of (80% v/v) acetone, and the temperature was maintained at 4°C for 24h under dark which was followed by the reading using a spectrophotometer at different nm 647 and 665 nm as per the procedure [21].
Chlorophyll stability index measurement
The banana leaf samples were collected from 30th days up to 240 days. One gm of fresh banana leaves was taken and heated at 65 0C in 25 mL of distilled water using a water bath. The chlorophyll content was extracted using 80% acetone in 25 mL quantity, followed by filtration using Whatman no. 1 filter paper. Filtered samples were read at 660 nm using a spectrophotometer. The chlorophyll stability index was calculated using the formula mentioned by [22].
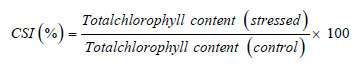
Measurement of leaf succulence
Measurement of leaf area, and fresh and dry weight of leaves were subjected to oven drying at 70 oC for a period until the leaves get reduced its moisture content. The leaf succulence was recorded using the following equation as mentioned by [23].
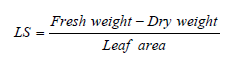
Morphological parameters
The parameters attribute to plant height, the number of leaves, total hands/bunch of whole fingers, and bunch yield, such as yield per plant, were recorded during the maturity period [24].
Copper release through in vitro condition
In vitro experiments were conducted to study the effect of time and pH to check the release of copper content from Cu-ChNPs. The Cu-ChNPs were dissolved in distilled water, and pH was adjusted between the range of 3 to 9. Later the contents were centrifuged at 6000 rpm for 15 min, and supernatants were collected for further analysis. The supernatant was analyzed for copper contents using atomic absorption spectrophotometer (Bio-Rad) [25].
Evaluation of Mineral content in banana fruit
To evaluate the mineral content of banana fruit, uniform sized undamaged fruits were selected at stage six (light yellow) of the maturity stage according to the color scale of USDA [26]. Furthermore, fruits were peeled and kept at -20 oC for 72 h in a deep freezer. These samples were ground to a fine powder and used to perform further analysis [27].
Estimation of vitamin C content in banana fruit
The use of a meta-phosphoric acid-acetic acid extraction solution has been reported to efficiently extract 99% of ascorbic acid from banana fruit samples, according to [28].
Induction of defense enzymes and pathogenesis-related (PR) proteins
The expression of defense-related proteins for peroxidase (POD), chitinase, polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) activities was examined after 240 DAT and 300 DAT against Foc pathogen.
Protein extraction
Treated banana samples were collected at the stage of 240 days after treatment and 300 days after treatment. Fungicide (SAAF)was used as a positive control, and negative control was maintained by spraying plants with distilled water.
Chitinase (EC 3.2.1.14) assay
The chitin powder (5 gm) (Hi-media Laboratories Pvt. Ltd. India) was dissolved in HCl (60 ml) with constant stirring for 30minand kept for precipitation at 40C overnight. The filtered were used for further use [29].
Peroxidase (EC. 1.11.1.7) assay
Peroxidase activity was estimated in the banana leaf samples. The reaction mixture was prepared by using 3mL of pyrogallol solution and 0.5 mL of enzyme extract. The reading was taken at 430 nm, according to the slight modification method of Chance and Maehly [30].
Polyphenol oxidase (EC.1.10.3.2) assay
Polyphenol oxidase (PPO) activity was determined by preparing the reaction mixture (1.5mL of 0.1M sodium phosphate buffer (pH 6.5) and 100μL of the enzyme extract). 0.01M catechol was added to start the reaction According to the procedure of Taneja and Sachar [31]. The activity was expressed as a change in absorbance at 495nm per minute/g/of protein.
Phenylalanine ammonia-lyase (PAL) (EC 4.3.1.5) assay
The deamination of L-phenylalanine determined phenylalanine ammonia-lyase to trans-cinnamic acid, and ammonia was measured at 290nm according to the procedures prescribed by Moerschbacher [32]. All enzymes’ activities were expressed in μmol/min/g tissue.
Results and Discussion
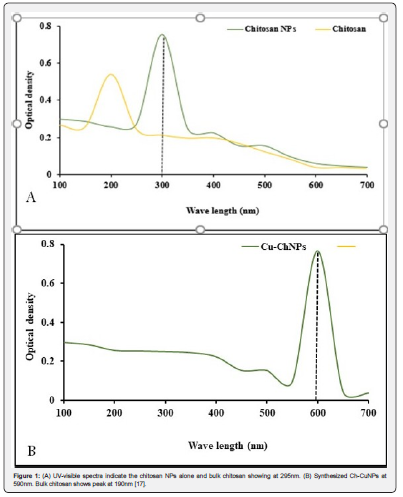
The formation of copper nanoparticles was confirmed through UV-visible spectroscopy at 590 nm due to surface plasmon resonance (SPR), according to our previous report [17] (Figure 1).
Effect of Cu-ChNPs on Fusarium wilt disease in field experiment
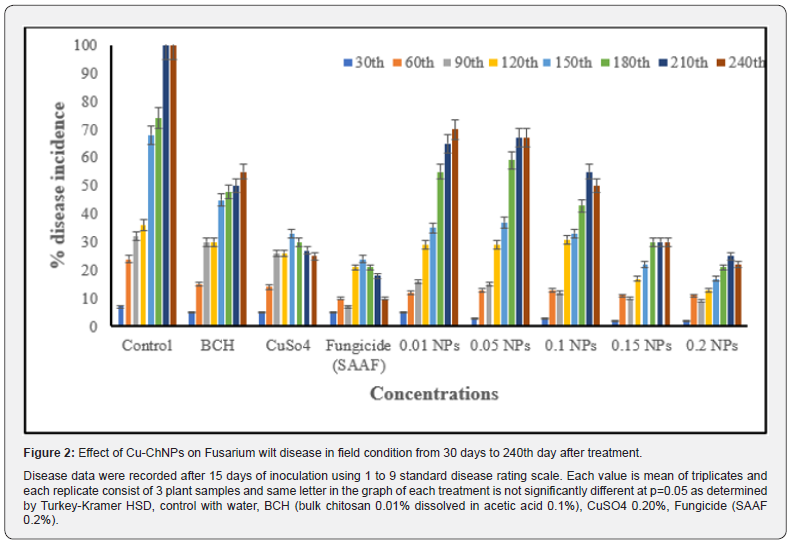
After transferring treated plants (Nanjanagudurasabale, a susceptible banana cultivar) from pot to the field condition [17]. Banana plants were subjected to foliar spray by following standard agronomic conditions. After the 30thDAT of pathogen inoculation, symptoms were recorded. Treatments were continued every 30 days intervals up to 180 days, and results were recorded up to 240 days. The symptoms appeared with wilt symptoms which gradually extended to the entire banana plant. The maximum disease incidence was recorded in control plants which showed 98% disease symptoms at 240 DAT and chitosan alone treated plants. The maximum PEDC was recorded in fungicide treated banana plants (22%), followed by 0.20mg/mL of Cu-ChNPs (24%) when recorded at 240 DAT. The least PEDC was recorded in 0.01mg/mL followed by BCH alone (65 and 55%),as shown in Figure 2. It was noticed that Cu-ChNPs were an improved version of chitosan and fungicide alone when used, and also, the concentrations used for chitosan and fungicides were higher than NPs used in the experiments. This may be due to the chitosan biopolymer, which acts as a strong elicitor of plant defense mechanisms and has the highest antifungal activity when coated with copper NPs [33]. Furthermore, significant growth promotion and antifungal activity capabilities of Cu-ChNPs confirmed the result in agricultural crops [34,35].
Plant physiological parameters
The data on CSI, CC and LS contents of treated banana plants were recorded accordingly. The CC of the banana plant was measured after 30th DAT up to 240th DAT. The data in Figure 3 revealed the effect of Cu-NPs on chlorophyll content. The highest Chl a content was observed in Cu-ChNPs at 150 DAT when compared to other treatments. Likewise, the highest Chl-b was recorded in Cu-ChNPs treated plants at 240 DAT, as shown in Figure 4. Stability and leaf succulence was reported, as shown in Table 1. The different treatments showed different effects on the physiological attributes of the banana fruits. The different concentrations of Cu-ChNPs were designed according to the response of the banana plants to the lower concentration. The CSI and LS contents showed a marked decrease from 36.57 to 21.54% in control, whereas increased CSI was observed in 0.2mg/mL NPs treated plants which showed 50.40 at 30th DAT reaches to 58.08% at 240th DAT, respectively. The treatment with fungicide (SAAF) showed 46.78% at 30th DAT, which increased up to 56.54% on 240th DAT, respectively.
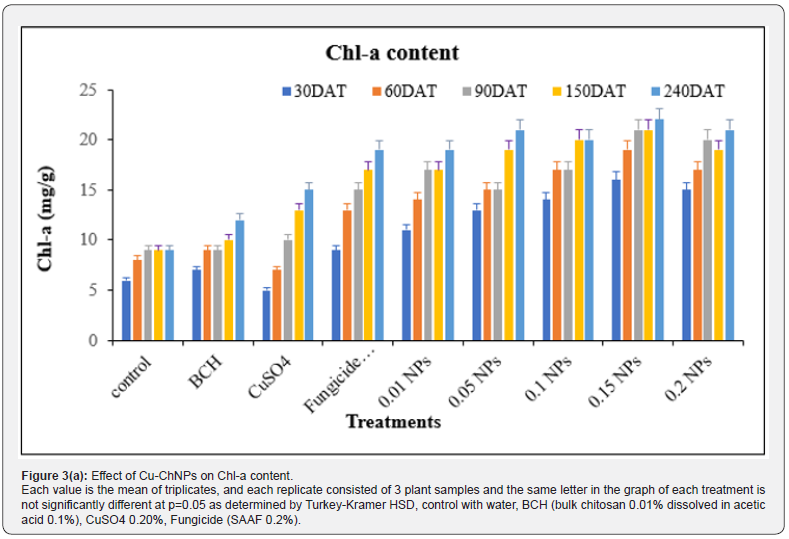
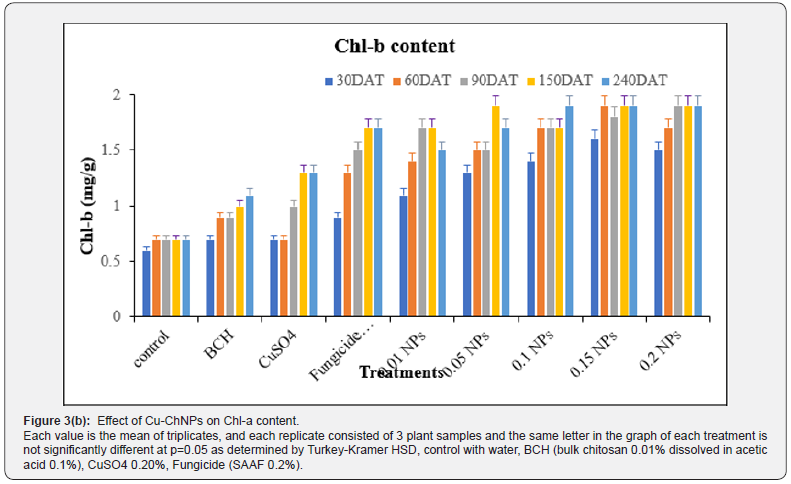
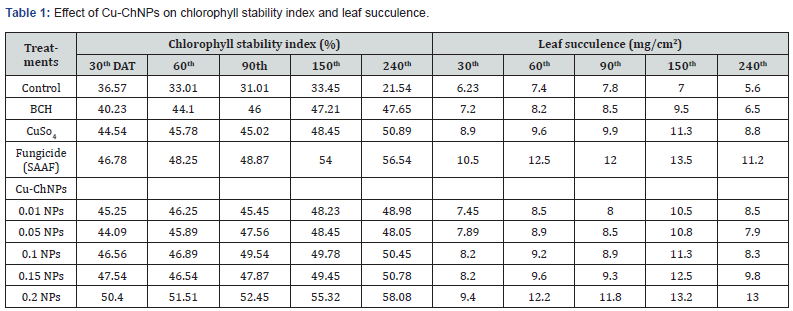
Each value is the mean of triplicates, and each replicate consisted of 3 plant samples and the same letter in the table of each treatment is not significantly different at p=0.05 as determined by Turkey-Kramer HSD, control with water, BCH (bulk chitosan 0.01% dissolved in acetic acid 0.1%), CuSO4 0.20%, Fungicide (SAAF 0.2%). The growth of banana plants increased remarkably, shown in the form of physiological parameter changes when treated with 0.20mg/mL Cu-ChNPs used. At the same time, the Cu- ChNPs plants exhibit better stress tolerance represented by physiological traits. The CSI was found maximum through the usage of 0.20mg/mL NPs, showed 58%, and also improved the LS, 13.0 g/cm2 at 240th DAT. Whereas positive control fungicide treatment showed 56.54% and LS showed 11.2 g/cm2 at 240th DAT. This proves that banana plants treated with 0.20mg/mL NPs showed improved CSI, and LS content when compared to other treatments. Some of the previous reports revealed that copper contributes to both micronutrients as well as disease management [36]. Plastocyananin is an important component of copper in photosynthetic electron transport chains in the form of Cu2+ which helps plants to tolerate stress conditions [37]. The treatment with NPs can improve the electron exchange efficiency in the cells, which reduces the formation of reactive oxygen species (ROS) by arresting electron leakage [38,39].
Significant differences were observed in the parameter studies, as shown in Table 2. The Cu-ChNPs treated plants showed an increase in plant height, a total number of leaves, total bunches, total fingers/hand, and also an increase in bunch weight. This indicates that the application of Cu-ChNPs necessarily generates Cu content in the plants and increases the plant height and other parameters. An accumulation of Cu in the fruits. Control treatment showed 10ft height, with 06 leaves without bunch due to fusarium wilt disease. The application of 0.20% NPs showed increased plant height with 10.6 ft, an increase in the number of leaves, with a total number of 11 leaves, when recorded after 300 DAT. Highest number of fingers/hand was also observed with 15 fingers/hand was recorded at 340 DAT. Finally, the bunch weight was found to be 15.5kg and was recorded after 360 DAT compared to different treatments of NPs, as shown in Table 2. Whereas positive control SAAF (fungicide) showed a plant height of 10.5ft, the total number of 11 leaves was recorded after 300 DAT. A total number of fingers/ hands observed with 15 fingers/hands was noticed at 340 DAT. The bunch weight was found to be 15.0kg and was recorded after 360 DAT. This may be due to the fact that chitosan directly delivers Cu that can penetrate tissues and move through the phloem to other organs, increasing the plant height and weight of the bunch [34]. In this study, applied as a foliar spray, the Cu-ChNPs were directly accumulated inside the leaves. Considering this, the application of Cu-ChNPs did not produce any accumulation of nanoparticle residues inside the banana fruits, and thus, there is no risk of consumption of the fruits [34].
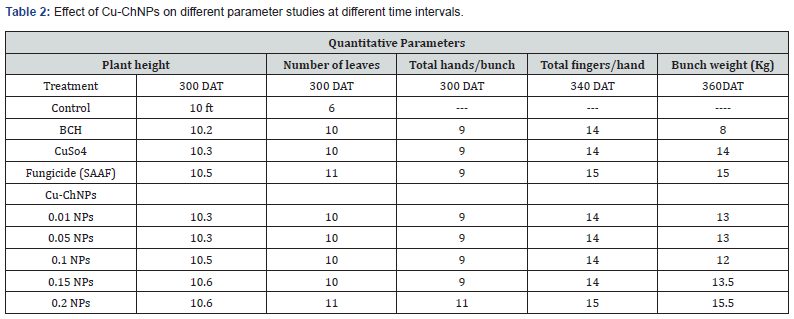
Plant height, number of leaves, total hands/bunch, total fingers, and bunch weight. Each value is the mean of triplicates, and each replicate consisted of 3 plant samples and the same letter in the table of each treatment is not significantly different at p=0.05 as determined by Turkey-Kramer HSD, control with water, BCH (bulk chitosan 0.01% dissolved in acetic acid 0.1%), CuSO4 0.20%, Fungicide (SAAF 0.2%).
Fruit Quality
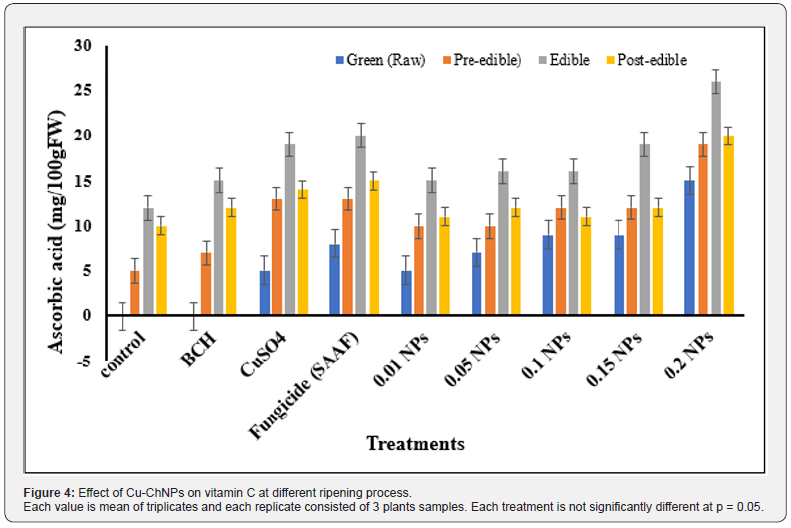
The concentration of vitamin C in 100g of banana fruit pulp was higher at 0.20mg/mL NPs treated showed 14mg in the raw green banana fruit, 16mg in semi-ripened banana fruit, and the highest level of 25mg at the ripened stage, followed by decreasing the concentration of vitamin C to 18mg at post ripened stage. The treatment with fungicide showed 7mg in the raw green banana fruit, 7mg in semi-ripened banana fruit, and the highest level of 18mg at the ripened stage, followed by a decrease in vit C of 16mg. The control showed 11mg/100g pulp at the edible stage, whereas BCH alone showed 13mg at the edible ripened stage as shown in Figure 4. Lokesh [40] reported the highest content of carotenoids such as alpha-carotene and beta-carotene in banana fruit. Furthermore, Abhishek [41] reported the highest content 45mg/100g of vitamin C content in Nanjangud rasabale fruit variety when compared to other varieties. Juarez Maldonada. [42] reported that the application of Cu NPs + chitosan increased the firmness of banana fruits by 9%. This is consistent with the results as shown in the present study. The NPs may be translocated through the vascular tissues with the subsequent dissolution to Cu2+ ions gives rise to the observed lignification and may increase the carotenoid substances in the banana fruit pulp [43].
In vitro copper release
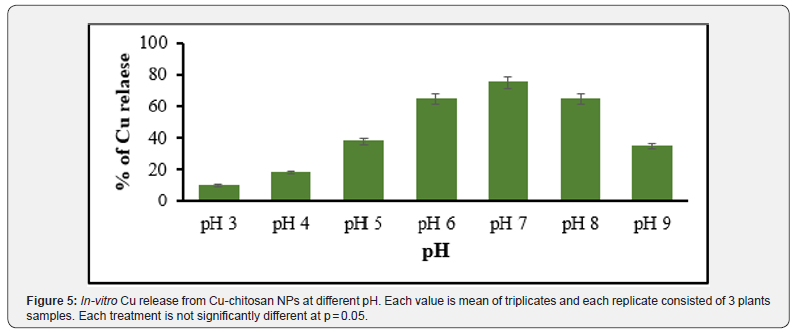
The release of copper from Cu-ChNPs was studied in the pH range from 3 to 9 with a decrease in pH from 3 to 5, the release of Cu increased rapidly at pH 7 when compared with 6 and 8 pH. pH 7 showed the highest Cu release of 78% as shown in Figure 5. This is due to the protonation of the amino group of chitosan and helps the Cu to release into the delivery system rapidly, as shown in Figure 6. Cu release increased from pH 6 to 8 from Cu-Ch NPs, and slow, sustained release of Cu was observed at pH 9 and pH 4. Choudhary et al. [25] reported a pH with a decrease in pH from 3 to 1 and an increase in the release of Cu rapidly from 21.5 to 44.11 at pH 7 to 8.
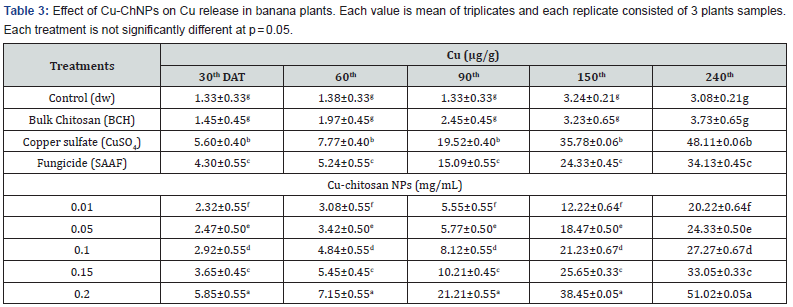
However, at higher concentrations of Cu-ChNPs (0.20) and CuSO4 treatment were significantly increased due to the accumulation of Cu (48.11 and 51.02) at 240DAT as shown in Table 3. Whereas BCH showed very less accumulation of Cu (3.73) and fungicide treatment showed (34.13) Cu at 240DAT, respectively.
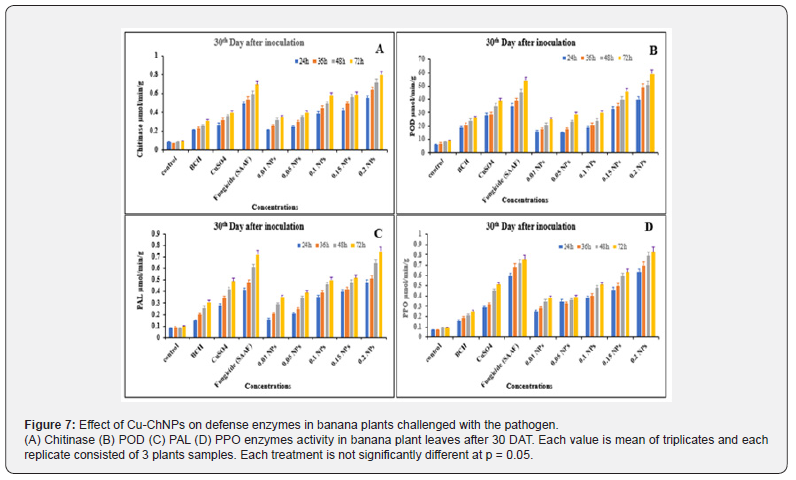
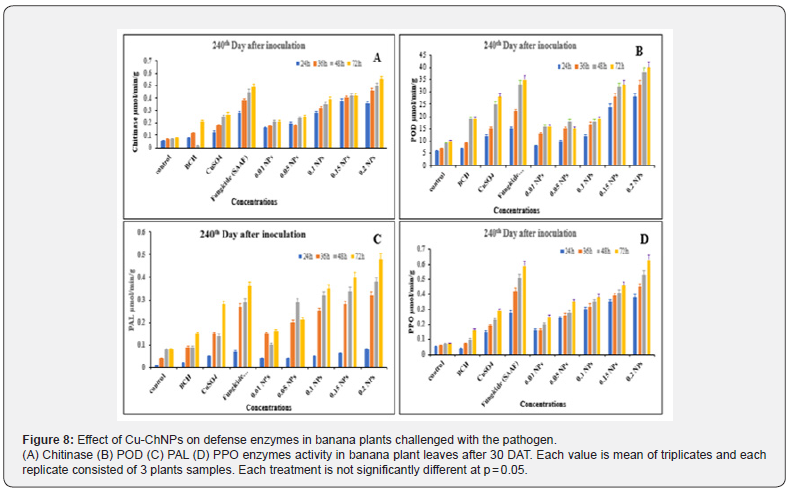
Effect of Cu-ChNPs on the activities of plant defense enzymes under field conditions
The plant defense enzyme activities in Cu-ChNPs treated plants were examined on the 30th day after treatment, and the 240th day after treatment were recorded. Chitinase activity was recorded for Cu-ChNPs at different concentrations, and CuSO4 treated plants. Chitinase activity was significantly Cu-ChNPs (0.20) treated plants showed 1.5 folds higher chitinase activity at 72 h was recorded when compared to fungicide-treated plants at 30th DAT as shown in Figure 7A. Applications of bulk chitosan, CuSO4 treated alone, and 0.01, 0.05 0.10 treated NPs did not show much activity. Similarly, the control treated with distilled water does not show any activity. At 240th DAT, chitinase activity was decreased to 1.0 fold in Cu-ChNPs (0.20) treated plants 72h. Whereas fungicide treated and other treated plants also showed gradual decrease in activity when compared to 30th DAT as shown in Figure 8A Chitinase induction was elicited in banana-treated nanoparticles plants with Cu might provide more protection at the initial level at 30th DAT and might provide higher crop protection against fusarium disease at the initial stage. Kouzai [44] reported that chitinase proteins are involved in the defense response of the host plant against phytopathogens. Likewise, Cu-ChNPs (0.20) treated plants showed 2-3 folds increased peroxidase activity when compared to fungicide-treated plants at 30th DAT as shown in Figure 7B. Whereas 1.5 to 2.0 folds increased, peroxidase activity was recorded at 72h in 240th DAT as shown in Figure 8B. Similarly, 1.0- 1.5 folds peroxidase were recorded in fungicide treated plants as compared to BCH, and CuSO4 treatments. Peroxidase is one of the important enzymes involved in defense enzymes which converts H2O2 scavenging radicles, which are highly toxic converts to H2O2 and O2. The elevated activities of peroxidase after the treatment with Cu-ChNPs (0.20) treatments might be responsible for balancing, degeneration, and reactive oxygen species (ROS), protecting plants from oxidative stress during pathogen invasion [45]. Likewise, Cu-ChNPs treated plants showed 1.5 to 2.0 folds increased PAL activity at 30th DAT as compared to fungicide and other treatments, as shown in Figure 7C. While PAL showed 1.0-1.5 folds increased activity at 72h in 240th DAT when compared with fungicide, bulk chitosan treated, CuSO4 and NPs with (0.01. 0.05 and 0.10) as shown in Figure 8C.
PAL might be associated with the accumulation of copper in the cell wall and the responses of phenylalanine ammonia-lyase (PAL). This catalyzes L-phenylalanine to cinnamic acid, synthesizing suberin and lignin in the phenylpropanoid pathway, which further acts as a mechanical barrier to invading plant pathogens [46,47]. In contrast, the activity of PPO was also enhanced by Cu- ChNPs treated plants at 30th DAT as shown in Figure 8D. The activity showed 2.0-2.5 folds higher at 72h at 240th DAT when compared to fungicide, bulk chitosan treated, CuSO4, and NPs with (0.01. 0.05 and 0.10) as shown in Figure 8D. The PPO might be associated with the production of suberin and lignin, which is responsible for the strengthening of the cell wall [48]. Previously in in vitro experiments Cu-ChNPs treated plants found effective in inhibiting the mycelial growth of fusariumoxysporum sp. cubense (foc) and in pot experiments, substantially induced antioxidant and defense enzyme activity in banana plants treated with foc [17].
Huge demand for food crops free from chemical components has exponentially increased in recent years to avoid the hazardous effects of chemical components and to evade the development of resistance to pathogens. A new approach is more important to strengthen plant innate immunity to manage plant pathogens. These NPs will also reduce chemical use and alongside enhances sustained plant growth. Cu-ChNPs at 0.20% has been proven as a promising plant protectant to manage plant pathogens and also enhances growth promotory agent in our past and recent studies against fusarium wilt. An increase in the physiological parameters such as chlorophyll content and leaf succulence, also indicated the growth-promoting effects of improving the crop yield. These biobased nanomaterials could be pivotal toward sustainable agriculture without harming the ecosystem. The biopolymer-derived NPs have immense potential to be commercially explored for agriculture use.
References
- Ploetz RC, Evans EA (2015) The future of global banana production. Horticultural reviews 43: 311-352.
- Falcomer AL, Riquette RFR, de Lima BR, Ginani VC, Zandonadi RP (2019) Health benefits of green banana consumption: A systematic review. Nutrients 11(6): 1222.
- Wall MM (2006) Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. Journal of Food Composition and analysis 19(5): 434-445.
- Apostolopoulos V, Antonipillai J, Tangalakis K, Ashton JF, Stojanovska L (2017) Let’s go bananas! Green bananas and their health benefits. prilozi 38(2): 147-151.
- Stover RH (1987) Somaclonal variation in Grand Naine and Saba bananas in the nursery and field. Banana and plantain breeding strategies 21: 136-139.
- De la Rosa G, García CC, Vázquez NE, Alonso CÁJ, Basurto IG, et al. (2017) Physiological and biochemical response of plants to engineered NMs: Implications on future design. Plant Physiol Biochem 110: 226-235.
- Peng C, Zhang H, Fang H, Xu C, Huang H, et al. (2015) Natural organic matter-induced alleviation of the phytotoxicity to rice (Oryza sativa L.) caused by copper oxide nanoparticles. Environ Toxicol Chem 34: 1996-2003.
- Zuverza MN, Martínez DF, Du W, Hernandez VJA, Bonilla BN, et al. (2017) Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol Biochem 110: 236-264.
- Judy JD, Bertsch PM (2014) Bioavailability, toxicity, and fate of manufactured nanomaterials in terrestrial ecosystems. Advances in agronomy 123: 1-64.
- Solanki P, Bhargava A, Chhipa H, Jain N, Panwar J (2015) Nano-fertilizers and their smart delivery system. In Nanotechnologies in food and agriculture, Springer, Cham, pp. 81-101.
- Usmam M, Farooq M, Wakeel A, Nawaz A, Cheema SA, et al. (2020) Nanotechnology in agriculture: Current status, challenges and future opportunities. Science of the Total Environment 721: 137778.
- Shalaby TA, Bayoumi Y, Abdalla N, Taha H, Alshaal T, et al. (2016) Nanoparticles, soils, plants and sustainable agriculture. In Nanoscience in food and agriculture, Springer, Cham 1, 283-312.
- Cumplido CF, González MS, Ortega OH, Cadenas PG, Benavides MA, et al. (2019) The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Scientia horticulturae 245(9): 82-89.
- Tamez C, Hernandez MM, Hernandez VJA, Gardea TJL (2019) Uptake, transport, and effects of nano-copper exposure in zucchini (Cucurbita pepo). Science of the Total Environment 665: 100-106.
- Keller AA, Adeleye AS, Conway JR, Garner KL, Zhao L, et al. (2017) Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 7: 28-40.
- Steiner AA (1961) A universal method for preparing nutrient solutions of a certain desired composition. Plant and soil 15(2): 134-154.
- Kumar NV, Basavegowda VR, Murthy AN, Lokesh S (2022) Synthesis and characterization of copper-chitosan based nanofungicide and its induced defense responses in Fusarium wilt of banana. Inorganic and Nano-Metal Chemistry, p. 1-9.
- Madden (2006) Botanical epidemiology: some key advances and its continuing role in disease management. European Journal of Plant Pathology 115(1): 3-23.
- Wheeler (1969) An introduction to plant diseases. An introduction to plant diseases. John Wiley and Sons Limited, London, pp. 301.
- Qi CH, Chen M, Song J, Wang BS (2009) Increase in aquaporin activity is involved in leaf succulence of the euhalophyte Suaeda salsa, under salinity. Plant science 176(2): 200-205.
- Khan ZS, Rizwan M, Hafeez M, Ali S, Javed MR, et al. (2019) The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environmental Science and Pollution Research 26(19): 19859-19870.
- Rai RV, Parthiban KT, Kumaravelu G (1995) Studies on the drought tolerance of Eucalyptus at seedling stage. Journal of Tropical Forest Science, pp. 155-160.
- Behboudi F, Tahmasebi SZ, Kassaee MZ, Modarres SAM, Sorooshzadeh A, et al. (2019) Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. Journal of Plant Nutrition 42(13): 1439-1451.
- Mustaffa R, Osman A, Yusof S, Mohamed S (1998) Physico-chemical changes in Cavendish banana (Musa cavendishiiL var Montel) at different positions within a bunch during development and maturation. Journal of the Science of Food and Agriculture 78(2): 201-207.
- Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, et al. (2017) Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays). Scientific Reports 7(1): 1-11.
- (1997) United States. Department of Agriculture. Office of Communications. (1997). Agriculture Fact Book. Office of Communications, US Department of Agriculture.
- Hernández HH, González MS, Benavides MA, Ortega OH, Cadenas PG, et al. (2018) Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules 23(1): 178.
- Hernández Y, Lobo MG, Gonzalez M (2006) Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chemistry 96(4): 654-664.
- Mauch F, Mauch MB, Boller T (1988) Antifungal hydrolases in pea tissue II. Inhibition of fungal growth by combinations of chitinase and beta-1,3-glucanase. Plant Physiol 88(3): 936-942.
- Chance B, Maehly A (1995) Assay of catalases and peroxidases. Methods Enzymol 1: 357-424.
- Taneja SR, Sachar R (1974) Induction of polyphenol oxidase in germinating wheat seeds. Phytochem 13(12): 2695-2702.
- Moerschbacher BM, Noll UM, Flott BE, Reisener HJ (1988) Lignin biosynthetic enzymes in stem rust infected, resistant and susceptible near-isogenic wheat lines. Physio and Mol Plant Pathol 33(1): 33-46.
- Saharan V, Sharma G, Yadav M, Choudharya MK, Sharma SS, et al. (2015) Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Int J of Biol Macromol 75: 346-353.
- Jaiswal M, Chauhan D, Sankararamakrishnan N (2012) Copper chitosan nanocomposite: synthesis, characterization, and application in removal of organophosphorous pesticide from agricultural runoff. Environmental Science and Pollution Research 19(6): 2055-2062.
- Brunel F, El Gueddari NE, Moerschbacher BM (2013) Complexation of copper (II) with chitosan nanogels: toward control of microbial growth. Carbohydrate polymers 92(2): 1348-1356.
- Kranjc E, Drobne D (2019) Nanomaterials in plants: a review of hazard and applications in the agri-food sector. Nanomaterials 9(8): 1094.
- Ahmed F, Javed B, Razzaq A, Mashwani ZUR (2021) Applications of copper and silver nanoparticles on wheat plants to induce drought tolerance and increase yield. IET nanobiotechnology 15(1): 68-78.
- Khan Z, Upadhyaya H (2019) Impact of nanoparticles on abiotic stress responses in plants: an overview. Nanomaterials in plants, algae and microorganisms, pp. 305-322.
- Dimkpa CO, Singh U, Bindraban PS, Elmer WH, Gardea TJL, et al. (2019) Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Science of the Total Environment 688: 926-934.
- Lokesh V, Divya P, Puthusseri B, Manjunatha G, Neelwarne B (2014) Profiles of carotenoids during post-climacteric ripening of some important cultivars of banana and development of a dry product from a high carotenoid yielding variety. LWT-Food Science and Technology 55(1): 59-66.
- Abhishek AB, Chandankumar KP, Vinay BR, Bhagyalakshmi N (2019) Vitamin C content in Indian dessert bananas and their antioxidant potential. Int J Sci Eng Res 7(5): 58-62.
- Hernández HH, Benavides MA, Ortega OH, Hernández AD, Juárez MA (2017) Cu Nanoparticles in chitosan-PVA hydrogels as promoters of growth, productivity and fruit quality in tomato. Emirates Journal of Food and Agriculture 29(8): 573-580.
- Nair PMG, Chung IM (2014) A mechanistic study on the toxic effect of copper oxide nanoparticles in soybean (Glycine max L.) root development and lignification of root cells. Biological trace element research 162(1): 342-352.
- Kouzai Y, Mochizuki S, Saito A, Ando A, Minami E, et al. (2012) Expression of a bacterial chitosanase in rice plants improves disease resistance to the rice blast fungus Magnaporthe oryzae. Plant Cell Reports 31: 629-636.
- Bowler C, Montagu MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annual review of plant biology 43(1): 83-116.
- Day R, Buschmann H, Randles S, Beeching JR, Cooper RM (2004) Phenylpropanoids, phenylalanine ammonia lyase and peroxidases in elicitor‐challenged cassava (Manihot esculenta) suspension cells and leaves. Annals of botany 94(1): 87-97.
- Fugate KK, Ribeiro WS, Lulai EC, Deckard EL, Finger FL (2016) Cold temperature delays wound healing in postharvest sugarbeet roots. Frontiers in plant science 7: 499.
- Kuźniak E, Urbanek H (2000) The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiologiae Plantarum 22(2): 195-203.






























