Bacteria Bacillus velezensis BIM В-439 D as the Basis of Biopreparation Betaprotectin to Control Root Rots of Cucumber
Mandryk-Litvinkovich MN1*, Sviridov AV2, Kuptsov VN1, Shinkorenko EG2, Ostapchik VV3 and Kalamiyets EI1
1Research and production association, Chemical synthesis and biotechnologies, Minsk, Belarus
2Grodno State Agrarian University, Grodno, Belarus
3Grodno vegetable factory, Grodno, Belarus
Submission: December 22, 2022; Published: January 17, 2023
*Corresponding author: Mandryk-Litvinkovich MN, Research and production association, Chemical synthesis and biotechnologies, Minsk, Belarus
How to cite this article: Mandryk-Litvinkovich MN, Sviridov AV, Kuptsov VN, Shinkorenko EG, Ostapchik VV, et al. Bacteria Bacillus velezensis BIM В-439 D as the Basis of Biopreparation Betaprotectin to Control Root Rots of Cucumber. Agri Res & Tech: Open Access J. 2023; 27 (3): 556370. DOI: 10.19080/ARTOAJ.2023.27.556370
Abstract
Molecular-genetic and functional characterization of bacterial strain B. velezensis BIM В-439 D as the basis of biopesticide Betaprotectin was carried out. Bacterial genome contained 10 loci determining synthesis of polyfunctional enzyme complexes responsible for production of several antimicrobial compounds and lacking genes of drug resistance and pathogenicity. It was shown that the microbial culture suppressed development of a broad spectrum of fungal and bacterial pathogens infecting agricultural crops, raised germination power and sprouting rate of cucumber seeds, displayed high survival rate in natural soil microbiocenoses accounting for elevated phytoprotective and growth-stimulating potential and attractive application prospects as the active principle of biopesticide Betaprotectin. Prolonged application record of biopesticide throughout 10-year vegetation period to promote greenhouse cucumber culture provided for stable phytosanitary control of root rots, caused favorable effect on plant status and increased yields of vegetable products.
Keywords: Bacteria Bacillus velezensis BIM В-439 D; Greenhouse cucumber; Root rots; Biopesticide betaprotectin
Introduction
Root rots of greenhouse cucumber are widely distributed and deleterious diseases affecting this crop in all cultivation areas. Typical pathogens are represented by soil-borne fungi Pythium debaryanum, Rhizoctonia solani, Ascohyta cucumis, genus Fusarium [1-3]. Pathogenic fungi apart from deterioration of rhizosphere, release toxic metabolites inhibiting growth and development of cucumber plants [4]. Strains of microbial antagonists and the derived biopreparations are successfully used to control population density of many phytopathogenic species, including causal agents of cucumber root rots [5-7]. Of special interest are sporulating bacteria of genus Bacillus exerting antagonistic impact on microbial pathogens and displaying phytoregulating activity, i.e. the ability to stimulate growth and development of cultivars, to induce their resistance to damaging factors, to initiate conducive influence on biological activity and make-up of soil microbiota. Such favorable effects are determined by the capacity of numerous bacilli to produce antibiotics, phytohormones, hydrolytic enzymes contributing to utilization of diverse substrates and to generate agents involved in mineralization of phosphororganic compounds resulting in plant-digestible ion РО42–. Growth-promoting activity of the bacilli may be also accounted for by the ability to synthesize vitamins and immunomudulators enhancing plant resistance potential [8-15]. Thus, the increased biological activity and phytostimulating function lay the basis for wide application prospects of Bacillus cultures in biotechnological sector as the promising sources of bioactive agents and the key ingredients of efficient biopesticides. Nowadays spore-forming bacteria of genus Bacillus serve as an active principle of a broad spectrum of biopreparations applied to curb root rots of cucumber crops around the world, e.g. Biosubtilin (Biotech International Ltd., India), RhizoVital (ABiTEP GmbH, Germany), Serenade (AgroGuest, USA), Sonata (AgroGuest, USA), etc [14-19]. To improve competitive edge of phytoprotective biopreparations it is vital to incorporate into their formulas superactive microbial strains capable to carry out long-term stable control of phytopathogen development. Investigation of genetic and physiological properties of the cultures enables to evaluate and predict biotechnological potential thereof. Aim of this research was characterization of the studied strain B. velezensis BIM B-439 D and assessment of long-term application efficiency of the derived biopesticide Betaprotectin for control of root rots attacking greenhouse cucumber crop.
Objects and Methods
Object of study - bacterial strain Bacillus velezensis BIM В-439 D distinguished by high antagonistic activity toward a broad spectrum of phytopathogenic fungi and bacteria. Test cultures to evaluate antimicrobial activity: fungi Penicillium expansum, Alternaria tenuis, Fusarium graminearum, Fusarium oxysporum, Fusarium culmorum, Fusarium avenaceum, Botrytis cinerea, Phoma betae and phytopathogenic bacteria Pseudomonas syringae var. lachrymans, Ps. fluorescens, Xanthomonas campestris subps. campestris, Pectobacterim carotovorum subsp. carotovorum, Clavibacter michiganensis subps. michiganensis, Agrobacterium tumefaciens, phytopathogenic deposited in Belarusian collection of non-pathogenic microorganisms, Institute of Microbiology, National Academy of Sciences of Belarus. Fungi cultures were maintained on agar nutrient media (MPA, LB, HBA, 2 % agar), the fungi were grown on PDA medium (2 % agar). Submerged culture of bacterial strain B. velezensis BIM В-439 D was carried out in liquid modified Meynell medium [19] with molasses (30 g/l) in Erlenmeyer flasks on the shaker-incubator (200 rpm) at temperature 30±2 °С during 48h.
Liquid submerged cultures of phytopathogenic bacteria were incubated in Hottinger broth in Erlenmeyer flasks on the shaker (180-200 rpm) at temperature 30±2 °С for 24h, phytopathogenic fungi were cultured in potato-glucose broth in Erlenmeyer flasks on the shaker-incubator (140 rpm) at temperature 24±2°С during 36-48h. Cell and spore titers of liquid bacterial culture B. velezensis BIM В-439 D were determined by Koch method [20]. To facilitate spore counts prior to inoculation on agar medium thermal treatment of bacterial dilutions was conducted at 80 °С for 10 min. Antagonistic activity of cultural liquid of strain B. velezensis BIM В-439 D was estimated by method of delayed antagonism (wells technique). Sterile water was used in control variants [21]. To evaluate enzymatic activity of bacterial cultures standard qualitative methods were engaged, taking into account utilization degree of specific substrates-inducers: starch for amylolytic activity assays, casein for proteolytic activity, xylan for xylanase measurements, Na+-carboxymethylcellulose for cellulase assays, colloid chitin – for chitinolytic activity [22].
Growth-stimulating activity of cultural liquid of bacteria B. velezensis BIM В-439 D was investigated on seeds and seedlings of tomato (hybrid variety Torrero F1) and cucumber (hybrid variety Rodnichok). Assessment of seed quality and germination was performed according to directions of State standard 12038- 84. Sterile water was taken as the control. Survival of bacteria B. velezensis BIM В-439 D in sod-podzol soil (рН 6.0-6.2) was judged by the results of model experiments (upon addition of 48h bacterial cultural liquid bearing spores in minimal concentration 1x107 CFU/g soil). The titer of bacterial spores in the samples was counted by Koch method after heating soil suspensions. Soil moisture content was kept throughout the experiment on the 50- 60 % level. Concentrations of micromycetes and aerobic bacterial microbiota in soil were determined by method of finite dilutions [20]. DNA libraries for full-genome sequencing were prepared with Nextera XT («Illumina», USA). Nucleotide sequences were identified using MiSeq («Illumina», USA) equipped with MiSeq Reagent Kit v3 (USA). Data quality was verified with FastQC [23]. Low-resolution reads were filtered with Trimmomatic-0.36 [24-27]. Readings were assembled with SPAdes-3.11.1 [28]. Preliminary genome annotation was realized with the aid of RAST 2.0 and PROKKA 1.12 package [26,27] Individual genetic loci were compared using web-resource antiSMASH [28]. Mauve (v. 20150226) software was applied for comparison of genomes [29]. Average Nucleotide Identity (ANI) was computed with web-service JSpeciesWS [30]. The search for genes of antibiotic resistance and pathogenicity relied on resources ResFinder 4.1 and PathogenFinder 1.1 provided by the web-server of the Center of Genome Epidemiology, Technical University, Denmark [31].
Large-scale efficiency trials of biopesticide Betaprotectin in regard to cucumber pathogenic agents were performed in greenhouse complex of Grodno vegetable factory, Belarus growing hybrid variety Mirabella F1. The technology of cucumber cultivation is standard for this agricultural zone. Betaprotectin efficiency tests were conducted on model vegetable culture against natural infection background. The dosage of Betaprotectin expense for treatment of greenhouse cucumber equaled 50 l/ ha. The vegetables were watered with 2 % working solution of biopesticide via installed irrigation system, 4-5 times during vegetation season with fortnight intervals. The unit dose of Betaprotectin working solution - 250 ml per plant. The treatment periods: for the first crop rotation cycle – February to May, for the second crop rotation cycle - July to October. Phytosanitary monitoring of greenhouse cucumber was accomplished throughout the whole period starting from initial supply of Betaprotectin until the end of vegetation season. Spread and severity rate of the diseases were controlled 10 days after plant treatment by checking 10 plants evenly distributed across field plot diameter in each sample repetition series. By the end of vegetation period root system and adjacent stem regions of plants were inspected. Economic efficiency of biopesticide was estimated as the amount of harvested cucumbers saved versus the control.
Results and Discussion
Sporulating bacterial strain Bacillus velezensis BIM B-439 D was isolated from sod-podzol soil sampled in Belarus and its bacterial genome was sequenced and deposited in database of GenBank NCBI under registration number CP032144 [16-18]. Genome of strain B. velezensis BIM B-439 D is represented by circular chromosome sized 3 978 954 b.p. containing 46.50 % of G/C pairs (Figure 1). The deciphered chromosome includes 3969 predicted genes, including 3769 protein-encoding sequences, 27 rRNA genes, 86 tRNA genes, 82 pseudogenes, 9 genetic loci responsible for synthesis of 27 rRNA molecules and 86 genes determining synthesis of tRNA. Analysis of B. velezensis BIM B-439 D genome revealed 10 loci common for genus Bacillus (the total length 336520 b.p.) defining synthesis of multifunctional enzyme complexes catalyzing production of several antimicrobial compounds. Using ResFinder 4.1 and PathogenFinder 1.1 resources, it was found that genome of strain B. velezensis BIM B-439 D lacked antibiotic resistance genes and islets of pathogenicity, accounting for their elevated phytoprotective potential and attractive application prospects as the basis of biopesticides.

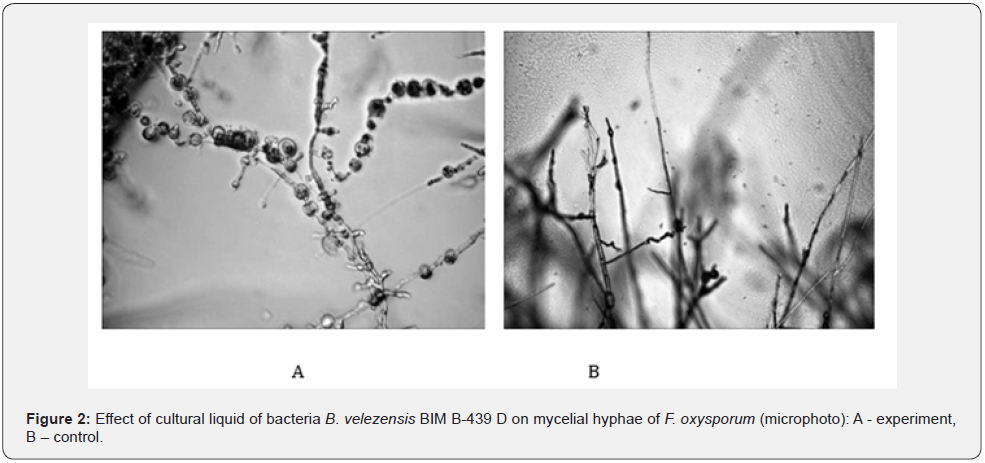
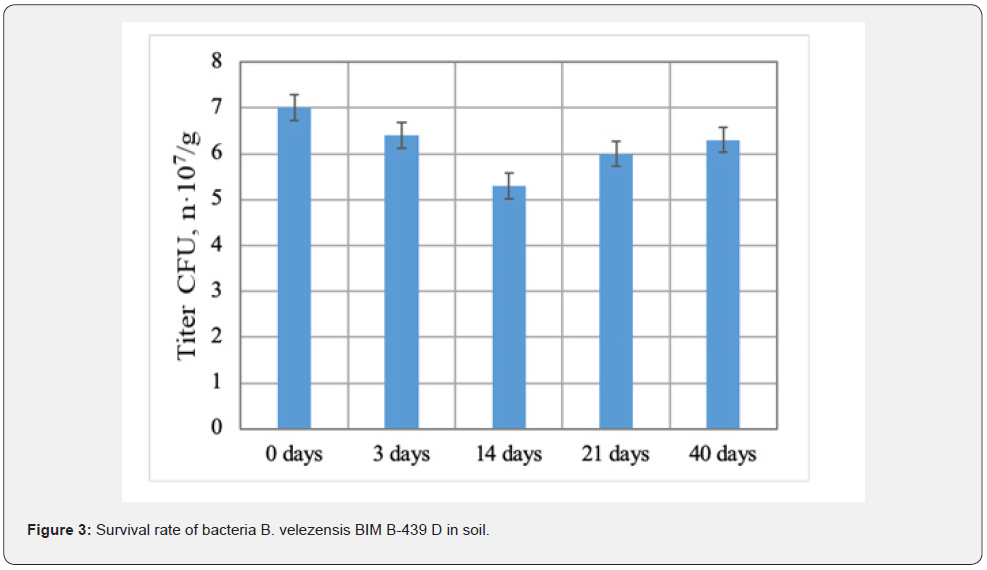
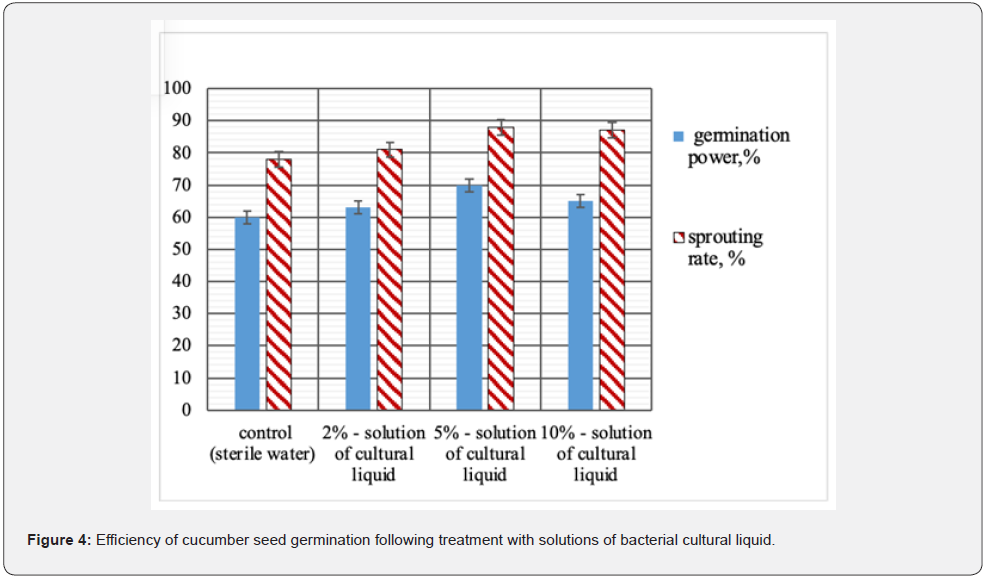
Biotechnological potential of strain B. velezensis BIM B-439 D was analyzed in laboratory and large-scale production experiments. Wells technique demonstrated (Table 1) that bacteria B. velezensis BIM B-439 D displayed inhibitory action toward a broad range of phytopathogens causing most wide-spread diseases of agricultural crops, including root rots of cucumber. It was observed that swelling and deformation of hyphae occurred in phytopathogenic fungi exposed to active metabolites of tested bacteria, ultimately resulting in their complete lysis (Figure 2). Bacterial strain B. velezensis BIM B-439 D shows the ability to utilize casein, starch, cellulose, colloid chitin and xylan, evidencing generation by bacterial cells of variegated enzyme complex: protease, amylase, β-endoglucanase, chitinase, xylanase. Production of a wide spectrum of hydrolytic enzymes may act as an additional antagonistic factor of bacteria B. velezensis BIM B-439 D augmenting their competitive efficiency in rivalry with pathogens from soil microbiota. To assess competitive and adaptive potential of strain B. velezensis BIM В-439 D, survival of the bacteria was traced in natural microbial cenoses. In the course of laboratory experiments bacterial cultural liquid was supplied into soil containing native strains of pathogenic and saprophytic micromycetes (cell titer at least 6.0·104 CFU/g) and bacteria (the minimal titer 3.0·103 CFU/g). Experimental results established that upon 40 days ≥β 90% of viable spores of B. velezensis BIM В-439 D survived in soil samples (Figure 3), evidencing enhanced adaptation rate and competitive ability of the culture introduced into natural soil microbiocenoses. Phytostimulating activity of bacteria B. velezensis BIM В-439 D was also evaluated. The effect of liquid bacterial culture on seed germination and development of cucumber seedlings was tested. The results have shown that seed treatment with bacterial cultural liquid significantly upgraded germination efficiency. The best results were achieved under the 20-min impact of 5 % solution of cultural liquid of B. velezensis BIM В-439 D: germination power of cucumber seeds soaked in 5% solution of bacterial liquid culture rose by 16 % on the average, sprouting rate by 14 % (Figure 4).

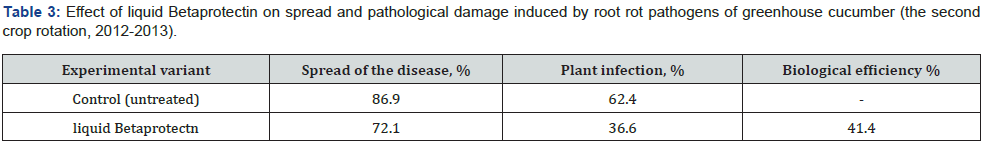
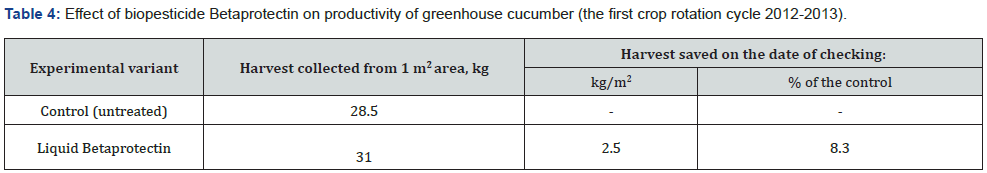
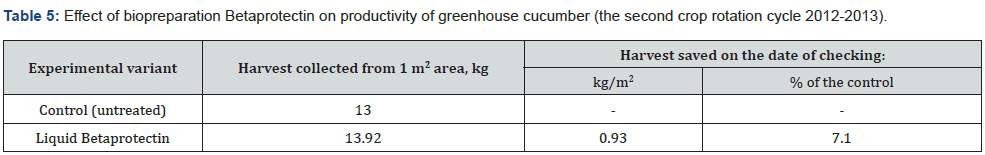
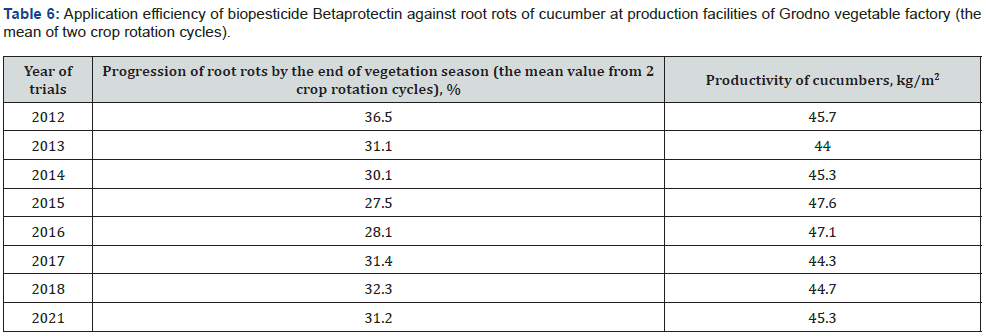
Owing to the pronounced phytoprotective and growth-stimulating activity bacterial strain B. velezensis BIM В-439 D was applied as the basis of biopesticide Betaprotectin intended for control of microbial infections in cucumber and tomato cultivars. Efficiency trials of biopesticide Betaprotectin (expense 50 l/ha) on cucumber seedlings grown in nurseries in small-scale hydroponic culture during the first crop rotation cycle diminished distribution of the pathologies by 25.6%, whereas the intensity of root rot damage fell by 20.9%. The level of biological efficiency of applied biopesticide reached 40.3% (Table 2). According tj the Table 3, the similar relationship was recorded likewise during the second rotation cycle of cucumber culture. Application of biopesticide Betaprotectin curtailed the average spread of root rots by 14.8%, and the extent of root damage was 25.8% lower than in the non-treated control variant. Biological efficiency of Betaprotectin reached the average mark 41.4%. Betaprotectin supply enabled to raise harvests of cucumbers during the first crop rotation cycle from 28.5 kg/m2 to 31.0 kg/m2. The saved vegetable yield equaled 2.5 kg/m2, or 8.3 % up the control (Table 4). Application of Betaprotectin during the second crop rotation cycle resulted in extra 0.93 kg/m2 yield of cucumber, gaining additional 7.1 % above the control harvest (Table 5). During 2012-2021 regular supply of biopesticide Betaprotectin 4-5 times in the course of cucumber vegetation season, with fortnight intervals in each crop rotation cycle provided for stable phytosanitary control of root rot diseases (Table 6). Extent of cucumber root damage within the framework of Betaprotectin testing program ranged from 36.5 % in 2012 to 27.5 % in 2015. In contrast, intensity of control plant lesions constituted 53.8-60.4 %. The improved phytosanitary status considerably upgraded cucumber yields varying from 44.0 kg/m2 in 2013 to 47.6 kg/m2 in 2015 as compared with the control values 40.4-42.5 kg/m2

Conclusion
Summing up, the analysis of genetic and functional characteristics of bacterial strain B. velezensis BIM В-439 D indicates that its promising biotechnological potential is appropriate for use in microbiological industry as the active principle of biopesticide Betaprotectin. Prolonged 10-year application record of biopesticide Betaprotectin in greenhouse practice secures stable phytosanitary control of root rot diseases, causes beneficial effect on cucumber seedlings and promotes output of top-grade vegetable products.
References
- Karbozova RD (1993) Root rot of greenhouse cucumber and methods to diminish its infectivity. Synopsis of Ph.D thesis in agrosciences, specialty 06.01.11-plant protection. Almata, p. 21.
- Grishechkina LD (2003) Diagnostics of vegetable crop diseases in greenhouse culture. Journal of Phytoprotection and quarantine 3: 45-50.
- Rudakov OL, Rudakov VО (2016) Modification of methods to diagnose root rots of cucumber. Journal of Plant protection 4: 21-23.
- Woloshuk CP (2013) Aflatoxins, fumonisins, and trichothecenes: a convergence of knowledge. Journal of FEMS Microbiol. Rev. 37(1): 94-109.
- Gravel V, Martinez C, Antoun H, Tweddell RJ (2006) Control of greenhouse tomato root rot [Pythium ultimum] in hydroponic systems, using plant-growth-promoting microorganisms Can. Journal Plant Patho 28(3): 475-483.
- Bosmans L, Burijn ID, Gerards S, Rob M, Lore V, et al. (2017) Potential for biocontrol of hairy root disease by a Paenibacillus clade 8: 447.
- Punja ZK, Yip R (2003) Biological control of damping-off and root rot caused by Pythium aphanidermatum on greenhouse cucumbers. Can. Journal Plant Pathol 25(4): 411-417.
- Harwood CR, Mouillon JM, Pohl S, Arnau J (2018) Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. Rev 42(6): 721-738.
- Saharan BS, Nehra V (2011) Plant Growth Promoting Rhizobacteria: A Critical Review. Journal of Life Sci Med Res 21: 1-30.
- Dragovoz VI (2014) Exometabolites of strain Bacillus amyloliquefaciens IMB В-7100 defining its phytostimulating activity. Journal of Plant physiology and genetics 46(6): 516-524.
- Morgun VV, Kots YS, Kyrychenko EV (2009) Growth promoting rhizobacteria and their use in practice. Journal of Fiziol. Biokhim. Kul’t Rast 41(3): 187-207.
- Zabokritskiy NA (2015) The biologically active substances produced by probiotic microorganisms of the genera Bacillus and Lactobacillus. Journal of Scient Art 17(3): 8-19.
- Bhushan BJ (2000) Production and characterization of a ther mostable chitinase from a new alkalophilic Bacillus sp. BG-11. Journal of Appl Microbiol 88(5): 800-808.
- Abbas A, Khan SU, Wasim UK, Saleh TA, Hafeez M, et al. (2019) Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticide. C R Biologies: 342(5-6): 124-135.
- Aloo BN, Makumba BA, Mbega ER (2019) The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability Journal of Microbiological Research 219: 26-39.
- Berezhnaya AV, Evdokimova OV, Valentovich LN, Sverchkova NV (2019) Molecular-genetic analysis of chromosome loci determining antimicrobial properties of bacteria B. velezensis BIM В-439 D. Journal of Prikl. biochem. Microbial 55(4): 366-377.
- Berezhnaya AV (2015) Molecular-genetic analysis of determinants encoding synthesis of antimicrobial metabolites by bacteria of genus Bacillus IX Int Sci conf, Belarus.
- Titok MA, Valentovich LN, Berezhnaya AV, Kolomiets EI (2018) Genome analysis of bacterial strain Bacillus amyloliquefaciens BIM В-439 D. Doklady Nat Acad Nauk Belarusi 62(5): 592-600.
- Meynell D (1967) Experimental microbiology. Journal of Mir Publ, pp.347.
- Aseyeva IV (1984) Methods of soil microbiology and biochemistry. pp. 304.
- Segy J (1983) Methods of soil microbiology Мoscow. Kolos Publ, pp. 296.
- Rukhlyadeva AP (1981) Methods to assay activity of hydrolytic enzymes. Legpishcheprom Publ, pp. 288.
- Babraham Bioinformatics - FastQC a quality control tool for high throughput sequence data.
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: А flexible trimmer for Illumina sequence data. Journal of Bioinforma. Oxf Engl 30(15): 2114-2120.
- SPAdes 3.11.1 Manual.
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75.
- Seemann T (2014) Prokka: Rapid prokaryotic genome annotation. Journal of Bioinformatics 30(14): 2068-2069.
- Darling AE, Mau B, Perna NT (2010) Progressive Mauve: Multiple Genome Alignment with Gene Gain, Loss and Rearrangement. PLOS ONE 5(6): e11147.
- Richter M, Móra RR, Glöckner FO, Peplies J (2016) SpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Journal of Bioinformatics 32(6): 929-931.
- CGE Server.






























