Mycoselectivity of Mushroom Compost and Surfaces, Disinfected with Gas Chlorine Dioxide
Wiesław Hreczuch1, Joanna Szumigaj Tarnowska3, Nikodem Sakson2, Arkadiusz Chruściel1, Zbigniew Uliński3 and Katarzyna Kiedos1*
1MEXEO, ul. Energetyków 9, 47-225 Kędzierzyn-Koźle, Poland
2Expert, Poznań, Poland
3The National Institute of Horticultural Research, ul. Konstytucji 3 Maja Skierniewice, Poland
Submission: August 12, 2022; Published: August 23, 2022
*Corresponding author: Katarzyna Kiedos MEXEO; ul. Energetyków 9, 47-225 Kędzierzyn-Koźle, Poland
How to cite this article: Wiesław H, Joanna Szumigaj T, Nikodem S, Arkadiusz C, Zbigniew U, et al. Mycoselectivity of Mushroom Compost and Surfaces, Disinfected with Gas Chlorine Dioxide. Agri Res & Tech: Open Access J. 2022; 26 (5): 556355. DOI: 10.19080/ARTOAJ.2022.26.556355
Abstract
In order to limit losses in mushroom cultivation, various methods and measures are used to prevent the development of pathogens. However, frequently occurs the effective disinfection does not result directly a positive response in the form of increased yielding or at least reduction of the problems of infections with green mould or bubble diseases. The studies presented in this paper show that even with a relatively high level of inoculation of phase II compost containing Trichoderma aggressivum (105/cm3), significantly exceeding the potential intensity of natural infections, Agaricus bisporus remained dominant in the environment. On the other hand, an excessive disinfection disturbed this balance in favor of the more disinfectant-resistant pathogen. High efficacy of the innovative gas ClO2 disinfection, at the applied balance concentration 1000 ppm (m/m) was determined on the surfaces of the cultivation hall. However, the study demonstrates that effective disinfection of compost may give the effects of weakening of mushroom spawn mycelium and domination of its environment by the competing pathogens.
Keywords: Agaricus bisporus; gas ClO2 disinfection; Trichoderma aggressivume
Introduction
Pathogens have contributed to huge crop losses in mushroom cultivations. The most important fungal diseases are: dry bubble (Verticillium fungicola), dactylium (Cladobotryum dendroides), wet bubble (Mycogone perniciosa) and green mould, caused in Europe by the species Trichoderma aggressivum f. europaeum. The source of success or problems in mushroom yielding are mostly the internal factors of the mushroom farm, including: cultivation methods, technical condition and equipment of the facility. External factors include the location/neighborhood of the facility, the hot or wet season or periodic epidemics. Secondary infections introduced from outside with the compost, casing, from the air or by the staff and flycatchers may also occur objectively [1]. Therefore, several overtures introducing disinfection of casing, compost and/or cultivation hall have been practiced. The essence of the optimal action for the success in yielding mushrooms is to create a mycoselective environment conducive to the growth of mycelium and mushroom fruiting bodies. Mycoselectivity is understood in this paper as a feature of casing, compost and the farm ensuring a non-sterile mushroom growing environment with rich symbiotic microflora, enzymes and mineral substances supporting defense mechanisms against pathogens limiting or eliminating growth and welfare of mushrooms.
There are three phases in the production of mushroom compost. In the first phase compost fermentation processes occur, in the second phase the compost (phase II compost) is conditioned at 45-50 oC, until it is free of ammonia and to enable microorganisms other than Agaricus bisporus to grow. The third phase (phase III compost) begins with the inoculation of phase two compost with mushroom mycelium and its growth [2,3]. It appears, that many modern button mushroom farms, which use the most effective disinfection methods, struggle with the problems of diseases and underestimated yield effects. At the same time, many mushroom farms using the simplest hygienic treatments, without advanced disinfectants and/or plant protection products achieve good results, and vice versa, as well. This issue is worth clarifying.
Disinfection measures applied in the cultivation hall before mushroom cultivation begins to play an important role for good yields. The use of chlorine releasing agents (CRAs) or formalin is still a common practice of chemical disinfection in mushroom cultivation process, but their nuisance due to both human and environmental health issues is well known [4,5]. Steam sterilization, steam pasteurization and hot water immersion are the next methods of disinfection in mushroom cultivation, but because of high costs of implementing they are not profitable [6]. It is quite easy to demonstrate the biocidal efficacy of the disinfectants and plant protection products in a laboratory and even in real objects during cultivation. However, frequently occurs the effective disinfection does not result directly a positive response in the form of increased yielding or at least reduction of the problems of infections, if not applied properly. The aim of the study is to demonstrate that direct disinfection of II-phase mushroom compost gives the effects of weakening of mushroom spawn mycelium and domination of the environment by the competing pathogens. Additionally, the applied chlorine dioxide gas fumigation was demonstrated, as an effective disinfection tool in mushroom farm.
Materials and Methods
Crop details
In the experiments, phase II compost produced by STEC Sp. z o.o., Poland, was used. The mushroom spawn (737 SPRINT) came from SYLVAN POLSKA Sp. z o.o., Poland. The cultivation was carried out in an experimental hall with a volume of 180 m3 which was at the disposal of the Laboratory of Vegetable and Edible Mushrooms Cultivation, The National Institute of Horticultural Research, Skierniewice, Poland (abbr. InHort). The breeding tests were performed in transparent polypropylene pots with a total volume of 2 dm3 each, filled with 350 g of mushroom phase II compost, spawned with Agaricus bisporus 7373 Sprint mushroom strain in the amount of 1% by weight. After inoculation, the mycelium of A. bisporus grew within the compost until complete colonization, than phase III compost was obtained. The conditions in the mushroom house chamber were fully controlled as for routine production that is, the compost temperature was maintained at 24-25 °C and the relative humidity was kept high to minimize drying of the compost surface or the spawn.
The prepared pots filled with phase III mushroom medium were inoculated with green mould. T. aggressivum isolate, CBS 100.526, used in this study, were obtained from international culture collections Centraalbureau voor Schimmelcultures (CBS,Utrecht, The Netherlands). The isolate was maintained on potato dextrose agar at 24 °C for 5 days. Spore suspensions of 5-day-old culture was prepared by flooding the plates with sterile distilled water. The Trichoderma spores were counted in Thoma chamber and the spores concentration was adjusted to 105 spores per ml.
Inoculation with the pathogen of green mould Trichoderma aggressivum
The compost surface of the investigated trials was sprayed with 1 ml of T. aggressivum spores suspension containing 1 × 105 spores per ml, which corresponded to 250 spores per 1 g of substrate. Two series of experiment with five replications were carried out.. The compost was incubated at 25°C, relative humidity 95% for 12 days. During incubation the pots were covered with light polystyrene trays in order to reduce evaporation. The development of A. bisporus mycelium overgrowth was visually assessed on the scale: 0 - none, 1 - weak, 2 - medium and 3 - strong. The development of T. aggressivum isolates in the infected pots was evaluated with the same scale.
ClO2 gas disinfection
Five of the inoculated pots were transferred to the experimental cultivation hall and exposed for 180 minutes to the atmosphere of chlorine dioxide generated in the growing room environment at concentration of 1000 ppm [m/m]. The disinfection was performed with the use of chlorine dioxide gas produced in the reaction of sodium chlorite (commercial preparation ARMEX 5 pink) and hydrochloric acid (commercial preparation MEXACID yellow) introduced to the ClO2 gas generator MEX-5 (Figure 1), developed by MEXEO in Kędzierzyn-Koźle, Poland.
The emission of the assumed mass of gas chlorine dioxide to the atmosphere of the experimental hall was quantified on the basis of the stoichiometry of the reaction equation (1), taking into account the amount of reagents introduced into the reactor of MEX-5 generator used:
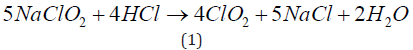
The expected balance concentration of ClO2 was 1000 ppm in the air [m/m]. However, the actual concentration of generated gas ClO2 in the space is not stable, due to its oxidative reactivity, especially in the presence of an organic material the concentration of chlorine dioxide is constantly decreasing. Therefore, the actual concentration in the air was monitored with a periodic measurement. The determination of the ClO2 gas concentration in the disinfected room of the cultivation hall was carried out with the use of the GASTEC GV-100 measuring device, with measuring tubes 8H (25 - 1000 ppm ClO2), produced by GASTEC Corporation, Japan. After the disinfection and ventilation of the cultivation hall, five remaining pots were also transferred into the experimental hall (Figure 2).


Disinfection control tools
Monitoring of the effectiveness of disinfection on the tested surfaces was carried out with the use of:
I. Count-Tact TSA agar contact plates with lecithin for general microbial counting, (Graso Biotech, Starogard Gdański, Poland), of which:
a. place of sample collection was flooring (1 location)
b. number of tests included 2 plates each, before and after ClO2 gassing.
II. Count-Tact agar contact plates with chloramphenicol and streptomycin - mycoselective, (Graso Biotech, Poland), of which:
a.. place of sample collection was flooring (2 locations), shelving (1 location)
b. number of tests included 3 plates each in one location, before and after ClO2 gassing.
III. Standard tests of spor Bacillus atrophaeus, which is recognized as a reference organism for determining disinfection efficiency, ACE test, (Fukuzawa Shoji Co., Ltd., Japan), of which:
a. exhibition area was on the shelf at the entrance to the hall (1 location) and
b. number of tests included 3 sachets. The negative test results indicated 6 log level of the microorganism reduction, equivalent to sterilization standard.
Results
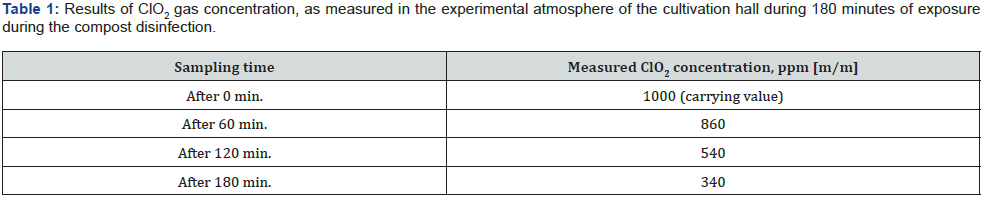
Chlorine dioxide is a known biocide of high disinfection efficiency. It is commonly used in the form of aqueous solutions for disinfecting drinking water in municipal networks. It is also used to disinfect technological water in mushroom farming. Within the framework of the described studies, chlorine dioxide was successfully used for the first time in gaseous form to disinfect cultivation hall. For this purpose, specially constructed generator and preparations developed by MEXEO company (www.dezynfekcja-pieczarka.pl) were used. The actual concentration of ClO2 in the atmosphere of the cultivation hall during the disinfection procedure, exposed for 180 minutes, is presented in Table 1.
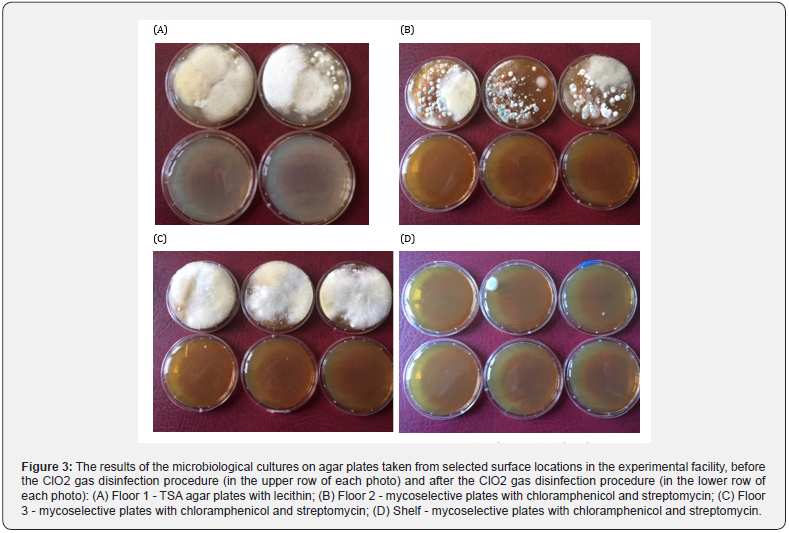
During the exposure of the open experimental pots to chlorine dioxide gas, all areas of the cultivation hall were also disinfected. In this respect, phytosanitary monitoring was carried out by microbiological cultures on agar plates, by taking prints in several places of the floor, walls and shelves, before and after the disinfection. The biocidal effectiveness of the performed disinfection procedure was verified simultaneously with the participation of standard biological tests for survival of the model spores Bacillus atrophaeus. The obtained results of cultures in this field are presented in Figure 3 and the results of verification of the effectiveness of neutralization of the model spore forms with use of the ACE tests are presented in Figure 4.
High disinfection efficiency of the monitored surfaces and air can be observed as a result of the ClO2 gasification carried out under the conditions described. The results of the tests presented on Figure 3. and Figure 4 show the microbiological purity of the floor surfaces as the most troublesome in terms of microbiological load. Moreover the full effectiveness of disinfection in relation to B. atrophaeus spores, reflecting the fulfillment of the criterion of high hygienic regimes adopted for the disinfected surfaces in medical areas, was demonstrated. Thanks to the ability of the gaseous ClO2 to penetrate into the microcracks that are difficult to access, an effectiveness of the disinfection of the mushroom compost was also assumed.

After 12 days period of incubation and overgrowth, the examined objects were subjected to visual assessment. The results are shown in Figure 5 & Figure 6. Based on the observation of the results of mushroom mycelium overgrowth and Trichoderma isolate development, it can be unequivocally stated that the second phase substrate, inoculated with a pathogen that was not disinfected with gas ClO2, perfectly coped with the infection and the environment was fully colonized by the A. bisporus mycelium.
Mushroom mycelium overgrowth was efficient in the entire volume of the experimental pots. In the disinfected pots, the lack of symptoms of mushroom mycelium overgrowth as well as the lack of green mould development can be observed in the upper part of the phase II compost. However, in the lower part of the experimental pots (about 50% by volume) the environment was completely colonized by the pathogen.


Discussion
Microorganisms play an important role in high-yielding production of A. bisporus. Compost preparation is a critical step in the cultivation process, where the biochemical transformations that take place during composting, provide the nutrients that determine mushroom growth [7-9]. Viera and Pecchia (2018) and many other authors [11,12] described the influence of pasteurization conditions of compost, and additives or external microorganisms on the composition of the fungal growth and the fruiting environment. Better understanding of the properties of the compost and the role of the microbial community will increase the efficiency of the cultivation A. bisporus. To that end, the mushroom spawn should be dominant in its growth environment from the beginning to the end of the growing cycle. It is hypothesized that properly shaped native microorganisms facilitate the colonization of the compost and help to limit the growth of competitors and parasites in the cultivation [13].
It is also known that on the surface of A. bisporus mycelium and in its direct vicinity there is a selected and stimulated symbiotic microflora from the phase II compost. However, the exact role of bacteria in the development of fungi has so far been recognized only to a small extent [14,15]. The conducted experiments demonstrated that the mushroom compost of phase II disinfected with gas ClO2 was deceased from the top and colonized by green mould in the bottom part of the experimental pots. It was observed that the impact of gas ClO2 was significantly weaker with the depth of the pot, as the result of oxidant consumption reactions of ClO2 with organic material. In this lower area, the decreased concentration of chlorine dioxide weakened the mushroom mycelium, but less threatened pathogenic fungi, which is known to be the more resistant to disinfectants . As a result, T. aggressivum, along exhaustion of ClO2 and in the absence of defense mechanisms of weakened mushroom mycelium or the symbiotic microflora, could soon dominate the environment. Such a phenomenon was also described in the literature [16-19].
What is more, the presented studies showed that the inoculation of phase II compost with the pathogen T. aggressivum (250 spores per 1 g compost), significantly exceeding the potential intensity of natural infections, remained A. bisporus dominant in the environment, when not disinfected with ClO2. The applied ClO2 disinfection disturbed this balance in favor of a more disinfectant-resistant pathogen. However, it should be noted that in the presence of Trichoderma aggressivum even fully colonized compost (spawn-run compost) by the mushroom mycelium may still suffer yield reduction, which has not been examined in this study. Such results were presented in the work of O’Bien et al. where severity of the effect of T. aggressivum infection on mushroom yield was depended on an infective inoculum as well as intensive mixing treatment after adding the pathogen. It was concluded that low inoculum of Trichoderma spores has no impact on the yield after two flushes, irrespective of the level of mixing. Furthermore, identification of T. aggressivum in these trials was difficult, suggesting that this inoculum level was close to the limit of detection. Thus T. aggressivum is likely to go undetected on surfaces, but compost creates good conditions for growth of Trichoderma mycelium to a point when the inoculum is high enough to cause the mushroom yield reduction. The effect of compost infestation with different fungal isolates of Trichoderma genus on the mushrooms yielding was also studied by Górski et al. [17], proving that different Trichoderma isolates reduced the yield of the edible mushrooms to varying severity. An unaffected growth of A. bisporus mycelium in the presence of the inoculated Trichoderma might also resulted from the mutation of isolate used in this study if had lower pathogenicity, which could have an influence on disease symptoms in the compost pots not exposed to ClO2 disinfection. Nevertheless, the conducted experiments confirmed unambiguously the occurrence of an internal strong defense mechanisms of mushroom mycelium in relation to even the most aggressive pathogen.
Conclusion
It can be concluded that the disinfection of the phase II compost weakened the mushroom mycelium and its symbiotic microbiome, favoring colonization of the cultivation by green mould as its main competitor. Therefore, direct disinfection of phase II compost is not recommended. High efficiency of the innovative gas chlorine dioxide disinfection at concentration 1000 ppm (m/m) and 3 h exposition was confirmed on the investigated surfaces of mushroom hall. As the result, the findings allow to pose further questions concerning the role of disinfection and stimulation of the symbiotic microflora in the scope of mycoselective action and directions of further optimization of cultivation technology to improve efficiency in mushroom production.
Acknowledgement
The research was carried out within the framework of the Operational Program Intelligent Development 2014-2020 co-financed by the EFRR, the project entitled: Innovative, Mycoselective Casing and Compost for Mushroom Cultivation.
References
- Largeteau ML, Savoie JM (2010) Microbially induced diseases of Agaricus bisporus: biochemical mechanisms and impact on commercial mushroom production. Applied Microbiology and Biotechnology 86(1): 63-73.
- Jurak E, Kabel MA, Gruppen H (2014) Carbohydrate composition of compost during cpomposting and mycelium growth of Agaricus bisporus. Carbohydrate Polymers 101: 281-288.
- Mouthier TMB, Kilic B, Vervoort P, Gruppen H, Kabel MA (2017): Potential of a gypsum-free compostin process of wheat straw for mushroom production. PLoS One 12(10): e0185901.
- Todorović B, Milijasević MS, Potočnik I, Stepanović M, Rekanowić E, et al. (2012) In vitro activity of antimicrobial agents against Pseudomonas tolaasii, pathogen of cultivated button mushrooms. Journal of Environmental Science and Health 47(3): 175-179.
- Atila F (2020) Chlorine dioxide as an alternative disinfectant for disinfection of oyster mushroom growing media. The Journal of Horticultural Science and Biotechnology 95(1): 121-127.
- Atila F (2016) Effect of different substrate disinfection method on the production of Pleutorus ostreatus. Journal of Agricultural Studies 4(4): 52-64.
- Kertesz MA, Thai M (2018) Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Applied Microbiology and Biotechnology 102(4): 1639-1650.
- Cao G, Song T, Shen Y, Jin Q, Feng W, et al. (2019) Diversity of bacterial and fungal communities in wheat straw compost for Agaricus bisporus cultivation. American Society for Horticultural Science 54(1):100-109.
- Ranjbar ME, Ghahremani Z, Carrasco J (2019) Effect of compost formulation and postharvest management on quality parameters of button mushroom. International Journal of Recycling of Organic Waste in Agriculture 8: 507-513.
- Vieira FR, Pecchia JA (2018) An exploration into the bacterial community under different pasteurization conditions during substrate preparation (composting–phase II) for Agaricus bisporus cultivation. Microbial Ecology 75(2): 318-330.
- White PF (2000) The effect of covering compost with paper on yield of the cultivated mushroom Agaricus bisporus (Lange) Imbach. The Journal of Horticultural Science and Biotechnology 75(6): 667-671.
- Milijašević MS, Stepanović M, Todorović B, Duduk B, Stepanović J, et al. (2017) Biological control of green mould on Agaricus bisporus by a native Bacillus subtilis strain from mushroom compost. European Jorunal of Plant Pathology 148(3): 509-519.
- Carrasco J, Preston GM (2020) Growing edible mushrooms: a conversation between bacteria and fungi. Environ Microbiol 22(3): 858-872.
- Singh AV, Sharma A, Johri BN (2012) Phylogenetic profiling of culturable bacteria associated with early phase of mushroom composting assessed by amplified rDNA restriction analysis. Annals of Microbiology 62: 675-682.
- Halsey JA, Silva MDCP, Andreote FD (2016) Bacterial selection by mycospheres of Atlantic Rainforest mushrooms. Antonie Van Leeuwenhoek 109(10): 1353-1365.
- Sobieralski K, Siwulski M, Frużyńska JD, Górski R (2009) Impact of Trichoderma aggressivum f. europaeum Th2 on the yielding of Agaricus bisporus. Phytopathologia 53: 5-10.
- Górski R, Sobieralski K, Siwulski M, Frąszczak B, Sas Golak I (2014) The effect of Trichoderma isolates, from family mushroom growing farms, on the yield of four Agaricus bisporus (Lange) Imbach strains, Journal of Plant Protection Research 54 (1): 102-105.
- Brien M, Kavanagh K, Grogan H (2017) Detection of Trichoderma aggressivum in bulk phase III substrate and the effect of T. aggressivum inoculum, supplementation and substrate-mixing on Agaricus bisporus yields. European Journal of Plant Pathology 147: 199-209.
- Aydoğdu M, Kurbetli I, Kitapçı A, Sülü G (2020) Aggressiveness of green mould on cultivated mushroom (Agaricus bisporus) in Turkey. Journal of Plant Diseases and Protection 127: 695-708.






























