GA3 and KNO3 Efficiency of Breaking Dormancy In Wild Brassicaceae (Brassica napus and Raphanus raphanistrum) Seeds for Enhanced Phytoremediation
Salia S Sheriff1,2*, Lexa Matasyoh3, Gelas M Simiyu1 and Barno Faith Jeruto1
1Department of Environmental Biology and Health, School of Environmental Studies, University of Eldoret, Kenya
2Department of Chemistry, College of Science, Technology, Environmental and Climate Change (COSTECC), University of Liberia, Liberia
3Department of Biological Sciences, School of Science, University of Eldoret, Kenya
Submission: May 16, 2022; Published: June 10, 2022
*Corresponding author: Salia S Sheriff, Department of Environmental Biology and Health, School of Environmental Studies, University of Eldoret, Kenya Agri
How to cite this article: Salia S S, Lexa M, Gelas M S, Barno F J.GA3 and KNO3 Efficiency of Breaking Dormancy In Wild Brassicaceae (Brassica napus and Raphanus raphanistrum) Seeds for Enhanced Phytoremediation. Agri Res & Tech: Agri Res & Tech: Open Access J. 2022; 26 (5): 556349. DOI: 10.19080/ARTOAJ.2022.26.556349
Abstract
Brassicaceae family are largely stress resistant to extreme environmental conditions particularly heavy metals laden soils. They are found in post-harvest agricultural field and sometimes during farming season costing economic loss to farmers. The species, Raphanus raphanistrum (wild radish) and Brassica napus (canola) were collected from agricultural fields within the University of Eldoret farmlands. The morphometric characteristics, longitudinal length, transverse length (width), perimeter, areas, and seed weight were assessed. Matured seeds within siliques were sundried in a glasshouse (25-45°C) for several days until constant weights were obtained. About a hundred seeds of each, wild radish and canola were mechanically removed and portioned into five germination test groups. The seeds presoaked in distilled twenty-four hours before treatment with plain water (control), concentrated acid solutions (H2SO4 and HCL 5 minutes and 30 minutes, respectively), Chilling at 4°C, 0.25mg/mL Gibberellic Acid (GA3), and 0.1% alkaline (KNO3). Pretreated seeds were transferred into sterilized petri dishes containing double-layer filter papers. The germination experiments were carried out in biotechnology lab at 20-25oC under normal lightening. In all setups, germination was effective in Brassica napus yielding 80% and 90% germination rate for GA3 and KNO3, compared to Baphanus raphanistrum, 70% and 10%, respectively. Priming with H2O, H2SO4, HCL, and chilling were ineffective in this trial.
Keywords: Induced Germination; Breaking Dormancy; Seed Priming
Introduction
Cruciferae are economically essential crop family that includes fodder crop, oilseed plants, and vegetables for food, organic fertilizers, and biofuels. Many species of Brassicaceae are tolerant to environmental stress conditions and agrochemicals application such that they are wildly used as model species [1]. There are over 3000 species from more than 300 genera of the mustard family, amongst them is the most used experimental plant, Arabidopsis thaliana. This simple angiosperm has paved ways to understanding growth and development of plants [2 Table 1: Morphological characteristics of raphanus raphanistrum.]. Numerous studies have been conducted to traceably establish the origin of the mustard family with many indicating Eurasia as the family origin before it was spread to the northern hemisphere and other parts of the world. The ancestors of brassicaceae are evidently believed to have originated from northeastern Mediterranean and later spread to Asia and Europe evidenced by the biogeographic events in the area [3]. Brassica napus is deliberated to have formed around 5000-10, 0000 mya most likely originating from the interspecific hybridization of the genotypes Brassica rapa and brassica oleracea [4]. It is one of the most cultivated medicinal crops in Eurasia and Saharan Africa, and also has attracted huge commercial value from its enriched oilseed potential to produce cooking and renewable energy [5].
Raphanus raphanistrum (wild radish), a widely known distributed weeds and it is understood to belong to the brassica plants though with some disputes of any link [6]. It has been reported as a troublesome weed for cereals in some parts of Australia, growing sporadically from multitude of seeds produced seasonally and interferes with yields of production [7,8]. It has herbicidal resistance to some agrochemicals use around the world, including Acetolactate, chlorusulfuron, and metsulfuron-methyl [9-11]. Wild radish observed some dormancy in the soil seedbank by physical restriction such as climatic conditions. The siliques provide additional adaptive mechanisms maximizing endurance during dormancy [12]. Wild radish also contains healthy and nutritious and bioactive compounds comprising of phenolic and hydroethanolic extracts that have supplementary antioxidant potential that can be added to human diet [13,14]. The bioactive compounds in Raphanus raphanistrum have long been used for the medicinal values. Several studies have reported different uses of wild radish in different areas around the world to treat different health conditions [15,16].
Materials and Method
Seeds collections
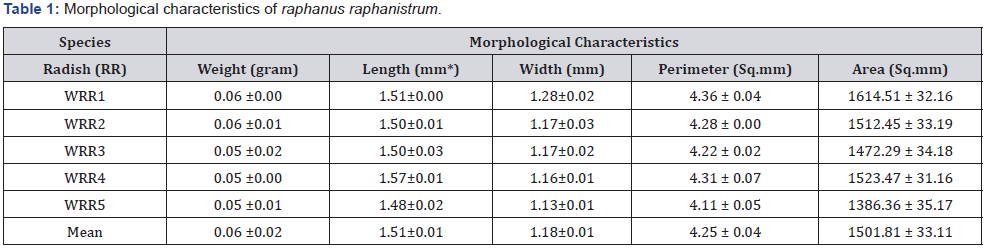
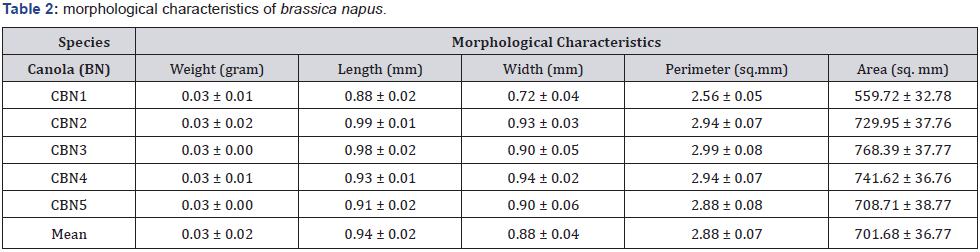
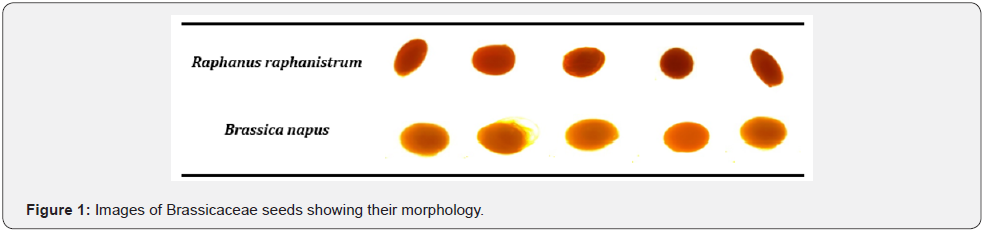
Matured Seeds of Raphanus raphanistrum (wild radish) and brassica napus (canola) were collected from three wheat farms at the University of Eldoret during postharvest seasons where they grow wildly as weeds. The seeds were garnered while still green though fully matured/raped within the siliques. They were taken to the biotechnology laboratory, where they were stratified, labelled and spread on tray for sun drying. The seeds were weighed and transferred into the glasshouse for drying at temperature ranging 25-450C. Some seeds were randomly selected to measure morphometries (Figure 1) through a microscope including longitudinal length (length), transverse length (width), perimeter and area in millimeter. The seeds, together the with their pods were measured every 24hrs for fourteen (14) days until a consist dry weight was achieved, then they were mechanically removed from the siliques and kept in paper bags at 20-250C before experiment. Individual seed of each species was measured in five replications (Table 1 & 2) and were subdivided into experimental groups, comprising 100 seeds/species and treated with different priming techniques, chemical germination agents (CGA) in order to induce laboratory germination. The laboratory test materials and reagents included 100 by 15 mm petri dishes, doubled-layer tissue papers, wash bottles, 70% ethanol, 0.25 mg/L of gibberellic acid (GA3), 0.1% potassium nitrate (KNO3), concentrated hydrochloric (HCl) and sulfuric (H2SO4) acids. The priming techniques efficiency to break dormancy in the selected cruciferae seeds were measured using equations 1, 2 and 3, to calculate Germination Index, Time for 50% germination of seedlings, Mean Germination Time, respectively [17].
Germination index (GI) was used to compute daily germination counts for the 10day trial, adopting the Association of Official Seed Analyst method as indicated in equation1 [18].
The time required for 50% of germination of the seedlings (T50) to occur was calculated using equation 2 below (Coolbear, FRANCIS, & Grierson, 1984). Though, percent of the seedling germinated at half of the experimental time, that is, in 5 days was calculated.
Where N is the final number of germinated seeds, ni and nj are ti and tj, respectively, when ni < N/2 < nj.
Similarly, the mean germination (MGT) was computed according to equation3, where n is the number of germinated seeds or emerging seedlings on day D, where D is the sum of days counted from the start of germination (Ellis & Roberts, 1981):
As indicated in Table 3 below, twenty (20) seeds of each crop were placed in 50 mL beakers and presoaked in distilled water H2O for 24hrs before treatment with chemical germination agents. The seeds were removed from the water and desiccated for 2-3 hours (hrs.) prior to treatment. The seeds were then transferred to petri dishes disinfected with 70% ethanol and filled with doubled-layer soft tissues. The seeds were unselectively divided into five (5) groups, that is, 20 seeds per petri dish (5 replicates); and treated with 10 mL of GA3, KNO3, HCl, H2SO4 and H20 in separate setups. Hydropriming using distilled H2O was the control treatments for all induced germination in these trials.
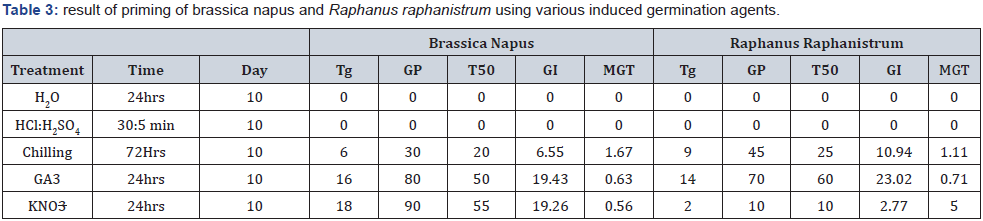
Tg=total seeds germinated, Day=numbers of days of trial, GP=germination percent, T50= percent germination at half of trial day, GI=germination index, MGT=mean germination time
Results
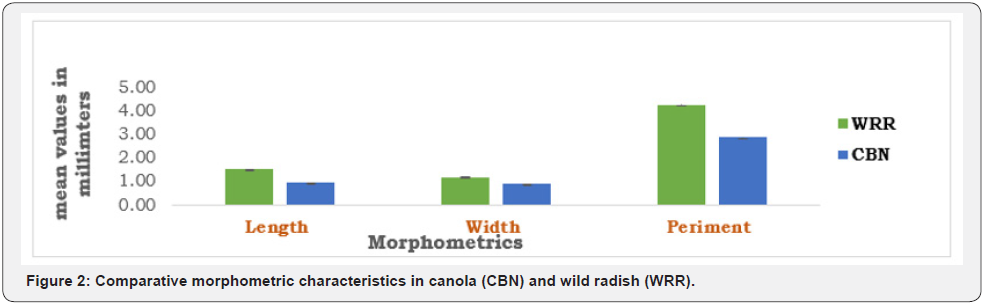
The results, Tables 1 & 2 showed simsilar morphological characteristics within species of Raphanus raphanistrum and Brassica napus, respectively.
When compared one-to-one, the morphometrics of the species showed wider difference in their perimeters, lengths and weights, with R. raphanistrum showing dominance in all physical characteristics measured as seen in Figure 2.
From the experiment, treatments of brassica napus seeds with chemical germination agent performance rate were 90, 80, 30, 0 and 0 for KNO3, GA3, chilling, HCl: H2SO4, and H2O, respectively (Table 3). Similarly, treatment of Raphanus raphanistrum with same agents gave the following results: 70, 45, 10, 0 and 0 for GA3, chilling, KNO3, HCl:H2SO4, and H2O, respectively.
Discussion
Various seed priming techniques were used to induce germination in the two crucuferae species-brassica napus and Raphanus raphanistrum. Priming is a simple, less expensive and maneuverable techniques that helps to improve seed germination, enhance early seedling emergence and stand formation against environmental stress conditions [19]. There are several means of seed priming, including osmo-priming, hydro-priming, chemical priming, physical priming, nano-priming and hormonal priming [20-22].
Chemical Priming
There are several chemical germination agents use to prime seeds including KNO3, H2O, HCl, H2SO4 using different concentrations for different seeds depending on the dormancy and stress resistance. Halopriming using 0.1mM of KNO3 used for the two species was very effective for brassica napus compared to Raphanus raphanistrum which agreed similar studies [23,24]. Also, use H2SO4 and HCl on the same crops. The seeds were exposed to the acids for a shorter time compared to KNO3, that is, 5 minutes and 30 minutes, respectively. Hydrochloric and sulfuric acids blocked germinations in both species compared to the halochemical, KNO3, and this is also true for other related studies [25,26]. On the contrary, other studies using same acids showed high germination rates compared [27-29,25].
Hormonal priming using gibberellic acid (GA3)
Gibberellic acid (GA3) is plant hormone that stimulates diverse metabolic synthesis in plant including seed germination, extensive growth, flowering; and its targeted site of bioactivity is the stamens [30,31]. A single concentration, 0.25mg/L, of GA3 was used to prime and induce germination in brassica napus and Raphanus raphanistrum. The results, 80 and 70, for CBN and WRR, respectively were the most effective priming agent overall, confirming similar finding/studies on GA3 use to enhance germination in Brassicaceae [32-34]. Though in certain plant species, and particular concentrations, GA3 slows germination of seeds [35-37].
Conclusion
The hormonal priming carried out by means of 0.25mg/L Gibberellic acid (GA3) was the most effective agent that induced germination in brassica napus and Raphanus raphanistrum followed chemical priming, 0.1M KNO3 then chilling. Hydropriming was completely ineffective to break the dormancy in the seeds. This finding is true for many seeds that go through secondary dormancy as results of building environmental resistant and adaptation.
Conflict of Interest
The authors declare that there are no known financial or personal competing interests that could have seemed to influence the work in this paper.
References
- Warwick SI (2011) Brassicaceae in agriculture. Genetics and Genomics of the Brassicaceae 33-65.
- Meinke DW, Cherry JM, Dean C, Rounsley SD, Koornneef M (1998) Arabidopsis thaliana: a model plant for genome analysis. Science 282(5389): 662-682.
- Arias T, Beilstein MA, Tang M, McKain, MR, Pires JC (2014) Diversification times among Brassica (Brassicaceae) crops suggest hybrid formation after 20 million years of divergence. Am J Bot 101(1): 86-91.
- Iniguez-Luy FL, Federico ML (2011) The genetics of Brassica napus. In Genetics and Genomics of the Brassicaceae (pp. 291-322.
- Saeidnia S, Gohari AR (2012) Importance of Brassica napus as a medicinal food plant. Journal of Medicinal Plants Research 6(14): 2700-2703.
- Yamagishi H (2017) Speciation and diversification of radish. In The radish genome (pp. 11-30).
- Cheam A, Code G, (1995) The biology of Australian weeds 24. Raphanus raphanistrum L. Plant Protection Quarterly 10(1): 2-13.
- Piggin C, Reeves T, Brooke H, Code G (1978) Germination of wild radish (Raphanus raphanistrum L.). Paper presented at the Proceedings of the First Conference of the Council of Australian Weed Science Societies 233-240.
- Costa L, Rizzardi M (2014) Resistance of Raphanus raphanistrum to the herbicide metsulfuron-methyl. Planta Daninha 32(1): 181-187.
- Smit J, Cairns A (2001) Resistance of Raphanus raphanistrum to chlorsulfuron in the Republic of South Africa. Weed research 41(1): 41-47.
- Yu Q, Han H, Li M, Purba E, Walsh M, et al. (2012) Resistance evaluation for herbicide resistance–endowing acetolactate synthase (ALS) gene mutations using Raphanus raphanistrum populations homozygous for specific ALS mutations. Weed research 52(2): 178-186.
- Tricault Y, Matejicek A, Darmency H (2018) Variation of seed dormancy and longevity in Raphanus raphanistrum L. Seed Science Research 28(1): 34-40.
- Iyda JH, Fernandes Â, Ferreira FD, Alves MJ, Pires TC, et al. (2019) Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Research International 121: 714-722.
- TURAN NN, AYDIN A (2012) Antioxidant activity and total phenolic content of aqueous extract from Raphanus Raphanistrum L. Turk J Pharm Sci 9(1): 93-100.
- Jbilou R, Ennabili A, Sayah F (2006) Insecticidal activity of four medicinal plant extracts against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). African Journal of Biotechnology 5(10).
- Lim HW, Song KY, Chon JW, Jeong D, Seo KH (2019) Antimicrobial action of Raphanus raphanistrum sativus (radish) extracts against foodborne bacteria present in various milk products: A Preliminary Study. Journal of Dairy Science and Biotechnology 37(3): 187-195.
- Ali HH, Tanveer A, Nadeem M (2012) Evaluation of some seed dormancy breaking methods on germination of Rhynchosia capitata (Roth DC). Pakistan Journal of Weed Science Research 18(4).
- Isely D (1965) Rules for testing seeds.
- Ashraf MA, Akbar A, Askari S H, Iqbal M, Rasheed R, et al. (2018) Recent advances in abiotic stress tolerance of plants through chemical priming: an overview. Advances in seed priming 51-79.
- Lutts S, Benincasa P, Wojtyla L, Kubala S, Pace R, et al. (2016) New challenges in seed biology-basic and translational research driving seed technology 1-46.
- Nawaz J, Hussain M, Jabbar A, Nadeem GA, Sajid M, et al. (2013) Seed priming a technique. International Journal of Agriculture and Crop Sciences 6(20): 1373-1381.
- Waqas M, Korres NE, Khan MD, Nizami AS, Deeba F, et al. (2019) Advances in the concept and methods of seed priming. In Priming and pretreatment of seeds and seedlings (pp. 11-41): Springer.
- Abdollahi F, Jafari L (2012) Effect of NaCl and KNO3 priming on seed germination of canola (Brassica Napus L.) under salinity conditions. International Journal of Agriculture Research and Review 2(5): 573-579.
- Omidi H, Heshmat Omidi S H, Fardin K (2011) The improvememt of seed germination traits in canola (Brassica napus L.) as affected by saline and drought stress. Journal of Agricultural Technology 7(3): 611-622.
- Rincón-Rosales R, Culebro-Espinosa N, Gutierrez-Miceli F, Dendooven L (2003) Scarification of seeds of Acacia angustissima (Mill.) Kuntze and its effect on germination. Seed Science and Technology 31(2): 301-307(7).
- YÜCEL E, DURAN A, Cengiz T, BÖCÜK H, ÖZAYDIN B (2008) Effects of different salt NaCl, nitrate KNO3 and acid HCl and H2S04 concentrations on the germination of some Hesperis species seeds. Biyolojik Çesitlilik ve Koruma 1(2): 91-104.
- Barmukh R, Nikam T (2008) Promotion of seed germination in Pterocarpus marsupium Roxb. Indian J Plant Physiol 13(2): 143-150.
- Bhoyar MS, Mishra GP, Singh R, Singh S (2010) Effects of various dormancy breaking treatments on the germination of wild caper (Capparis spinosa) seeds from the cold arid desert of trans-Himalayas. Indian Journal of Agricultural Sciences 80(7): 621-625.
- Kanmegne G, Fotso DAM, Omokolo DN (2017) Effects of substrates, different pretreatment protocols and dehydration on the induction of seeds germination of Xylopia aethiopica (Dunal) A. Rich. International Journal of Biological and Chemical Sciences 11(2): 597-608.
- Gupta R, Chakrabarty S (2013) Gibberellic acid in plant: still a mystery unresolved. Plant signaling & behavior 8(9): e25504.
- Schwechheimer C (2008) Understanding gibberellic acid signaling-are we there yet? Current opinion in plant biology, 11(1): 9-15.
- Bojovic B, Ðelic G, Topuzovic M, Stankovic M (2010) Effects of NaCl on seed germination in some species from families Brassicaceae and Solanaceae. Kragujevac J Sci 32: 83-87.
- Chauhan BS, Gill G, Preston C (2006) African mustard (Brassica tournefortii) germination in southern Australia. Weed Science 54(5): 891-897.
- Li Z, Lu G, Zhang X, Zou C, Cheng, et al. (2010) Improving drought tolerance of germinating seeds by exogenous application of gibberellic acid (GA3) in rapeseed (Brassica napus L.). Seed Science and Technology 38(2): 432-440(9).
- Ghodrat V, Rousta MJ (2012) Effect of priming with Gibberellic acid (GA3) on germination and growth of corn (Zea mays L.) under saline conditions. International Journal of Agriculture and Crop Sciences (IJACS) 4(13): 882-885.
- Coolbear P, FRANCIS A, Grierson D (1984) The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. Journal of Experimental Botany 35(11): 1609-1617.
- Ellis R, Roberts E (1981) The quantification of ageing and survival in orthodox seeds. Seed Science and Technology (Netherlands) (2): 373-409.






























